Abstract
δ-Catenin is a member of the p120-catenin subfamily of armadillo proteins. Here, we describe distinctive features of δ-catenin localization and its association with E-cadherin in HEK293 epithelial cells. In HEK293 cells maintained in low cell densities, approximately 15% of cells overexpressing δ-catenin showed dendrite-like process formation, but there was no detectable change in RhoA activity. In addition, δ-catenin was localized mainly in the cytoplasm and was associated with p190RhoGEF. However, at high cell densities, δ-catenin localization was shifted to the plasma membrane. The association of δ-catenin with E-cadherin was strengthened, whereas its interaction with p190RhoGEF was weakened. In mouse embryonic fibroblast cell, ectopic expression of E-cadherin decreased the effect of δ-catenin on the reduction of RhoA activity as well as on dendrite-like process formation. These results suggest that δ-catenin is more dominantly bound to E-cadherin than to p190RhoGEF, and that δ-catenin’s function is dependent on its cellular binding partner.
Keywords: δ-catenin, cadherin, dendrogenesis, adherens junction, RhoA
Introduction
δ-Catenin is mainly expressed in the brain [1], and its associated protein partners in cells include Presenilin-1 [2], E-cadherin [3], 14-3-3 [4; 5], Kaiso [6], and others. Recently, we found that p190RhoGEF, a Rho-specific guanine nucleotide exchange factor (GEF), interacts with δ-catenin. We provided evidence that δ-catenin decreases RhoA activity by sequestering p190RhoGEF and results in increases both in the branching of dendrite-like process in NIH 3T3 fibroblasts and in the lengths and numbers of mature mushroom shaped spines in primary hippocampal neurons [4; 7].
Epithelial cells express E-cadherin, whereas fibroblasts and neuronal cells express mainly N-cadherin [8]. E-cadherin is a homophilic cell adhesion molecule. It is found at adherens junctions and associates with actin-cytoskeleton via α- and β-catenin. Moreover, these associations are critical for maintaining an epithelial cell architecture [9]. δ-Catenin binds to the juxtamembrane domain of E-cadherin [3]. In contrast to the secure and stable cell-cell interface formed by epithelial cells, fibroblasts and neuronal cells form rather temporary, functional contacts involving N-cadherin at limited cell-cell interfaces. Interestingly, N-cadherin plays a pivotal role in synaptic plasticity [10]. Even though both β- and δ-catenin can interact with N-cadherin [3], pools of these molecules at the cell-cell interfaces of fibroblasts and neuronal cells are significantly fewer and smaller than those at epithelial cell interfaces. Moreover, δ-catenin is known to induce remarkable dendritic branches in both fibroblasts and primary hippocampal neurons [7]. However, morphological changes induced by δ-catenin overexpression in epithelial cells are relatively minimal compared to those induced in fibroblasts and neuronal cells. Lu et al. found that MDCK epithelial cells overexpressing δ-catenin tend to lose their polygonal morphology and assume either irregular shapes or an elongated fibroblastic appearance [3].
Because δ-catenin affects cell morphology differently in epithelial cells compared to those in fibroblasts, we decided to test the hypothesis whether the binding partner protein of δ-catenin is altered in epithelial cells. The present study demonstrates that E-cadherin competes with p190RhoGEF for the interaction with δ-catenin in a cell density-dependent manner, and that overexpressed δ-catenin induces noticeable morphological changes in HEK293 epithelial cells only at low cell density. By ectopic expression of E-cadherin, the effect of δ-catenin on morphological changes and on reduction RhoA activity in mouse embryonic fibroblast (MEF) cell was abolished. Our results suggest that E-cadherin dominates the interaction with δ-catenin over p190RhoGEF in epithelial cells, and the cell type specific binding partner for δ-catenin is a determinant of its function.
Experimental procedures
Plasmids and Antibodies
The construction of δ-catenin in pEGFP-C1 has been previously described [7]. The expression plasmid containing a dominant negative (DN) form of RhoA was provided by K.Y. Lee (Chonnam National University, Gwangju, Korea), and E-Cadherin was provided by C. Vanderburg. The antibodies used were as follows: anti-δ-catenin (C98320) (BD Biosciences); anti-RhoA (sc-418n) (Santa Cruz Biotechnology); anti-GFP (632376) (Sigma). HA epitopes were detected using media from 12CA5 hybridoma.
Cell culture and transfection
Mouse embryonic fibroblasts (MEF) and HEK293 cells were cultured in Dulbecco’s modified Eagles medium (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. Cells were transfected using calcium phosphate or Lipofectamine Plus reagent (Invitrogen), according to manufacturers’ instructions.
Affinity-precipitation of cellular GTPases
Cellular RhoA activities were determined using GST-RBD, as we previously described [4]. Briefly, cells were lysed in lysis buffer. Lysates were cleared by centrifugation and then incubated with GST-RBD beads (Cytoskeleton Inc.) at 4°C for 1 hr. Beads were then washed four times with the washing buffer, and bound RhoA proteins were detected by immunoblotting using a monoclonal antibody against RhoA. To determine the relative activities of RhoA proteins, GTP-bound RhoA bands and total RhoA were quantified using the TINA 2.09 software program (Raytest). GTP-bound RhoA bands were normalized versus total RhoA. Recombinant protein quantities were confirmed using Direct blue 71 staining kits (EZBiopaQ, Co., Ltd.).
Immunoblotting
Immunoprecipitation/immunoblotting was performed as previously described [11]. Briefly, proteins were boiled at 95°C for 2 min with sample buffer and subjected to electrophoresis. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore) and probed with antibodies. Bound antibodies were visualized using horse-radish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL) western blotting detection reagents (Millipore).
Image acquisition
Cells were visualized under an inverted fluorescent microscope (Eclipse TE2000-U; Nikon). Photographs were acquired using the ACT-2U program (Nikon) using a DS-U1 device-controlled camera (DS-5M; Nikon) and compiled in Adobe Photoshop.
Results
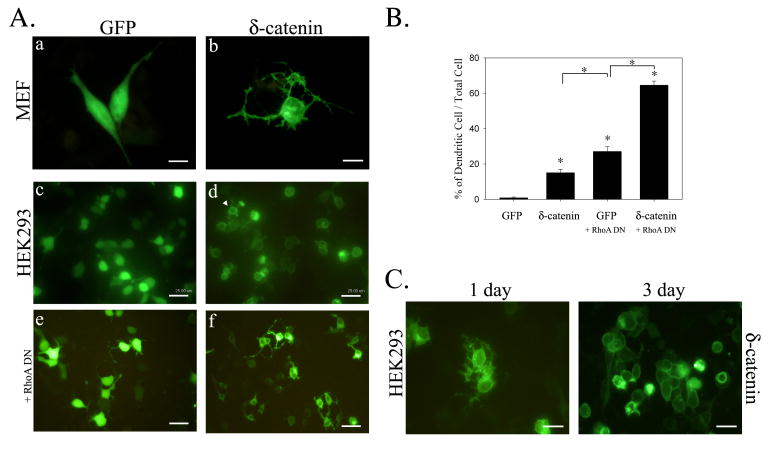
Our previous study showed that δ-catenin overexpression induces dendrite-like processes with branches in NIH 3T3 fibroblasts and that it enhances dendritic morphogenesis in primary hippocampal neurons [7]. To determine the effects of δ-catenin overexpression on epithelial cells, we first compared the morphological changes in HEK293 cells transfected with GFP tagged δ-catenin to those of mouse embryonic fibroblasts (MEF). Initially, cell morphologies were examined at fewer than 50% confluences where cell-cell contact was limited, and was further observed 3 days after transfection when monolayer was formed with complete cell-cell contact. While all GFP positive MEF cells with δ-catenin overexpression had a typical dendritic morphology even at 50% confluence, only 15 ± 7% of GFP positive HEK293 cells showed dendrite-like process formation at 50% confluence (right panel, Fig. 1A, a–d, and Fig. 1B). In comparison, the overexpression of dominant negative RhoA mutant (RhoA DN) induced dendrite-like process formation in 27 ± 8% of 50% confluent HEK293 cells, and co-transfecting dominant negative mutant of RhoA (RhoA DN) with δ-catenin enhanced δ-catenin induced dendrite-like process formation to 65 ± 7% in these cells (Fig. 1A, e – f, and Fig. 1B). Moreover, in 50% confluent HEK293 cells, δ-catenin was localized mainly in the cytoplasm, as was observed for fibroblasts. On the other hand, when 50% confluent HEK293 cells were allowed to culture for 3 days to fully form cell-cell contacts, δ-catenin was found to be predominantly localized at the plasma membrane and cell-cell junction. In addition, these cells showed no noticeable morphologic changes (Fig. 1C).
Figure 1. δ-catenin-induced morphological changes in MEF and HEK293 cell.
(A) MEF (a-b) and HEK293 (c-f) cells were transfected with either GFP or GFP-δ-catenin, and fluorescent images were taken after 1 day post-transfection. HEK293 cells were also co-transfected with GFP-δ-catenin and dominant negative (DN) mutant of RhoA (e-f), and fluorescent images were taken after 1 day post-transfection. Almost all GFP-δ-catenin positive MEF cell showed dendrite-like process formation, but approximately 15% of GFP-δ-catenin positive HEK293 cells showed such morphological changes. (B) Percentage of cells showing dendrite-like process formation among total GFP-positive cells. The data are represented as the mean ± S.E.M (*, p<0.0001) (C) HEK293 cells were transfected with GFP-δ-catenin and fluorescent image was taken after 1 and 3 day post-transfection. Scale bars, 25 um.
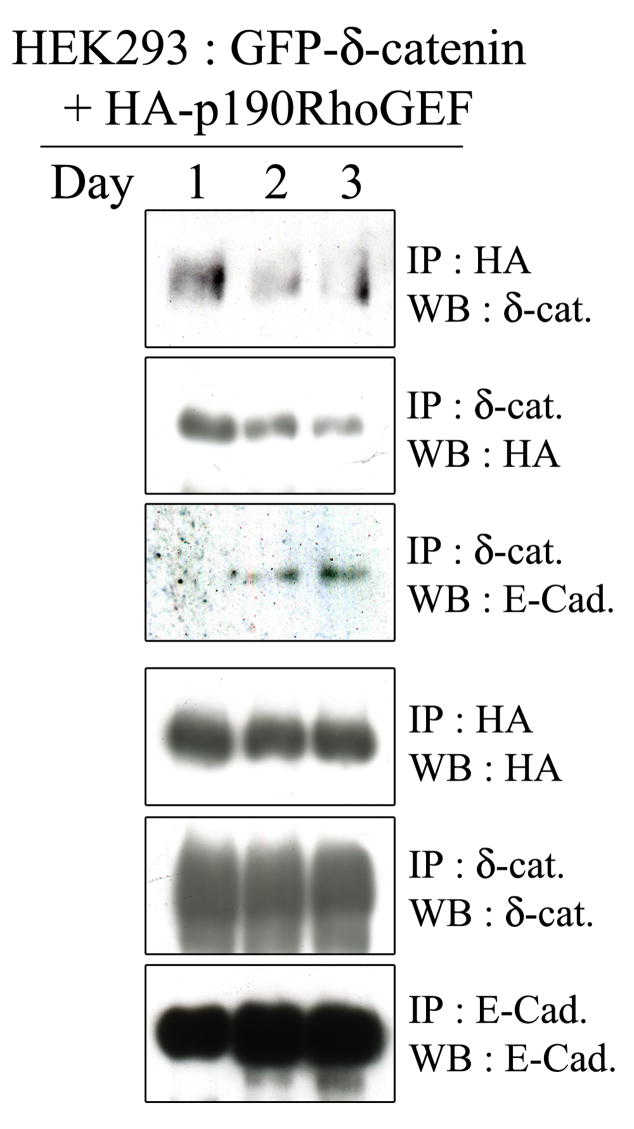
Based on the results shown in Figure 1, we hypothesized that δ-catenin’s binding partners in HEK293 epithelial cells change in a cell density-dependent manner. In other words, when cell densities are low and cell junctions are not fully formed, δ-catenin may localize to the cytoplasm and bind to cytoplasmic proteins such as p190RhoGEF. Alternatively, at high densities when stable cell-cell junctions were fully formed, δ-catenin binds to E-cadherin at adherens junctions. To investigate this possibility, we transfected δ-catenin with p190RhoGEF into HEK293 cells, and harvested cells daily for 3 days. Protein extracts were then subjected to immunoprecipitation assays. We found that δ-catenin was mainly associated with p190RhoGEF at day 1, but this association reduced noticeably with time (Fig. 2, upper first and second panels). The total levels of δ-catenin and p190RhoGEF remained constant (Fig. 2, bottom second and third panel). On the other hand, the association between δ-catenin and E-cadherin was gradually increased with time (Fig. 2, upper third panel). Total E-cadherin levels were increased with time, which could have been the result of E-cadherin stabilization due to the formation of a mature zonula adherens.
Figure 2. Interaction of δ-catenin with E-cadherin is increased as cells reach confluence, whereas the interaction with p190RhoGEF is decreased.
HEK293 cells were transfected with GFP-δ-catenin and p190RhoGEF, and proteins were extracted at the indicated day of post-transfection. Cell lysates were then subjected to immunoprecipitation followed by immunoblotting using specific antibodies to detect δ-catenin, p190RhoGEF, and E-cadherin.
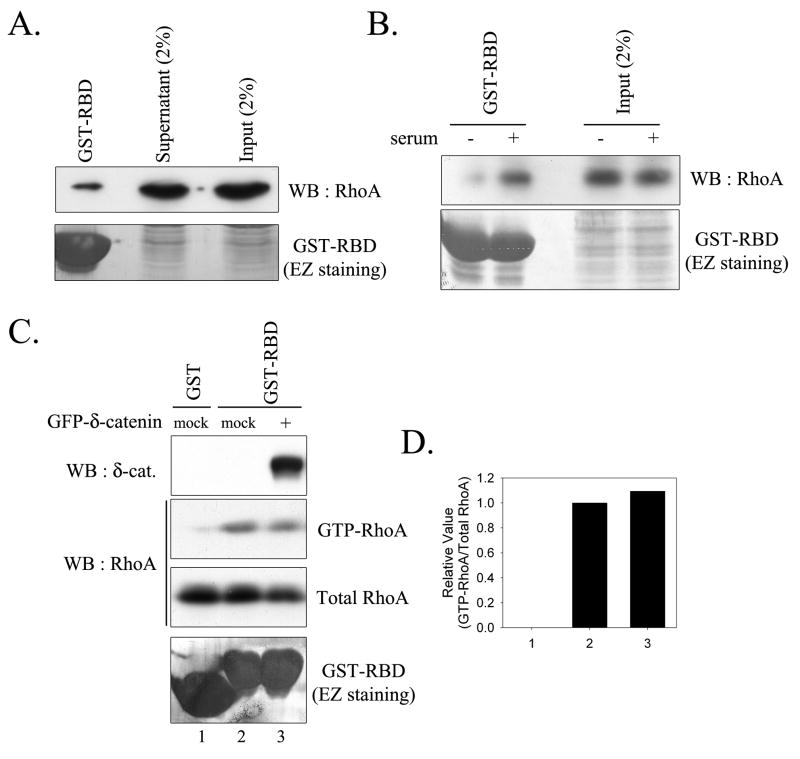
Because δ-catenin overexpression has been previously reported to reduce RhoA activity by 40% in MEF cells [4], we examined the effect of δ-catenin overexpression on RhoA activity in epithelial cells. Accordingly, we performed RhoA assays using the lysates of HEK293 cells transfected with either mock or GFP-δ-catenin. Quantitative analysis revealed that although GST-RBD retained GTP bound RhoA accounted for less than 1% of cellular RhoA in HEK293 cells, RhoA activity sensitively responded to serum deprivation/activation (Fig. 3, A and B). As shown in Figure 3C, δ-catenin overexpression did not reduce RhoA activity in HEK293 cell. Furthermore, quantitative analyses for GTP-RhoA and total RhoA indicated no significant difference between active GTP-RhoA levels in mock and δ-catenin transfected HEK293 epithelial cells (Fig. 3D).
Figure 3. δ-catenin overexpression does not induce any significant changes in RhoA activity in HEK293 cells.
(A) HEK293 cell lysates were subjected to RhoA assay to analyze cellular RhoA activity. (B) HEK293 cells were incubated without serum for overnight, and cells were incubated either with or without serum for additional 30 min, then harvested and subjected to RhoA assay. (C) Levels of GTP-RhoA were measured in HEK293 cells transfected with either mock or GFP-δ-catenin. (D) Relative activity of RhoA was determined as described under “Experimental procedures”. Number under graph is identical to the lane number in Figure 3C. No significant change was observed.
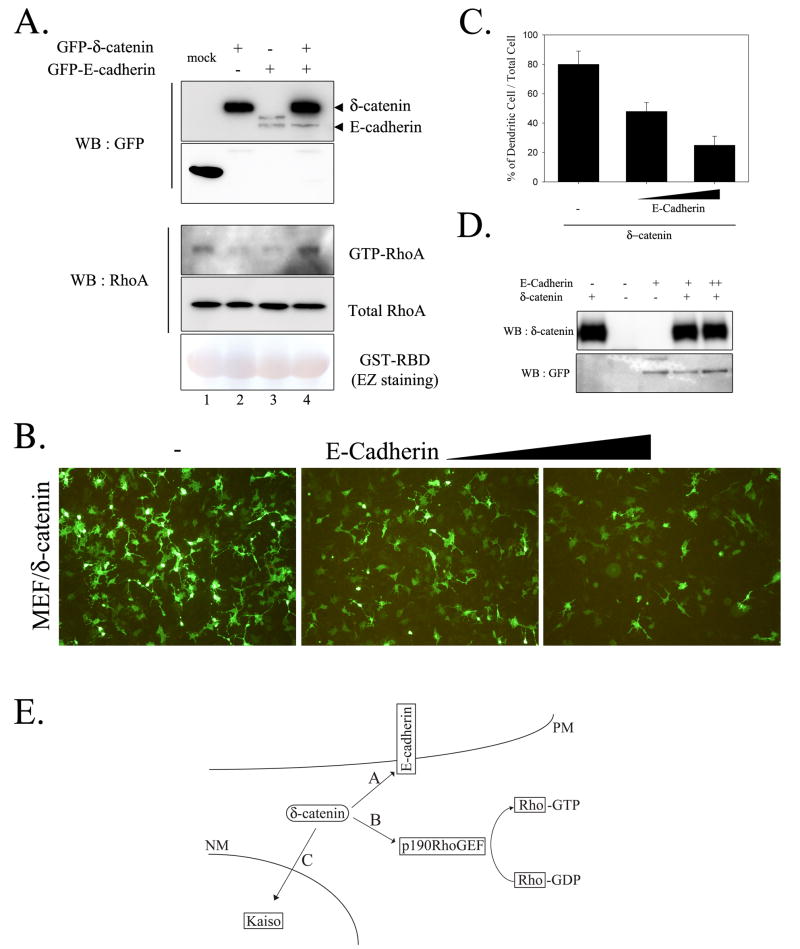
The presence of E-cadherin in HEK293 cell may sequester δ-catenin, thus abolish δ-catenin induced morphological changes and reduced RhoA activity. We examined the possibility whether ectopic E-cadherin expression in MEF cell, which does not contain endogenous E-cadherin, abolishes the reduced RhoA activity induced by δ-catenin overexpression and, thereby, reduces the dendrite-like process formation. As shown in Figure 4A and our previous report [4], δ-catenin overexpression reduced RhoA activity by 40% in MEF cell. However, E-cadherin co-expression with δ-catenin abolished this effect. Expression of E-cadherin alone also reduced the RhoA activity (Fig. 4A) but did not induce any noticeable dendrite-like processes in HEK293 cell (data not shown). Furthermore, the dendrite-like process formation by δ-catenin overexpression was noticeably reduced by E-cadherin co-expression in a dose-dependent manner (Fig. 4, B and C). On the other hand, δ-catenin level was not altered by E-cadherin expression in MEF cell (Fig. 4D). Based on these results, we proposed a model illustrating cellular δ-catenin localizations and interactions (Fig. 4E). In the absence of E-cadherin and/or E-cadherin-mediated cell-cell contacts, δ-catenin is located in the cytoplasm and binds to cytoplasmic proteins such as p190RhoGEF, which results in the downregulation of RhoA activity (B, Fig. 4E). However, in the presence of E-cadherin and/or E-cadherin-mediated cell-cell contacts, δ-catenin prefers to bind to E-cadherin (A, Fig. 4E).
Figure 4. Co-expression of E-cadherin with d-catenin reduced the effects of d-catenin on lowering RhoA activity and dendrite-like process formation.
(A) Levels of GTP-RhoA were measured in MEF cells transfected as indicated. (B) MEF cells were transfected with d-catenin or increased amount of E-cadherin expressing plasmid, and fluorescent image was taken after 1-day post-transfection. (C) Percentage of cells showing dendrite-like process formation among total GFP-positive cells. (D) MEF cell lysates were subjected to western blotting and levels of d-catenin and E-cadherin were shown. (E) Schematic illustration of δ-catenin interaction with different binding partners in cells. A) In the presence of E-cadherin and/or E-cadherin-mediated cell-cell contacts, cellular microenvironment is preferential for δ-catenin localization in the plasma membrane and for the association of δ-catenin with E-cadherin. B) In the absence of E-cadherin and/or E-cadherin-mediated cell-cell contact, δ-catenin is localized in the cytoplasm and binds cytoplasmic proteins such as p190RhoGEF, which results in the down-regulation of RhoA activity. C) δ-catenin enters the nucleus and interacts with Kaiso, a transcription factor. The signal for δ-catenin nuclear localization and/or function remains elusive. PM, plasma membrane; NM, nuclear membrane.
Discussion
δ-Catenin is mainly expressed in the brain, and therefore, has attracted research attention in the contexts of dendritic morphogenesis and cognitive function. However, recent reports showed thatδ-catenin is up-regulated in some carcinomas, which raises the possibility that δ-catenin plays a role in tumorigenesis. With the exception of the association between δ-catenin and E-cadherin, the roles of δ-catenin in epithelial cells are not fully understood. The present study has revealed following major findings concerning the role of δ-catenin, i.e., 1) δ-Catenin overexpression induces noticeable morphological changes in HEK293 cells only when the cell-cell interface is unstable; 2) The interaction between δ-catenin and E-cadherin is stronger than that between δ-catenin and p190RhoGEF; 3) The overexpression of δ-catenin does not induce any noticeable reduction in the levels of GTP bound RhoA in HEK293 cells; 4) Ectopic expression of E-cadherin in MEF cell abolishes δ-catenin-induced morphological changes and reduced RhoA activity.
Unlike epithelial cells, fibroblasts and neuronal cells form limited cell-cell contacts, which probably leads to free pools of δ-catenin in the cytoplasm, and increases the likelihood that δ-catenin will interact with other cytoplasmic binding partners such as p190RhoGEF. The presence of N-cadherin in these cells should not exert strong interaction with δ-catenin, as it remains free in the cytoplasm unless cells form functional junctions. In contrast to the dramatic effects of δ-catenin on the morphologies of fibroblasts and neuronal cells, HEK293 epithelial cells overexpressing δ–catenin show noticeable morphologic changes only when the cell-cell interface is unstable (i.e., cells located to the edges of epithelial cell islands or single cells). As was shown by Martinez et al. for PC12 cells [12], we also found that co-transfecting dominant negative mutant of RhoA in the presence of δ-catenin enhanced morphologic changes in epithelial cells, which strongly suggests that δ–catenin is capable of inducing morphologic changes even in epithelial cells, possibly by reducing RhoA activity. However, we could not biochemically measure such alterations in RhoA activity using RhoA assays. Theoretically, changes in RhoA activity should also be detected in lysates from δ-catenin overexpressed HEK293 cell when the cell-cell interface was unstable, i.e., at 1 day post-transfection. The intrinsic level of active RhoA is fairly low in HEK293 cell, and our quantitative analysis showed that less than 1 % of total RhoA was retained by GST-RBD in HEK293 cells. Moreover, Ren et al. previously described that RBD-bound Rho accounts for ~0.5–5% of total RhoA [13], and quantitative analysis performed by Kimura et al. revealed that about 5% of Val14-Rho expressed in COS cells was precipitated by mDia-RBD [14]. Furthermore, only 15% of HEK293 epithelial cells overexpressing δ-catenin showed noticeable dendritic morphogenesis. Thus, changes in RhoA activity in HEK293 ought to be much less than that of in MEF, and such small changes cannot be detected by GST-RBD pull-down assays.
Dendritic HEK293 epithelial cells may undergo cell death or morphologic transformation to classical cobblestone-shaped epithelial cells. According to our observations, δ-catenin was translocated from the cytoplasm to the plasma membrane as the cell population grew, which suggest that δ-catenin is translocated during the formation of stable epithelial islands. As demonstrated in Figure 2, the interaction between p190RhoGEF and δ-catenin weakened with time, whereas the association between E-cadherin and δ-catenin strengthened. Even though total levels of E-cadherin increases and level of p190RhoGEF remained relatively constant, their interactions with δ-catenin changed markedly with time. We propose the following mechanism for this process. First, p190RhoGEF and E-cadherin compete with each other for a common binding site on δ–catenin. β-Catenin, a prototype of Arm repeat proteins, contains 12 central Arm repeats, which mutually exclusively bind with interacting molecules, such as, APC, E-cadherin, and LEF-1/TCFs. As Lu et al. demonstrated by deletion analysis for the δ–catenin interaction with E-cadherin, the central Arm repeats (Arm 1–10) of δ–catenin are sufficient to allow it to interact with E-cadherin [3]. We previously demonstrated the importance of Akt1-mediated phosphorylation at Thr-454 residue of δ-catenin for the interaction between p190RhoGEF and δ–catenin [4]. It remains elusive whether the part of central Arm repeats is responsible for the interaction between p190RhoGEF and δ–catenin when Thr-454 residue of δ-catenin is phosphorylated by Akt. If p190RhoGEF and E-cadherin compete with each other for δ-catenin, the binding affinity of E-cadherin to δ–catenin should be stronger than that of p190RhoGEF in order to explain our results. Otherwise, as cell-cell interface formed, cellular microenvironment would favor the association between δ-catenin and E-cadherin. Indeed, p120-catenin, a prototype of 10 Arm repeat subfamily proteins, localizes to the cytoplasm in cadherin-deficient cells, and cadherins are both necessary and sufficient for recruitment of p120-catenin to membrane/junctions [15]. Second, the interaction of δ-catenin with E-cadherin may occur earlier than that with p190RhoGEF during protein transports from endoplasmic reticulum to elsewhere in the cells. Third, the stability of δ-catenin may depend on the binding partner in cells. In the case of β-catenin, it remains stable when it binds to E-cadherin. When it is released from E-cadherin complexes, it is degraded by GSK3/Axin/APC complexes before it binds to another binding partner such as LEF-1 [16]. Interestingly, we recently found that GSK3 negatively regulates the stability of δ-catenin as well (unpublished result of Oh et al.).
Because fibroblast cells do not express E-cadherin, it is worth verifying whether ectopic expression of E-cadherin in this cell affects the action of d-catenin. Interestingly, co-expression of E-cadherin with δ-catenin restores reduced RhoA activity by δ-catenin overexpression and decreases δ-catenin-induced dendritic morphogenesis in MEF cell. However, expression of E-cadherin alone in this cell decreases RhoA activity as well. This can be comprehended by previous literature demonstrating that cadherin signaling activates Rac1 and Cdc42 while suppresses RhoA activity [17; 18]. Thus, we speculate that E-cadherin and δ-catenin may mutually sequester each other from their own signaling.
It still remains elusive what the major function of δ-catenin is in epithelial cells or epithelial-derived carcinoma cells. During brain development, δ-catenin staining is most intense at the apical end of neuroepithelial cells in the cortical ventricular zone where adherens junctions are prominent [19], and the overexpression of δ-catenin has been reported in tumors [20; 21]. These results suggest that δ-catenin indeed play certain roles in epithelial cells. Interestingly, p120-catenin regulates the stability of E-cadherin by controlling its endocytosis, is localized aberrantly to the cytoplasm in metastatic cell lines that had lost E-cadherin, and is implicated in the regulation of tumorigenesis and metastasis [22]. Further, p120-catenin localization shifts from cell-cell junction to the cytoplasm during epithelial to mesenchymal transition (EMT) [23]. Along the same line, it is reasonable to deduce that downregulation of E-cadherin in tumor triggers the shifts of δ-catenin from cell-cell interface to the cytoplasm, which may favor tumor invasion and metastasis. As cadherin complexes play pivotal roles in the maintenance of epithelial architecture, the regulation of cell proliferation and the suppression of tumor metastasis, it is of great interest to determine the role of δ-catenin in E-cadherin complexes. Future experiments will be required to answer this question and to further define the role of δ-catenin in epithelial-derived carcinoma cells.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-521-E00024) (to K.K.), by Grant KOSEF S&T Graduate Scholarship (to H.K.), and by National Institutes of Health CA111891 and AG 026630 (to Q. L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paffenholz R, Franke WW. Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation. 1997;61:293–304. doi: 10.1046/j.1432-0436.1997.6150293.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–90. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]
- 3.Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS. delta-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol. 1999;144:519–32. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Han JR, Park J, Oh M, James SE, Chang S, Lu Q, Lee KY, Ki H, Song WJ, Kim K. {delta}-Catenin-induced Dendritic Morphogenesis: AN ESSENTIAL ROLE OF p190RhoGEF INTERACTION THROUGH AKT1-MEDIATED PHOSPHORYLATION. J Biol Chem. 2008;283:977–87. doi: 10.1074/jbc.M707158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie S, Aitken A. Novel brain 14-3-3 interacting proteins involved in neurodegenerative disease. FEBS J. 2005;272:4202–10. doi: 10.1111/j.1742-4658.2005.04832.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodova M, Kelly KF, VanSaun M, Daniel JM, Werle MJ. Regulation of the rapsyn promoter by kaiso and delta-catenin. Mol Cell Biol. 2004;24:7188–96. doi: 10.1128/MCB.24.16.7188-7196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Sirota A, Chen YH, Jones YhSB, Dudek R, Lanford GW, Thakore C, Lu Q. Dendrite-like process formation and cytoskeletal remodeling regulated by delta-catenin expression. Exp Cell Res. 2002;275:171–84. doi: 10.1006/excr.2002.5503. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Daniels KJ, Hay ED. Tissue-specific expression of beta-catenin in normal mesenchyme and uveal melanomas and its effect on invasiveness. Exp Cell Res. 1998;245:79–90. doi: 10.1006/excr.1998.4238. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–7. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Ki H, Park HS, Kim K. Presenilin-1 D257A and D385A mutants fail to cleave Notch in their endoproteolyzed forms, but only presenilin-1 D385A mutant can restore its gamma-secretase activity with the compensatory overexpression of normal C-terminal fragment. J Biol Chem. 2005;280:22462–72. doi: 10.1074/jbc.M502769200. [DOI] [PubMed] [Google Scholar]
- 12.Martinez MC, Ochiishi T, Majewski M, Kosik KS. Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho. J Cell Biol. 2003;162:99–111. doi: 10.1083/jcb.200211025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J Biol Chem. 2000;275:17233–6. doi: 10.1074/jbc.C000212200. [DOI] [PubMed] [Google Scholar]
- 15.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–8. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 18.Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–46. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- 19.Ho C, Zhou J, Medina M, Goto T, Jacobson M, Bhide PG, Kosik KS. delta-catenin is a nervous system-specific adherens junction protein which undergoes dynamic relocalization during development. J Comp Neurol. 2000;420:261–76. [PubMed] [Google Scholar]
- 20.Burger MJ, Tebay MA, Keith PA, Samaratunga HM, Clements J, Lavin MF, Gardiner RA. Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer. Int J Cancer. 2002;100:228–37. doi: 10.1002/ijc.10468. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q, Dobbs LJ, Gregory CW, Lanford GW, Revelo MP, Shappell S, Chen YH. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer. Hum Pathol. 2005;36:1037–48. doi: 10.1016/j.humpath.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 22.van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta. 2007;1773:78–88. doi: 10.1016/j.bbamcr.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938–45. doi: 10.1158/0008-5472.CAN-05-1947. [DOI] [PubMed] [Google Scholar]