Abstract
Ultraviolet radiation (UV) induces apoptosis and functional maturation in skin dendritic cells (DCs). However, the molecular mechanisms through which UV activates DCs have not been thoroughly investigated. In this study, we examined the mechanisms of activation and apoptosis of DC after UV irradiation by focusing on epidermal growth factor receptor (EGFR). Our previous studies have demonstrated that in addition to cognate ligands, EGFR is also activated by UVB irradiation in cultured human skin keratinocytes in vitro and in human skin in vivo. We found for the first time in this study that UV also induces EGFR activation in cultured mouse skin DCs (XS 106 cell line) as well as mouse monocyte-derived dendritic cells (MoDCs). Pharmacological inhibition of EGFR tyrosine kinase significantly inhibits UV-induced ERK, p38, and JNK MAP kinases, and their effectors, transcription factors c-Fos and c-Jun. Inhibition of EGFR also suppresses UV-induced activation of PI3K/AKT/mTOR/S6K and NF-κB signal transduction pathways. Our data demonstrated that UV induces LKB1/AMPK pathway, also dependent on EGFR trans-activation. We further observed that MAPK, LKB1/AMPK, PI3K/AKT/mTOR/S6K as well as NF-κB activation are impaired in EGFR−/− cells compared to wide type MEF cells after UV radiation. Taken together, we conclude that UV induces multiple signaling pathways mediated by EGFR trans-activation leading to possible maturation, apoptosis and survival, and EGFR activation protects against UV-induced apoptosis in cultured mouse dendritic cells.
Keywords: UV, EGFR, signal transduction, skin dendritic cells
1. Introduction
Ultraviolet radiation (UV) impairs skin immune system both locally and systemically. In terms of the former, UV abrogates the contact hypersensitivity reaction at the irradiated site and induces tolerance for applied antigens [1]. UV also penetrates into papillary area of the dermis (~0.2 mm) and induces DNA damage on residing dendritic cells (DC), that is, epidermal Langerhans cells (LC) and dermal dendritic cells (DDC), as well as keratinocytes [2]. When LCs are isolated and cultured in vitro and irradiated with UVB, they are perturbed both phenotypically and functionally undergoing apoptosis [3]. In monocyte-derived dendritic cells (or MoDC) that activation of caspases 3, 8, and 9 is involved in the apoptotic processes. Some LCs, however, show maturation, when they are irradiated with UVB in vivo or ex vivo [4]. Thus far, the mechanisms through which UVB activates DCs have not been well studied.
Previous studies in skin keratinocytes have demonstrated that UV response comprises UV activation of cell surface growth factor and cytokine receptors and their attendant downstream signal transduction machinery such as MAPK and PI3K [5–7]. UV activation of four major families of growth factor receptors has been demonstrated: epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor, fibroblast growth factor receptor, and insulin receptor. Accumulating data have indicated that EGFR, among other growth factor receptors, remains a critical mediator of ultraviolet B radiation-induced signal transduction [6].
Binding of EGF family ligands to EGFR triggers a complex network of signaling pathways, culminating in responses ranging from cell division to death, and motility to adhesion proteolysis [8]. Dysregulation of EGFR family protein tyrosine kinases (HER, erbB) has been reported in multiple epithelial human cancers [9]. In addition to activation by their cognate ligands, EGFR proteins are trans-activated by several other molecules or conditions. For example, cellular stress conditions, treatment with inflammatory cytokines, oxidative stresses, as well as UV radiation, induce either tyrosine phosphorylation or serine/threonine phosphorylation of EGFR [5, 6, 10, 11]. However, whether EGFR can be trans-activated by UV in DCs and the effect of this trans-activation have not been studied.
Therefore, in this study, we examined the mechanisms of activation or apoptosis of DC after UVB irradiation by focusing on EGFR using specific inhibitors for EGFR and EGFR genetic manipulation, and clarified the roles played by EGFR and downstream signal transduction cascades in the maturation and apoptosis of DC. We conclude, based on our data, that EGFR is a critical mediator of UV-induced signal transduction in cultured mouse skin dendritic cells.
2. Materials and methods
2.1. UV light apparatus
As previously reported [11, 12], UV-irradiation apparatus used in this study consisted of four F36T12 EREVHO UV tubes. A Kodacel TA401/407 filter was mounted 4 cm in front of the tubes to remove wavelengths <290 nm. Irradiation intensity was monitored using an IL443 phototherapy radiometer and a SED240/UV/W photodetector. Before UV irradiation, cells were washed with 1 ml PBS and changed to fresh 0.5 ml PBS each well. Cells were irradiated at the desired intensity without plastic dish lid. After UV irradiation, cells were returned to incubation in basal medium with treatments for various time points prior to harvest.
2.2. Chemicals and reagents
PD 153035, AG 1478, LY 294002, Wortmannin, Rapamycin, PD 98059, U 0126, SB 203580 and JNK inhibitor (JNKi) were from CalbioChem (San Diego, CA). EGFR (1005) antibody, IκBα antibody, goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse anti-β-actin was obtained from Sigma (St. Louis, MO). phospho-AKT (Ser473), phospho-AKT (Thr308), phospho-S6K (Thr389), phospho-4E-BP1(Ser65), total-AKT, phospho-EGFR (Tyr1068), phospho-EGFR (Tyr1045), phospho-mTOR (Ser2448), phospho-AMPK (Thr172), phospho-p38 (Thr180/Tyr182), phospho-LKB1 (Ser428), p-IκB (Ser32/36), SAPK/JNK, p38 antibody and AKT antibody were all from Cell Signaling Technology (Danvers, MA).
2.3. Cell culture
Spontaneously immortalized human keratinocytes (HaCaT cell line) were used as previously reported [12–14]. EGFR wild type MEFs (Mouse Embryonic Fibroblasts), EGFR knockout MEFs [15] from Dr. Zhigang Dong, AKT wild type MEFs, AKT1, AKT1/2 knockout MEFs were from Dr. Wen-ming Chu. Cells were maintained in a DMEM medium (Sigma, St. Louis, MO), supplemented with a 10% fetal bovine serum (Invitrogen, Carlsbad, CA), Penicillin/Streptomycin (1:100, Sigma, St. Louis, MO) and 4 mM L-glutamine (Sigma, St. Louis, MO), in a CO2 incubator at 37°C. Cultured mouse skin dendritic cell line XS-106 (DCs) from Dr. Takashima [16, 17]. Primary cultured mouse monocyte-derived dendritic cells (MoDCs) from Dr. Wenming Chu, were from mice bone marrow, maintained in a MEM medium (Sigma, St. Louis, MO) supplemented with a 10% FBS plus GM-CSF (50 ng/ml). For Western blot analysis, cells were reseeded in 6-well plates at a density of 0.2×106 cells/ml with fresh complete culture medium.
2.4. Western blot analysis
As reported previously [12, 13, 18], cultured cells with and without treatments were washed with cold PBS and harvested by scraping into 150 µl of RIPA buffer with protease inhibitor. 20 µg proteins were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, Bedford, MA). After blocking with 10% milk in TBS, membranes were incubated with specific antibodies in dilution buffer (2% BSA in TBS) overnight at 4°C followed by horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG at appropriate dilutions and room temperature for 1 h. Antibody binding was detected using enhanced chemiluminescence (ECL) detection system from GE Biosciences (Piscataway, NJ) following manufacturer’s instructions and visualized by fluorography with Hyperfilm.
2.5. Cell viability assay (MTT dye assay)
Cell viability was measured by the 3-[4,5-dimethylthylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) method [12]. Briefly, cells were collected and seeded in 96-well plates at a density of 2×105 cells/cm2. Different seeding densities were optimized at the beginning of the experiments (data not shown). After incubation for 24 h, cells were exposed to fresh medium containing reagents at 37°C. After incubation for up to 24 h, 20 µl of MTT tetrazolium salt (Sigma, St. Louis, MO) dissolved in Hank’s balanced solution at a concentration of 5 mg/ml was added to each well and incubated in CO2 incubator for 4 h. Finally, the medium was aspirated from each well and 150 µl of DMSO (Sigma, St. Louis, MO) was added to dissolve formazan crystals and the absorbance of each well was obtained using a Dynatech MR5000 plate reader at a test wavelength of 490 nm with a reference wavelength of 630 nm.
2.6. Assessment of the percentage of apoptotic cells
To detect apoptotic cells [12], cells were stained with DNA binding dye Hoechst 33342 (Sigma, St. Louis, MO). After the cells were exposed to UV and the test compounds for the allotted time periods, they were fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 10 min at 4°C, and then washed with PBS. To stain the nuclei, cells were incubated for 20 min with 20 µg/ml of Hoechst 33342. After washing with PBS, the cells were observed under a fluorescence microscope (Zeiss Axiophoto 2, Carl Zeiss, Germany). Cells exhibiting condensed chromatin and fragmented nuclei were scored as apoptotic cells. A minimum of 500 cells was scored from each sample.
2.7. Statistical analysis
The values in the figures are expressed as the means ± standard error (SE). The figures in this study were representatives of at least 3 different experiments. Statistical analysis of the data between the control and treated groups was performed by a student t test. Values of p < 0.05 were considered as statistically significant.
3. Results
3.1. UV radiation transactivates EGFR in cultured mouse skin dendritic cells
To investigate the cell signaling pathways in response to UV radiation in mouse skin dendritic cells, first we tested whether UV induces EGFR activation in cultured DCs (XS 106 cell line). As shown in Fig. 1A, UV (30 mJ/cm2) induces a transient EGFR activation (Tyr1068, but not Tyr1045) in a time dependent manner. Furthermore, pretreatment of cells with 1 µM of PD153035, an EGFR inhibitor, nearly completely blocks tyrosine phosphorylation of EGFR by UVB irradiation (Fig. 1A). As a control, EGF (100 ng/ml) activates EGFR (T1068) in DCs, which is blocked by PD 153035 and another EGFR tyrosine kinase inhibitor AG 1478 (Fig. 1B). As expected, UV also induces EGFR activation in cultured keratinocytes in both sites (T1068 and 1045), which is blocked by PD 153035 (Fig. 1C). To further confirm EGFR activation, EGFR knockout MEFs were applied. As shown in Fig. 1D, UV transactivates EGFR in wild type but not in EGFR knockout MEFs. Similar to cultured skin dendritic cell line (XS 106 cell line), UV also transactivates EGFR (T-1068) in monocyte-derived dendritic cell or MoDCs, which is inhibited by PD 153035 (Fig. 1E). Based on these data, we conclude that UV transactivates EGFR in cultured skin dendritic cells.
Fig. 1. UV radiation transactivates EGFR in cultured mouse skin dendritic cells.
Cultured mouse skin dendritic cells (DCs, XS 106 cells) were pre-treated with EGFR inhibitor PD 153035 (1 µM) for 1 hour, followed by UV radiation (30 mJ/cm2) (A) or EGF (100 ng/ml) (B) for indicated time points, p-EGFR (Tyr 1068, Tyr 1045) and T-EGFR were detected by Western blot. EGFR activation (Tyr 1068 and Tyr 1045) was detected in cultured human skin keratinocytes (HaCaT cell line) treated with UV or UV plus PD 153035 (C). Wild type and EGFR knockout MEFs (mouse embryonic fibroblasts) were treated with UV radiation (30 mJ/cm2) and cultured for indicated time points, p-EGFR (Tyr 1068), T-EGFR and β-actin were detected by Western blot (D). Primarily cultured mouse monocyte-derived dendritic cells (MoDCs) were treated with UV or UV plus PD 153035, p-EGFR and T-EGFR were detected by Western Blot (E). Data are presented as mean ± s.e.m for at least three independent experiments.
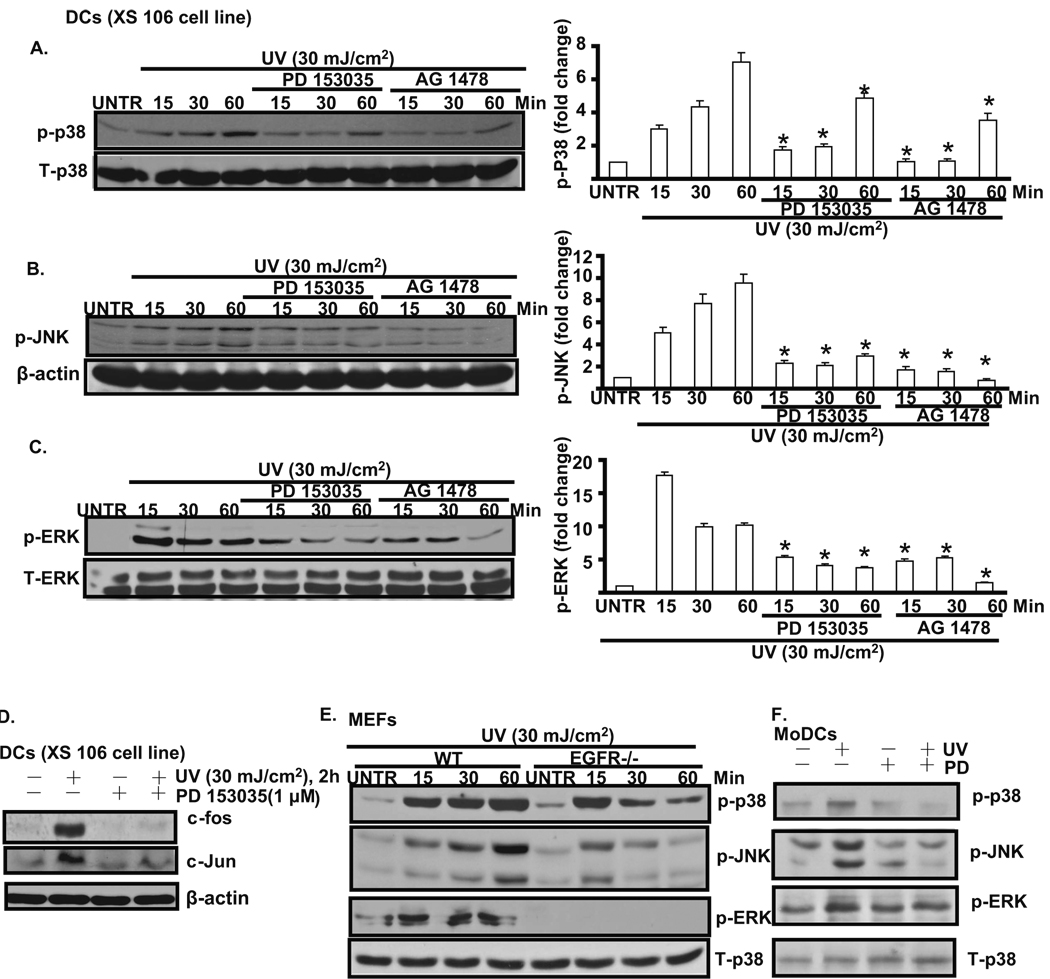
3.2. EGFR activation mediates UV-induced MAPK activation in cultured mouse skin dendritic cells
The data above show that UV induces EGFR activation in DCs (Fig. 1) and previous studies have demonstrated that MAPK plays important roles in UV-induced biological responses such as maturation and apoptosis of DCs [4, 19], we next examined the effect of EGFR activation on phosphorylation of p38, ERK1/2, and SAPK/JNK. As shown in Fig. 2A, B and C, UV activates p38, JNK, and ERK. Pretreatment with EGFR inhibitor PD 153035 and AG 1478 inhibits UV-induced MAPK activation in cultured DC cells (XS 106 cell line). Since UV-induced MAPK activation results in induction of c-Fos and c-Jun [20], we next investigated whether EGFR activation is required for stimulation of downstream c-Fos and c-Jun activation. As shown in Fig. 2D, UV radiation induces a marked increase in the level of c-Fos and c-Jun at 2 hours after UV. Pretreatment with PD 153035 before UV irradiation inhibits c-Fos and c-Jun induction. To further confirm the key role of EGFR in UV-induced MAPK activation, EGFR knockout MEFs were used. As shown in Fig. 2E, the induction of MAPK in EGFR knockout MEFs is much lower than in wild type MEFs. Furthermore, PD 153035 pretreatment also inhibits UV-induced MAPK activation in MoDCs (Fig. 2F). Based on these data, we conclude that EGFR plays important role on UV-induced MAPK activation in cultured mouse skin dendritic cells.
Fig. 2. EGFR mediates UV-induced MAPK activation in cultured mouse skin dendritic cells.
Cultured mouse skin dendritic cells (DCs, XS 106 cell) were treated with EGFR inhibitor PD 153035 (1 µM) or AG 1478 (1 µM) for 1 hour, followed by UV radiation (30 mJ/cm2) and cultured for 15, 30 and 60 min, p-p38 (A), p-JNK (B), p-ERK (C) were detected by Western blot and were quantified. c-fos and c-Jun level was detected in DCs treated with UV plus PD 153035 for 2 hours (D). Wild type and EGFR knockout MEFs (mouse embryonic fibroblasts) were treated with UV radiation (30 mJ/cm2) and cultured for 15, 30 and 60 min, p-p38, p-JNK, p-ERK were detected by Western blot (E). MAPK activation was detected in MoDCs treated with UV plus PD 153035 (F). *P<0.05 vs. UV treated group. Data are presented as mean ± s.e.m for at least three independent experiments.
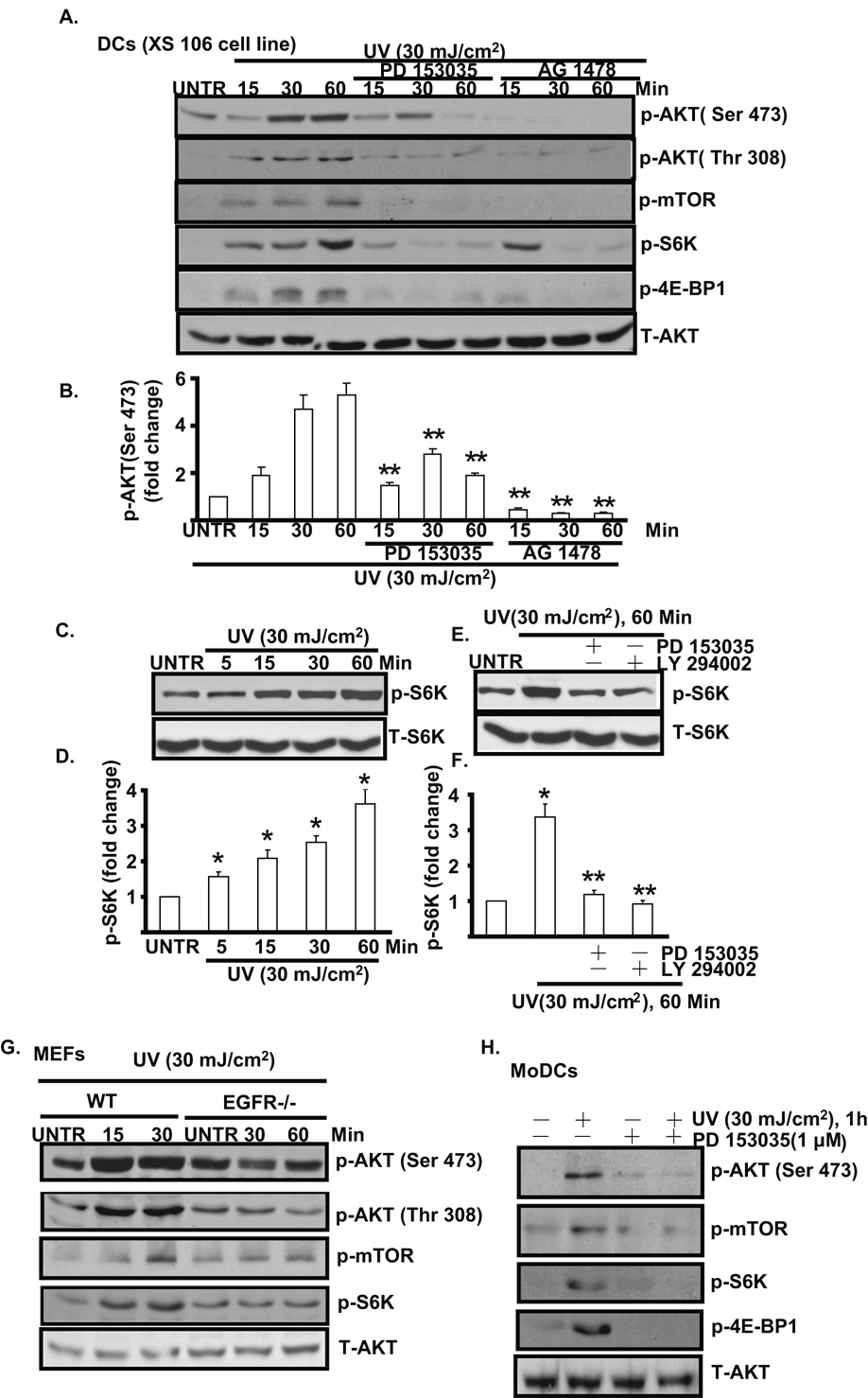
3.3. EGFR activation mediates UV-induced AKT/mTORC1/S6K/4E-BP1 activation in cultured mouse skin dendritic cells
Previous studies in human skin keratinocytes have demonstrated that in addition to MAP kinases, UV also activates PI3K/AKT [6, 21, 22] pathway, which protects against UV- induced widespread skin cell damage. However, whether UV induces AKT activation in DCs and possible signal pathway in this process are not well studied. As shown in Fig. 3A and B, UV activates AKT, mTOR and downstream components s6k and 4E-BP1. Pretreatment with EGFR inhibitor PD 153035 and AG 1478 almost completely blocks UV- induced AKT (Ser 473 and Thr 308) and downstream mTOR/S6K/4E-BP1 signal pathway. UV induces S6K activation in a time dependent manner (Fig. 3C and D). To further confirm that S6K is downstream of EGFR/AKT in UV-treated DCs, EGFR inhibitor PD 153035 and PI3K/AKT inhibitor LY 294002 were used. The results showed that PD 153035 and LY 294002 almost completely block UV-induced S6K activation (Fig. 3E and F). Further, the induction of AKT/mTOR signal is largely impaired in EGFR knockout MEFs as compared to wild type MEFs (Fig. 3G). Similar results were also seen in MoDCs, as PD153035 blocks UV- induced AKT/mTOR/S6K activation (Fig. 3H). Collectively, our data suggest that EGFR trans-activation mediates UV-induced AKT/mTOR activation, which might serve as a survival signal against UV-induced widespread skin cell damage.
Fig. 3. EGFR mediates UV-induced AKT/mTOR/S6K/4E-BP1 activation in cultured mouse skin dendritic cells.
Cultured mouse skin dendritic cells (DCs, XS 106 cell) were treated with EGFR inhibitor PD 153035 (1 µM) or AG 1478 (1 µM) for 1 hour, followed by UVB radiation (30 mJ/cm2) and cultured for 15, 30 and 60 min, p-AKT (Ser 473 and Thr 308), p-mTOR (Ser 2448), p-S6K (Thr 389), p-4E-BP1(Ser 65) and T- AKT were detected by Western blot (A) and AKT phosphorylation (Ser 473) was quantified (B). S6K phosphorylation was detected in DCs treated different time points after UV radiation (C and D). DCs were pre-treated with PD 153035 and LY 294002 for 1 hour, followed by UV radiation, S6K phosphorylation was detected by Western blot (E and F). UV-induced AKT/mTOR/S6K/4E-BP1 was detected in both wild type and EGFR knockout MEFs (G). AKT/mTOR/S6K/4E-BP1 activation was detected in MoDCs treated with UV plus PD 153035 (H). *P<0.05 vs. untreated group. **P<0.05 vs. UV treated group. Data are presented as mean ± s.e.m for at least three independent experiments.
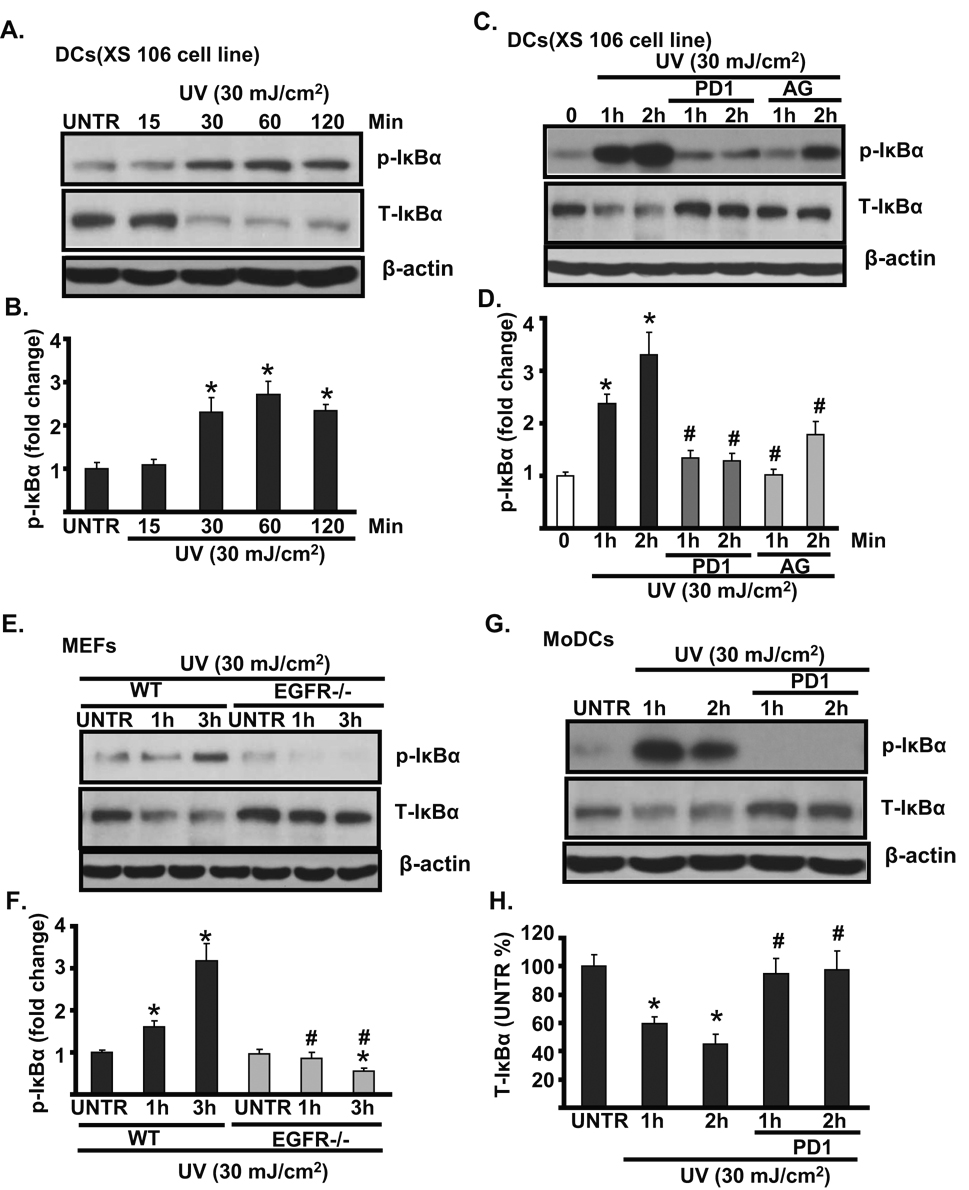
3.4. EGFR activation mediates UV-induced NF-κB activation in cultured mouse skin dendritic cells
Previous studies have suggested that NF-κB plays an important role in mediating UV-induced dendritic cell response [23–25]. We next tested whether EGFR is involved in UV-induced NF-κB activation in cultured skin dendritic cells. As shown in Fig. 4A and B, UV radiation induces IκBα phosphorylation and degradation in a time dependent manner in cultured skin dendritic cells (XS 106 cell line). Pretreatment with EGFR inhibitor PD153035 and AG 1478 largely impairs UV-induced IκBα phosphorylation and degradation (Fig. 4C and D). Furthermore, the activation of NF-κB (IκBα phosphorylation and degradation) was seen in wild type but not in EGFR knockout MEFs (Fig. 4E and F). PD 153035 also inhibits UV-induced NF-κB activation in MoDCs (Fig. 4G and H). Based on these data, we conclude that EGFR plays as a mediator in UV-induced NF-κB activation in cultured mouse skin dendritic cells.
Fig. 4. EGFR mediates UV-induced NF-κB activation in cultured mouse skin dendritic cells.
Cultured mouse skin dendritic cells (DCs, XS 106 cells) were treated with UV radiation (30 mJ/cm2) and cultured for 15, 30 60 and 120 min, p- IκBα (Ser 32/36), T-IκBα and β-actin were detected by Western blot (A and B). DCs (XS 106 cells) were treated with EGFR inhibitor PD 153035 (PD1, 1 μM) or AG 1478 (AG, 1 µM) for 1 hour, followed by UVB radiation (30 mJ/cm2) and cultured for 1 and 2 hours, p- IκBα (Ser 32/36), T-IκBα and β-actin were detected by Western blot (C and D). UV-induced NF-κB activation was detected in wild type but not in EGFR knockout MEFs (E and F). NF-κB activation was detected in MoDCs treated with UV plus PD 153035 (G and H). *P<0.05 vs. untreated group. #P<0.05 vs. UV treated group. Data are presented as mean ± s.e.m for at least three independent experiments.
3.5. EGFR mediates UV-induced AMPK activation in cultured mouse skin dendritic cells
AMPK is a heterotrimeric serine-threonine kinase that senses depletion of intracellular energy and responds by stimulating catabolic pathways that generate ATP [26, 27]. Our recent studies in cultured skin keratinocytes found for the first time that UV induces AMPK activation in a LKB1 dependent way (unpublished data), and this AMPK activation plays a key role in UV-induced skin cell damage by modulating downstream signals such as p38, p53 and ACC. In this study, using cultured skin dendritic cells, we also found that UV radiation activates LKB1/AMPK, which is abolished by EGFR inhibitors PD 153035 and AG 1478 (Fig. 5A). Furthermore, EGF also induces AMPK phosphorylation in an EGFR dependent manner (Fig. 5B). The induction of LKB1/AMPK was seen in wild type but not in EGFR knockout MEFs (Fig. 5C). PD 153035 also abolishes UV-induced AMPK activation in MoDCs (Fig. 5D). Collectively, our data suggest that EGFR mediates UV-induced LKB1/AMPK activation in cultured skin dendritic cells.
Fig. 5. EGFR mediates UV-induced AMPK activation in cultured mouse skin dendritic cells.
Cultured mouse skin dendritic cells (DCs, XS 106 cells) were treated with EGFR inhibitor PD 153035 (1 µM) or AG 1478 (1 μM) for 1 hour, followed by UVB radiation (30 mJ/cm2) (A) or EGF (100 ng/ml) (B) and cultured for 15, 30 and 60 min, p-LKB1 (ser 428), p-AMPKα (Thr 172) and T-AMPK were detected by Western blot. Wild type and EGFR knockout MEFs (mouse embryonic fibroblasts) were treated with UV radiation (30 mJ/cm2) and cultured for indicated time points, p-LKB1 (Ser 428), p-AMPKα (Thr 172) and β-actin were detected by Western blot (C). LKB1 and AMPK phosphorylation were detected in MoDCs treated with UV with or without PD 153035 (D). *P<0.05 vs. untreated group. #P<0.05 vs. UV treated group. Data are presented as mean ± s.e.m for at least three independent experiments.
3.6. Trans-activation of EGFR protects dendritic cells from UV-induced cell death
Next we tested the functional result of EGFR trans-activation after UV radiation in dendritic cells. As shown in Fig. 6A and D, EGFR activation protects against UV-induced dendritic cell death and apoptosis, since PD 153035 and AG 1478 enhance UV-induced dendritic cell death and apoptosis. Furthermore, while ERK and AKT inhibition enhances UV-induced cell death, inhibition of p38 and JNK protects it. All these inhibitors have no obvious effects on dendritic cell viability (Fig. 6B). As a positive control, EGFR ligand, EGF also protects against UV-induced dendritic cell death (Fig. 6C). To further confirm above findings, EGFR knockout MEFs were used. As shown in Fig. 6E, EGFR knockout MEFs are more sensitive to UV-induced cell death. AKT knockout and mTOR inhibition (by pretreatment with rapamycin) also enhance UV-induced MEF cell death (Fig. 6F). Finally, the protective effect of EGFR trans-activation against UV-induced cell death or apoptosis is also seen in MoDCs (Fig. 6G and H). Based on these data, we conclude that trans-activation of EGFR exerts protective effects against UV-induced dendritic cell death.
Fig. 6. Trans-activation of EGFR protects dendritic cells from UV-induced cell death.
Cultured mouse skin dendritic cells (DCs, XS 106 cells) were pre-treated with EGFR inhibitor PD153035 (PD1, 1 µM), AG1478 (AG, 1 µM), PI3K/AKT inhibitor LY294002 (LY, 1 µM), Wortmanin (WT, 1 µM), MEK/ERK inhibitor PD 98059 (PD9, 1 µM), U0126 (U, 1 µM), JNK inhibitor JNKi (JNKi, 1 µM) and p38 inhibitor SB 203580 (SB, 10 µM) for 1 hour, followed by UV radiation (30 mJ/cm2) for 24 hours, cell viability was detected by MTT assay (A). The effects of these inhibitors alone on cell viability were shown in (B). XS106 cells were pre-treated with or without EGF (100 ng/ml) for 1 hour, followed by UV radiation (30 mJ/cm2) for 24 hours, cell viability was detected by MTT assay (C). The apoptosis rate (Hoechst assay) of XS 106 cells treated with UV plus PD153035 (PD1, 1 µM) or AG 1478 (AG, 1 µM) for 18 hours was shown in (D). The cell viability of wild type and EGFR knockout MEFs treated with UV (30 mJ/cm2) with or without PD 153035 (1 µM) for 24 hours was shown in (E). Wild type, AKT1/2 knockout or AKT1 knockout MEFs were pre-treated with mTOR inhibitor rapamycin (20 nM) for 1 hour, followed by UV radiation (30 mJ/cm2), cell viability was detected by MTT assay (F). Cell viability and apoptosis rate (Hoechst assay) of MoDCs treated with UV (30 mJ/cm2) with or without PD1 (1 µM) were shown in (G) and (H) respectively. The data represent mean ± SE of triplicate experiments. *P<0.05 vs. untreated group. #P<0.05 vs. UV treated group. For the Hoechst experiment, a minimum of ten random fields and 500 cells were counted for apoptotic death rate.
4. Discussion
When high dose of UV was irradiated (as used in this study), apoptosis or cell damage instead of maturation was induced on skin dendritic cells [4, 28, 29]. Using Western-blotting analysis and the experiments using a specific inhibitor for p38 MAPK, it is clarified that apoptosis of MoDC is mediated at least partly by p38 MAPK [4]. However, the detailed signals, especially the upstream signals, involved in this activation have not been fully uncovered. In this study, we found for the first time that UV radiation induces EGFR trans-activation in cultured skin dendritic cell line (XS 106 cell line) and monocyte derived dendritic cells (MoDCs) (Fig. 1), which mediates activation of downstream signals such as MAPK (Fig. 2), AKT/mTORC1/S6K/4EBP1 (Fig. 3), NF-κB (Fig. 4) and LKB1/AMPK (Fig. 5). Furthermore, this EGFR trans-activation serves as internal protective effects against UV-induced dendritic cell death or apoptosis (Fig. 6). Our study provides evidence to support the notion that EGFR acts as a key mediator on UV-induced signal transduction in dendritic cells.
It has been reported that UV irradiation induces activation of EGF, tumor necrosis factor-α (TNF-α), and interleukin-1 (IL-1) receptors and that consequent downstream signaling resembled the sum of the three individual receptor-stimulated pathways in HeLa cells [10] and in human skin in vivo [30]. In cultured skin keratinocytes (HaCaT cell line), EGFR, among three of these cell surface receptors, plays a preeminent role in UV irradiation-induced activation of multiple signal transduction pathways, including MAPK, AKT and PKC [31]. This finding suggests that interconnections among cell surface receptor activation and downstream signaling pathways that are triggered in response to UV irradiation may differ depending on cell type and cellular context. It should be noted that our studies were conducted with human immortalized dendritic cells XS 106 cell line and Monocyte derived dendritic cells (MoDCs), which may differ in their responsiveness to UV irradiation compared to normal human skin dendritic cells in vivo.
In this study, we demonstrated that trans-activation of EGFR protects against UV-induced dendritic cell death (Fig. 6). It seems inconsistent with previous results since EGFR is also involved in UV-induced p38 and JNK activation, which is responsible of cell death or damage (Fig. 2). There are some explanations to address this issue. First of all, blocking EGFR (by pharmacological inhibition and genetic knockout of EGFR) only impairs (but not abolishes) UV-induced p38, ERK and JNK activation (Fig. 2), other signals and other growth factor receptors for examples, might be also involved in this activation. Second, EGFR trans-activation also mediates UV-induced AKT/mTOR (Fig. 3) and NF-κB (Fig. 4) activation, of which, at least AKT/mTOR serves as a protective mechanism against UV-induced cell death (Fig. 6F). Third, other undefined and critical pro-survival signals might be dependent on EGFR in UV-radiated dendritic cells. Taken all these together, we conclude that EGFR activation serves as a pro-survival signal against UV-induced cell death. However, the detailed mechanisms by which EGFR mediates pro-survival effects need further investigation.
In this study, we also demonstrated for the first time AMPK activation in UV-radiated skin dendritic cells (Fig. 5). AMPK is a heterotrimeric serine-threonine kinase that senses depletion of intracellular energy and responds by stimulating catabolic pathways that generate ATP [26, 27]. One mechanism for sensing cellular energy levels involves allosteric activation of the kinase activity of AMPK. Under conditions in which cellular energy demands are increased (such as enhanced cell work or cell stress) or when fuel availability is decreased (because of a reduced rate of glucose uptake), intracellular ATP is reduced and AMP levels rise. AMP then allosterically activates AMPK. In addition to allosteric activation, AMPK activity can be regulated by a mechanism involving covalent modification through the addition of a phosphate group, such as LKB1 [26, 27, 32–40]. Until now, lots of stimuli have been found that can induce AMPK activation. Our previous study using cultured skin keratinocytes (HaCaT cells) has demonstrated for the first time that UV radiation activates LKB1/AMPK pathway, which plays a critical role on UV induced skin cell pathology (unpublished data). In this study, we also found that UV activates AMPK in cultured dendritic cells in an EGFR dependent manner (Fig. 5). However, the role of this LKB1/AMPK activation in dendritic cell physiology warrants further study.
In conclusion, we, in this study, demonstrate for the first time that UV induces EGFR trans-activation in cultured skin dendritic cells, this EGFR trans-activation serves as an upstream signal and mediator for UV-induced downstream signals such as MAPK, AKT/mTOR, NF-κB and LKB1/AMPK pathway (Fig. 7). Furthermore, EGFR activation also protects dendritic cells against UV-induced dendritic cell death and apoptosis. Our studies provide insights into understanding of the molecular mechanism of UV-induced skin aging and skin cancer.
Fig. 7. Proposed model of UV-induced signal transduction pathways in mouse skin dendritic cells.
(A) EGFR mediates UV induced AKT/mTOR activation in cultured skin dendritic cells, possibly leading to cell survival, (B) EGFR mediates UV-induced LKB1/AMPK activation, inhibiting mTOR, (C) EGFR is involved in UV- induced NF-κB activation, possibly leading to cell survival, activation or maturation of DCs, (D) EGFR is involved in UV induced-MAPK activation, possibly leading to cell survival or apoptosis.
Acknowledgement
This research was supported in part by a grant from NIH (P20 RR016457 from INBRE Program of the National Center for Research Resources) and a grant for biomedical research from Rhode Island Foundation, and a grant from Slater Center for Environmental Biotechnology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Diabetes. 2006;55 Suppl 2:S48–S54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 2.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh DB, Kripke ML. J Exp Med. 1996;183(4):1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meunier L, Eur J. Dermatol. 1999;9(4):269–275. [PubMed] [Google Scholar]
- 4.Nakagawa S, Ohtani T, Mizuashi M, Mollah ZU, Ito Y, Tagami H, Aiba S. J Invest Dermatol. 2004;123(2):361–370. doi: 10.1111/j.0022-202X.2004.23238.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang L, Wang B, Shinder GA, Shivji GM, Mak TW, Sauder DN. J Immunol. 1999;162(3):1440–1447. [PubMed] [Google Scholar]
- 6.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Science. 2000;288(5467):870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 7.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ. Cell. 1994;78(6):963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 8.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Nat Rev Cancer. 2006;6(11):876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 10.Rosette C, Karin M. Science. 1996;274(5290):1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 11.Wan YS, Wang ZQ, Voorhees J, Fisher G. Cell Signal. 2001;13(2):139–144. doi: 10.1016/s0898-6568(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 12.Cao C, Healey S, Amaral A, Lee-Couture A, Wan S, Kouttab N, Chu W, Wan YJ. Cell Physiol. 2007;212(1):252–263. doi: 10.1002/jcp.21026. [DOI] [PubMed] [Google Scholar]
- 13.Cao C, Sun Y, Healey S, Bi Z, Hu G, Wan S, Kouttab N, Chu W, Wan Y. Biochem J. 2006;400(2):225–234. doi: 10.1042/BJ20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao C, Wan S, Jiang Q, Amaral A, Lu S, Hu G, Bi Z, Kouttab N, Chu W, Wan Y. J Cell Physiol. 2008;215(2):506–516. doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Bi Z, Yan B, Wan Y. Int J Mol Med. 2006;18(4):713–719. [PubMed] [Google Scholar]
- 16.Dickenson JM, Reeder S, Rees B, Alexander S, Kendall D, Eur J. Pharmacol. 2003;474(1):43–51. doi: 10.1016/s0014-2999(03)02041-7. [DOI] [PubMed] [Google Scholar]
- 17.Schuhmachers G, Ariizumi K, Kitajima T, Edelbaum D, Xu S, Shadduck RK, Gilmore GL, Taylor RS, Bergstresser PR, Takashima A. J Invest Dermatol. 1996;106(5):1023–1029. doi: 10.1111/1523-1747.ep12338592. [DOI] [PubMed] [Google Scholar]
- 18.Cao C, Wan S, Jiang Q, Amaral A, Lu S, Hu G, Bi Z, Kouttab N, Chu W, Wan Y. J Cell Physiol. 2007 doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Int Immunol. 2004;16(12):1701–1709. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. J Clin Invest. 1998;101(6):1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan YS, Wang ZQ, Shao Y, Voorhees JJ, Fisher GJ. Int J Oncol. 2001;18(3):461–466. doi: 10.3892/ijo.18.3.461. [DOI] [PubMed] [Google Scholar]
- 22.Nomura M, Kaji A, Ma WY, Zhong S, Liu G, Bowden GT, Miyamoto KI, Dong Z. J Biol Chem. 2001;276(27):25558–25567. doi: 10.1074/jbc.M101164200. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Tan M, Hu Y, Wang JL, Scheuner D, Kaufman RJ. J Biol Chem. 2004;279(33):34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 24.Wenk J, Schuller J, Hinrichs C, Syrovets T, Azoitei N, Podda M, Wlaschek M, Brenneisen P, Schneider LA, Sabiwalsky A, Peters T, Sulyok S, Dissemond J, Schauen M, Krieg T, Wirth T, Simmet T, Scharffetter-Kochanek K. J Biol Chem. 2004;279(44):45634–45642. doi: 10.1074/jbc.M408893200. [DOI] [PubMed] [Google Scholar]
- 25.Marwaha V, Chen YH, Helms E, Arad S, Inoue H, Bord E, Kishore R, Sarkissian RD, Gilchrest BA, Goukassian DA. J Biol Chem. 2005;280(37):32379–32388. doi: 10.1074/jbc.M503245200. [DOI] [PubMed] [Google Scholar]
- 26.Carling D. Trends Biochem Sci. 2004;29(1):18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Mittelbrunn M, Tejedor R, de la Fuente H, Garcia-Lopez MA, Ursa A, Penas PF, Garcia-Diez A, Alonso-Lebrero JL, Pivel JP, Gonzalez S, Gonzalez-Amaro R, Sanchez-Madrid F. J Invest Dermatol. 2005;125(2):334–342. doi: 10.1111/j.0022-202X.2005.23824.x. [DOI] [PubMed] [Google Scholar]
- 29.Janssens AS, Pavel S, Out-Luiting JJ, Willemze R, de Gruijl FR. Br J Dermatol. 2005;152(6):1268–1274. doi: 10.1111/j.1365-2133.2005.06690.x. [DOI] [PubMed] [Google Scholar]
- 30.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Voorhees JJ, Fisher GJ. Am J Pathol. 2006;169(3):823–830. doi: 10.2353/ajpath.2006.050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Diabetes. 2007;56(5):1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 33.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Am J Physiol Endocrinol Metab. 2002;282(5):E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Zhu T, Guan KL. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 35.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. Proc Natl Acad Sci U S A. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.01147.2005. [DOI] [PubMed] [Google Scholar]
- 37.Park Y, Lee SW, Sung YC. J Immunol. 2002;168(1):5–8. doi: 10.4049/jimmunol.168.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Zhu XJ, Feng CZ, Dai ZM, Zhang RC, Yang WJ. Stress. 2007;10(1):53–63. doi: 10.1080/10253890601130773. [DOI] [PubMed] [Google Scholar]
- 39.Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Free Radic Biol Med. 2007;42(1):64–78. doi: 10.1016/j.freeradbiomed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Tzatsos A, Tsichlis PN. J Biol Chem. 2007;282(25):18069–18082. doi: 10.1074/jbc.M610101200. [DOI] [PubMed] [Google Scholar]