Abstract
Purpose
The primary objective of this study was to determine whether markers of differentiation and activation of the Akt pathway are associated with metastasis in adenocarcinoma of the lung.
Experimental Design
Paired primary and metastatic tumor samples were obtained from 41 patients who had undergone resection of both primary lung adenocarcinoma and brain metastatic lesions. Paired samples were compared for relative expression of TTF-1 and E-cadherin as potential markers of differentiation. Activation of the Akt pathway was assessed by expression of p-Akt and p-S6. Biomarkers which showed relative discordance in expression between the matched pairs were then assessed in a cohort of 77 primary lung adenocarcinomas. Validation was performed in an independent cohort of 82 primary lung adenocarcinomas.
Results
Among the 41 matched pairs, E-cadherin (23 discordant pairs) and TTF-1 (18 discordant pairs) were overexpressed in primary tumors (20/23 and 15/18, respectively). In contrast, p-S6 overexpression was significantly associated with metastatic tumors (20 of 21 discordant pairs). The expression of E-cadherin, p-S6 and TTF-1 was evaluated in 77 primary lung adenocarcinomas, where high p-S6 expression was associated with shorter time to metastasis. The association of p-S6 with metastasis was then validated in an independent set of 82 tumors. In multivariable analysis, p-S6 expression was a negative independent predictor of metastasis-free survival after adjustment for tumor stage.
Conclusions
p-S6 is overexpressed in metastatic tumors. In primary tumors, higher p-S6 expression is associated with shorter metastatic-free survival. This biomarker has the potential for risk stratification in future clinical trials.
INTRODUCTION
The leading cause of cancer death in the U.S. is lung cancer. It has been estimated that there were more than 160,000 deaths from lung cancer in the U.S. during 2007, with more than 213,000 new cases diagnosed (1). About 80% of these cases were classified as one of the three major types of non–small cell lung carcinoma (NSCLC): adenocarcinoma, squamous cell carcinoma, or large-cell anaplastic carcinoma. The most important factor for survival in NSCLC patients is tumor stage, in part due to the potential for complete resection (2). Only patients who undergo complete tumor resection have a significant chance for cure (3).
Even with complete surgical resection, survival rates for NSCLC are disappointing. Depending on the pathologic stage, 5-year survival rates range between 23 to 67% (2). Thus, it is likely that at the time of diagnosis many cancers have already spread or metastasized at the microscopic level. About half of locally advanced lung adenocarcinoma patients will develop brain metastatic disease. (4, 5) Since resection of these lesions is the cornerstone of therapy, examining the differences between primary and metastatic tissue is possible.
Currently there are no diagnostic tests at this time to determine which patients will likely relapse after potentially curative surgery. Previous studies have been conducted to identify molecular markers with the potential for prognostic/diagnostic use. Among the different types of non-small cell lung carcinoma (NSCLC), TTF-1 expression has been found predominately in lung adenocarcinoma (6, 7) and has been shown to be a favorable, independent predictor of survival in lung adenocarcinoma patients (8). The phosphatidylinositol 3 (PI3) kinase/Akt pathway is frequently deregulated in cancer (9). Overexpression of activated or phosphorylated AKT (p-AKT) in pre-invasive lesions has been associated with severe dysplasia, suggesting early involvement of this pathway in lung cancer (10, 11). Over-expression of p-AKT has been shown to be a poor prognostic factor for NSCLC patients with lymph node involvement (12, 13). AKT is a known repressor of E-cadherin; reduced E-cadherin expression, along with EGFR expression, has also been associated with poorer survival in NSCLC (14).
These reports demonstrate the possibility that biomarkers associated with these pathways may be used to stratify lung adenocarcinoma patients. Unfortunately, few studies have included analyses of both primary tissue and corresponding metastatic tissue to further our knowledge of the development of lung tumor metastasis. To address issues related to metastasis in lung adenocarcinoma, studies using matched primary-metastatic pairs have been examined. Using a variety of markers (p53, bcl-2, Ki-67, EGFR, COX-2 and BAX), differences were not detected between the expression of these markers between the paired samples (15),(16). Since the publications of those studies, additional potential markers have been identified that may elucidate important factors regarding metastatic behavior.
In this study, we sought to determine whether markers of differentiation and activation of the Akt pathway are associated with metastasis in adenocarcinoma of the lung. Matched primary tumors and brain metastases from 41 patients were used to identify differentially expressed markers. We chose brain metastasis for this purpose because out of all potential metastatic sites, those occurring in the brain are the most frequently surgically excised and provide the best quality of tissue for molecular evaluation. Further investigation and validation of these markers was performed in an additional 159 primary lung adenocarcinoma samples.
METHODS & MATERIALS
Patient Population
This study was conducted under an Institutional Review Board-approved, retrospective laboratory protocol that was compliant under the Health Insurance Portability and Accountability Act. Patients that had primary thoracic tumor resection at the University of Texas M.D. Anderson Cancer Center (UTMDACC) between 1992 and 2004 for primary adenocarcinoma of the lung were considered for inclusion in this study. Forty-one patients were first ascertained that had additionally undergone metastatic neurosurgery at UTMDACC; these 41 patients also had sufficient samples of tissue for whole section assessment from both surgeries. The study and validation cohorts were comprised of an additional 159 patients that a) had a positive history of smoking, and b) did not receive induction chemotherapy. The difference between the study and the validation cohorts was based solely on tissue availability. Cases included in the test cohort had enough sample for whole section assessment, whereas cases included in the validation cohort were available for assessment only in tissue microarray (TMA) format.
The study cohort, in which the results of the matched-pair analysis were tested, were comprised of 41 women and 36 men diagnosed with primary lung adenocarcinomas (n=77). The staging of these patients at initial diagnosis were as follows: 40 Stage I, 15 Stage II, 21 Stage III and 1 Stage IV. The median age at primary tumor diagnosis was 66.3 years (range 40.1−83.9 years), and the median clinical follow-up was 104.4 weeks. Twenty-eight patients had suffered metastatic lesions at the time of this study, with 10 involving brain metastatic disease. The validation cohort (n=82), in which findings from the study cohort were tested, consisted of 43 women and 39 men. The staging of these patients at initial diagnosis were as follows: 61 Stage I, 12 Stage II, 8 Stage III and 1 Stage IV. The median age at primary tumor diagnosis was 66.0 years (range 33.5−84.3 years), and the median clinical follow-up was 98.0 weeks. Twenty-five patients had suffered metastatic lesions by the point of study, with 8 involving the brain.
All 241 tumor samples (200 primaries, 41 metastases) were obtained from formalin-fixed, paraffin-embedded tissue blocks collected by the UTMDACC Department of Pathology, and each sample was assessed histologically for tumor tissue by one or more pathologists (M.S., G.R., I.I.W., K.A.). A Beecher tissue arrayer was utilized to manufacture tissue arrays that comprised our validation set of 82 primary lung adenocarcinomas.
Immunohistochemistry
Immunohistochemistry was performed as described previously (17). Briefly, 5μm sections were deparaffinized, followed by microwave antigen retrieval in 10mM sodium citrate, pH 6.0 (except for TTF-1, which was retrieved in 10mM Tris-EDTA). For the matched primary/brain-metastatic pair cohort, the antibodies against E-cadherin (1:100 of Ab-3, Lab Vision, Inc., Fremont CA), p-AKT (1:250 of Thr308, New England BioLabs, Inc., Beverly MA), p-S6 (1:1000 of Ser235/6, Cell Signaling Technology, Inc., Danvers MA) and TTF-1 (1:50 of 8G7G3/1, Lab Vision, Inc.; 1:100 of NCL-L-TTF-1, Novocastra Laboratories, Newcastle upon Tyne, UK) were applied and incubated overnight at 4°C. The DAKO EnVision kit (Carpinteria, CA) was used to detect staining. The p-S6 antibody has been tested for specificity (18), and the 1:1000 dilution worked well when comparing primary tumor to metastatic tumor for differential expression. However, the amount of p-S6 antibody was increased 4-fold to enhance the detection of signal for the 2 cohorts of primary tumor samples. The increased sensitivity provided by the lower dilution allowed for better assessment of the primary cohorts. Negative control experiments were conducted by excluding primary antibodies from the protocol described above.
Scoring for expression was based on the percentage of positively stained tumor cells (Figure 1). Each slide was reviewed by one of us (K.A) under low magnification to evaluate staining intensity (e.g. positive and negative). Staining was considered diffuse if >50% of the tumor cells stained for the biomarker and focal if < 50% of the tumor cells stained for the biomarker. For scoring purposes, the most prominently stained area of the slide was chosen. The positive and negative expression of E-cadherin, p-AKT, p-S6 and TTF-1 were defined in accordance with the definition used in previous studies (12-14). Immunoreactivity for TTF-1 was detected as nuclear staining and scored as 0 to 1 based on the proportion of positively stained tumor nuclei (0 ≤ 5% positive tumor nuclei; 1 >5% positive tumor nuclei). Cytoplasmic staining was observed but disregarded, in accordance with prior studies (19). E-cadherin was scored as 0 to 1 based on positive membrane staining (0 ≤ 75% positive cells; 1 > 75% positive cells). Immunoreactivity of p-S6 and pAKT was scored as 0 to 2 based on positive cytoplasmic staining (0 < 5% positive cells, 1 was between 5−30% positive cells, 2 > 30% positive cells).
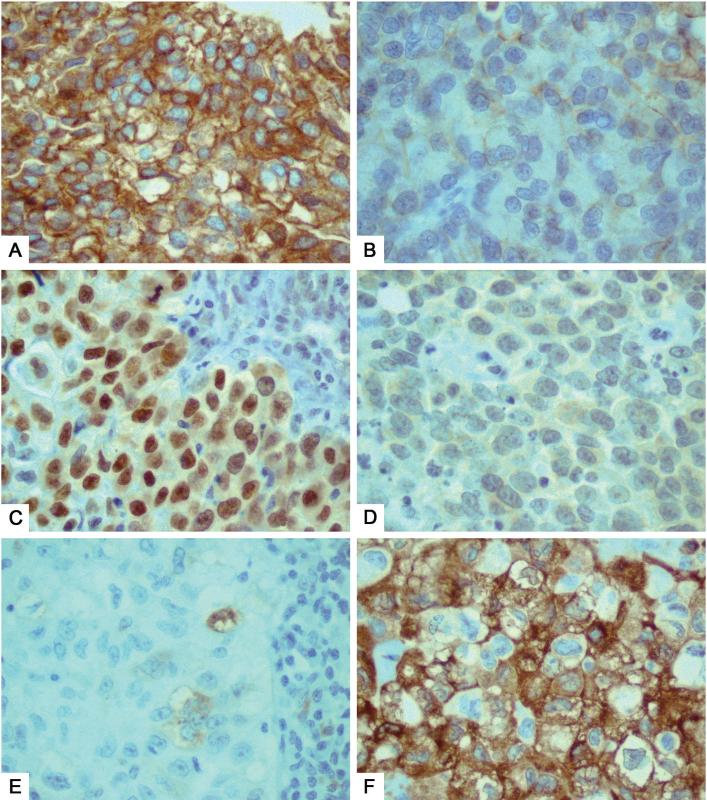
Figure 1.
Differential expression in primary-metastatic pairs. Loss of E-cadherin and TTF-1 expression was seen in these metastatic tumors, as compared to their corresponding primary tumors (A and B, respectively). In contrast, acquisition of p-S6 was observed for another metastatic tumor, as compared to its corresponding primary tumor (C).
Statistical Analysis
A paired 2-sided t-test was performed to determine the differences of markers scores between the matched primary and metastatic tumor specimens. Log-rank and Kaplan-Meier analyses were performed to determine the correlations of variables with time-to-metastasis in univariate analyses. Cox multivariate analysis was used to determine independent associations with time-to-metastasis. Correlations of independent variables with one another were determined using Spearman rank. Time-to-metastasis was defined as the time from initial diagnosis to the first clinically evident metastasis.
RESULTS
Markers in matched primary and brain-metastatic pairs
All 41 patients with matched primary/metastatic pairs in the current study were diagnosed with primary adenocarcinoma of the lung. The median age at initial diagnosis was 57.5 years (range 35.6−85.1 years). There were 16 women and 25 men in this test group, and the majority of patients were Caucasian (88%). Four patients presented with brain metastases at initial diagnosis. For the 37 remaining patients, brain metastases became clinically evident after surgical resection of the primary tumor at a median rate of 54.4 weeks. As described in the Methods section, these paired samples were evaluated for expression of 4 markers. In instances of IHC staining intensity discordance (example: the primary tumor was high while the metastatic tumor was low), the direction of the discrepancy was noted and a paired sample t-test was performed to determine significance. A summary of these results is given in Table 1. For TTF-1 there were 18 instances of discordant expression, of which 15 (83%) pairs had less TTF-1 expression in the metastatic tumor as compared to the corresponding primary tumor. There were 23 discordant pairs for E-cadherin, of which 20 (87%) pairs showed loss of expression in the metastatic lesion. There were 21 discordant examples of positive p-S6 staining of which 20 (95%) showed higher expression in the metastatic lesion. The distribution of the differences in expression among the discordant pairs for these 3 markers (TTF-1, E-cadherin and p-S6) was all statistically significant (Table 1). In contrast, among the 15 discordant p-AKT cases 5 were higher in the primary whereas 10 were higher in the metastasis, not a statistically significant difference.
Table 1.
Comparison of biomarkers in paired primary and metastatic pairs. Markers were determined to be either concordant or discordant, and if discordant, the direction of discordance is indicated. The significance of these differences, based on a 2-sided t-test, is shown.
| marker |
concordant (positive) |
concordant (negative) |
discordant |
2-sided t-test |
|
|---|---|---|---|---|---|
| higher in primary |
higher in met |
P-value |
|||
| E-cadherin |
18 |
0 |
20 |
3 |
<0.01 |
| TTF-1 |
13 |
10 |
15 |
3 |
<0.01 |
| p-AKT |
26 |
0 |
5 |
10 |
0.28 |
| p-S6 | 20 | 0 | 1 | 20 | <0.01 |
Association of biomarkers and metastasis in primary lung tumors
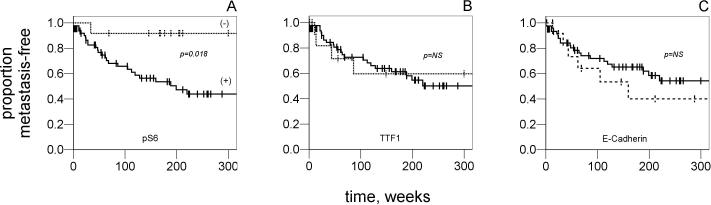
The finding of higher p-S6 expression and decreased TTF-1 and E-cadherin expression in metastatic lesions among matched primary-metastatic pairs suggested that these biomarkers may be predictive of metastasis in primary tumors. Specifically, these data generated the hypothesis that increased p-S6 expression and decreased TTF-1 and E-cadherin in primary lung tumors were associated with increased risk of metastasis. To test this, 77 primary lung adenocarcinoma cases were tested. Positive staining was detected in 61 (79%), 65 (84%) and 64 (83%) cases for E-cadherin, p-S6 and TTF-1, respectively. The proportion of tumors which were positive for p-S6 was higher in the primary tumors in this sample set compared to the 41 tumors in the matched pairs, as the antibody concentration for the IHC was raised in order to increase detection. The median time to metastasis for the p-S6-negative cases could not be ascertained since 11 of the 12 cases did not have metastatic disease at last follow-up. In the cases staining positive for p-S6, the median time-to-metastasis was 64.1 weeks. Kaplan-Meier survival curves and log-rank testing demonstrated a statistically significant correlation between p-S6 expression (negative versus positive) and time-to-metastasis (Figure 2A). The difference in time-to-metastasis was significant between p-S6-negative cases and positive cases (P=0.02). Significant differences were not observed in time-to-metastasis when stratified by TTF-1 (P=0.73) or E-cadherin (P=0.49) (Figure 2B and 2C). Interestingly, we did not observe a correlation between TNM stage and any of the 3 markers tested. Along with the p-S6 status, T-stage (P=0.02, data not shown), but not N-stage were predictive in univariate analysis. Cox proportional hazards analysis showed only positive p-S6 staining to be an independent predictive factor for shortened time-to-metastasis, after accounting for T-stage and patient age (Table 2).
Figure 2.
Kaplan-Meier time-to-metastasis curves of the study primary tumor set, when stratified by p-S6 status. For p-S6-negative cases (dashed line, n=12), a median survival could not be ascertained as all but 1 case were censored. For p-S6-positive (solid line, n=65), the median time-to-metastasis was 199 weeks. The difference in time-to-metastasis was significant between p-S6-negative cases and positive cases (P=0.02, log-rank). B. There was no significant difference in time-to-metastasis when stratified by TTF1 (P=0.73); or C. E-cadherin (P=0.49).
Table 2.
Cox proportional hazards analysis of marker expression and T-stage in study and validation cohorts of primary lung tumors.
| |
study set, n=77 |
validation set, n=82 |
Combined set, n=159 |
|||
|---|---|---|---|---|---|---|
| Factor |
HR* |
P-value |
HR |
P-value |
HR |
P-value |
| p-S6 |
7.6 |
0.05 |
2.6 |
0.04 |
3.2 |
<0.01 |
| T2−4 | NS** | NS | 2.0 | 0.09 | 2.0 | 0.02 |
Hazard ratio
Not significant
Validation of p-S6 as a marker of metastasis
To validate the finding that p-S6 was a biomarker of metastasis, p-S6 expression was examined tested an independent set of tumors on tissue arrays. We found 50 out of 82 cases positive for p-S6 (61%). The Kaplan-Meier time-to-metastasis curves of the validation set are shown in Figure 3. The difference in time-to-metastasis was significant between the cases with positive p-S6 staining and those with no staining (P=0.04, log-rank). As with the study set, a correlation between TNM stage and positive p-S6 staining was not observed. T-stage, was also predictive of a shortened time-to-distant metastasis in univariate analysis (P=0.03, data not shown). Multivariable analysis of this set demonstrated that positive p-S6 staining and a T-stage >1 were independent predictive factors for shortened time-to-metastasis (Table 2). Lastly, when both the study and validation datasets from the lung primary tumors are combined, positive p-S6 staining (HR: 3.2; [95% confidence interval 1.5−6.8], P<0.01) and a T-stage >1 (HR: 2.0; [95% confidence interval 1.1−3.7], P<0.01) were independent, adverse predictive factors for time-to-metastasis (Table 2).
Figure 3.
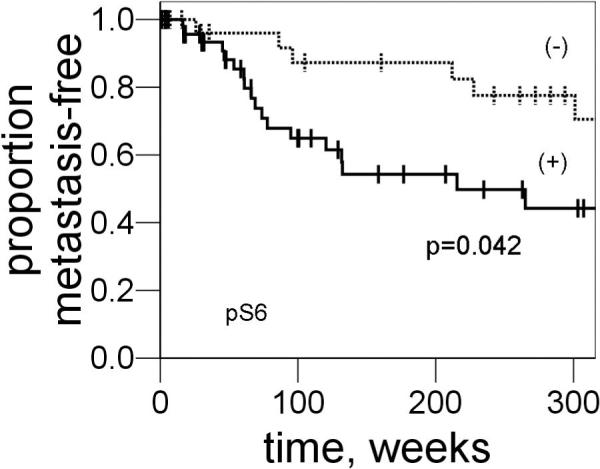
Kaplan-Meier time-to-metastasis curves of the validation set when stratified by p-S6 status. For p-S6-negative cases (dashed line, n=32), a median survival could not be ascertained as 25 cases were censored. For p-S6-positive (solid line, n=50), the median time-to-metastasis was 215 weeks. The difference in time-to-metastasis was significant between p-S6-negative cases and positive cases (P=0.04, log-rank).
Although not the primary endpoint of our study, the relationship of p-S6 status to overall survival was also examined. Analysis of the 159 combined cases (study and validation primary adenocarcinoma sets) demonstrated that the 44 cases with a negative p-S6 status had a 420-week median overall survival, compared to a 300-week median survival of the 115 p-S6-positive cases (P=0.04, log rank).
DISCUSSION
The PI3K/AKT pathway is critical for the regulation of cell proliferation, growth, differentiation, migration, and survival in many human cancers. Activation of this pathway appears to occur early in NSCLC development, since over-expression of p-AKT in pre-invasive NSCLC lesions have been detected (10, 11). The over-expression of p-AKT has been seen as a poor prognostic factor for NSCLC patients with lymph node involvement (12, 13). It has been demonstrated that AKT transmits some of its downstream effects through activation of the serine/threonine protein kinase mammalian target of rapamycin (mTOR) (20). The current model has p-AKT directly activating mTOR through phosphorylation of its Ser2448 residue (21, 22), which in turn, activates ribosomal S6K. S6K affects mRNA translation indirectly through intermediates such as the 40S ribosomal protein S6 (23). In fact, immunohistochemical testing for p-S6 has recently been used to detect S6K and mTOR activity in lung adenocarcinomas (24).
The major finding of this study is that high p-S6 expression was a negative prognostic factor for lung adenocarcinoma, as it was associated with time-to-metastasis in patients with early stage lung adenocarcinoma. We first tested p-S6 for concordant expression in 41 primary lung adenocarcinoma-brain metastatic pairs. Half of these cases had discordant expression, with a vast majority of the higher p-S6 expression in the metastatic tissues (20 of 21). This led to the hypothesis that high p-S6 expression in the primary tumor might be a predictor of metastatic behavior. We tested this by examining p-S6 expression levels in a set of 77 primary lung adenocarcinoma tissues. We found that high expression of p-S6 was associated with a shorter average time to metastasis (200 weeks vs. >400 weeks, post-thoracic surgery). This finding was confirmed in a validation group (120 weeks vs. 400 weeks, post-thoracic surgery), a tumor set consisting of 82 primary lung adenocarcinoma cases. Although the finding remained robust, the time-to-metastasis curves appear somewhat different in this set compared to the study set of primary tumors. We believe that this is largely due to technical reasons, in that the IHC on the study set was performed on whole sections, whereas the validation set was performed on tissue arrays, where 1−3 cores of tumor was evaluable for scoring. Whole-sectioning allowed more of the tumor to be assessed, and the area with the highest amount of staining was used. For this reason the under-sampling of tumor inherent to the tissue array format likely led to false-negatives (no staining observed in a tumor which actually expressed p-S6 if interrogated elsewhere). In support of this explanation, we found that the proportion of cases positive for p-S6 was higher when whole sections were used, rather than tissue cores (84% versus 61%, respectively), indicating that some of the cores where no expression was detected likely represented false negatives. Despite these technical differences, p-S6 remained associated with time-to-metastasis in both sets. In addition, the results indicate that the increased p-S6 seen in the brain-metastatic tissue samples (Table 1) could be due to an acquisition of PI3K/Akt signaling in the course of metastasis, but further study will be required to verify this.
While reports describing the relationship between clinical outcome to p-S6 status in lung cancer were lacking prior to this study, there are several studies that suggest that activation of the mTOR pathway, and hence, S6 activation, are indeed adverse prognostic characteristics in a variety of other malignancies. Activation of the mTOR pathway has been shown to be prognostic in renal cell carcinoma (RCC) (25). Immunohistochemical analysis of tumor specimens of 375 RCC patients showed a correlation between p-S6 positivity and the clear cell histologic subtype, higher histologic grade and other adverse pathologic features. Further, it was shown that p-S6 was an independent, adverse prognostic factor when accounting for patient performance status, tumor TNM stage and the status of the biomarkers, p-Akt and PTEN. One report indicated the presence of p-S6K (an upstream regulator of S6) to be much higher in 101 cases that presented with disseminated, stage IV (70%) compared to 51 cases with local-regional stage II-III disease (39%) (26). Lastly, results from our laboratory indicated that p-S6K was an adverse prognostic marker in glioblastoma (27). Collectively, these data suggest that pathways which lead to S6 activation confer clinical aggressiveness in solid tumors.
There is considerable evidence that loss of differentiation and unchecked proliferation are two mechanisms by which primary neoplastic lesions metastasize to distant organ sites. We chose two well-known differentiation markers for lung tissue as well as two well-characterized signal transduction markers to test for differential expression in a set of primary lung adenocarcinomas and their subsequent metastatic brain lesions. The basis for choosing these markers for study was taken from various reports. Increased E-cadherin expression, has been associated with favorable survival in NSCLC (14). Further, as stated previously, adenocarcinoma of the lung is known for its relatively high TTF-1 expression in comparison to the other types of NSCLC (6, 7), and increased expression has been reported as a favorable independent predictor of survival in lung adenocarcinoma patients (8). A recent meta-analysis of 10 studies examining the prognostic relevance of TTF-1 in NSCLC (28) found that, indeed, this is a favorable biomarker in this disease. While our observations do not support this conclusion, there may be several factors for this discrepancy. Since TTF-1 is a favorable, independent prognostic factor, the benefit of TTF-1 would be more pronounced in later-staged disease. In our study, only 31 out of 159 (19%) were Stage III-IV. Since the majority of the cases within our study were otherwise favorable (clinical stages I-II and medically operable), a greater number of cases would be needed to show the benefit of TTF-1 positivity. Also, our primary clinical measure was time-to-metastasis. Although loosely correlated with overall survival time, these are different biologic endpoints. Lastly, we only included patients with adenocarcinoma and a smoking history. This excludes other histologies, including bronchioalveolar carcinoma (BAC), which commonly affects nonsmokers (29). Out of the 10 reports that were included in this meta-analysis, only four were adenocarcinoma-specific, and only two showed a statistically significant survival advantage. One of these included BAC in their analysis.
In summary, analyses of matched primary-metastatic paired samples, as well as 2 cohorts of lung primary samples, suggest that activation of S6 protein is involved in metastasis of lung adenocarcinoma. Our findings further suggest that evaluation of p-S6 expression could contribute to the stratification of lung adenocarcinoma patients for potential treatment regimens. In addition, those found to have increased risk of early metastasis, by the use of p-S6 as well as other clinical and molecular factors, may benefit from particularly close clinical follow-up.
Acknowledgments
Grant Support: American Cancer Society Postdoctoral Fellowship grant PF-06-272-01-CCE (McDonald) and Specialized Program of Research Excellence in Lung Cancer grant P50CA70907, National Cancer Institute, Bethesda, MD (Wistuba and Aldape).
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
This retrospective study demonstrates the potential utility for using p-S6, identified by immunohistochemistry in tumor specimen thin-sections, as a predictive biomarker of a more rapid time to the development of distant metastasis in early stage adenocarcinoma of the lung. Obtaining the status of this biomarker may guide adjuvant therapy decisions in these patients after the initial, definitive surgery has been performed.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2007. American Cancer Society; Atlanta: 2007. [Google Scholar]
- 2.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 3.Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer. Implications for screening. Chest. 1992;101:1013–8. doi: 10.1378/chest.101.4.1013. [DOI] [PubMed] [Google Scholar]
- 4.Stuschke M, Eberhardt W, Pottgen C, et al. Prophylactic cranial irradiation in locally advanced non-small-cell lung cancer after multimodality treatment: long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol. 1999;17:2700–9. doi: 10.1200/JCO.1999.17.9.2700. [DOI] [PubMed] [Google Scholar]
- 5.Mamon HJ, Yeap BY, Janne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;1(23):1530–7. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 6.Tan D, Li Q, Deeb G, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34:597–604. doi: 10.1016/s0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 7.Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korean Med Sci. 2003;18:494–500. doi: 10.3346/jkms.2003.18.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad RS, Liu YL, Han H, Landreneau RJ, Silverman JF. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol. 2004;35:3–7. doi: 10.1016/j.humpath.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Miakotina OL, Goss KL, Snyder JM. Insulin utilizes the PI 3-kinase pathway to inhibit SP-A gene expression in lung epithelial cells. Respir Res. 2002;3:27. doi: 10.1186/rr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massion PP, Taflan PM, Shyr Y, Rahman SM, Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ, Gonzalez AL. Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med. 2004;170:1088–94. doi: 10.1164/rccm.200404-487OC. [DOI] [PubMed] [Google Scholar]
- 11.Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–32. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 12.Hirami Y, Aoe M, Tsukuda K, Hara F, Otani Y, Koshimune R, Hanabata T, Nagahiro I, Sano Y, Date H, Shimizu N. Relation of epidermal growth factor receptor, phosphorylated-Akt, and hypoxia-inducible factor-1alpha in non-small cell lung cancers. Cancer Lett. 2004;214:157–64. doi: 10.1016/j.canlet.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 13.David O, Jett J, LeBeau H, et al. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res. 2004;10:6865–71. doi: 10.1158/1078-0432.CCR-04-0174. [DOI] [PubMed] [Google Scholar]
- 14.Deeb G, Wang J, Ramnath N, et al. Altered E-cadherin and epidermal growth factor receptor expressions are associated with patient survival in lung cancer: a study utilizing high-density tissue microarray and immunohistochemistry. Mod Pathol. 2004;17:430–9. doi: 10.1038/modpathol.3800041. [DOI] [PubMed] [Google Scholar]
- 15.Bubb RS, Komaki R, Hachiya T, et al. Association of Ki-67, p53, and bcl-2 expression of the primary non-small-cell lung cancer lesion with brain metastatic lesion. Int J Radiat Oncol Biol Phys. 2002;53:1216–24. doi: 10.1016/s0360-3016(02)02861-4. [DOI] [PubMed] [Google Scholar]
- 16.Milas I, Komaki R, Hachiya T, et al. Epidermal growth factor receptor, cyclooxygenase-2, and BAX expression in the primary non-small cell lung cancer and brain metastases. Clin Cancer Res. 2003;9:1070–6. [PubMed] [Google Scholar]
- 17.Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001 Feb 1;61:1122–8. [PubMed] [Google Scholar]
- 18.Cell Signaling Technology, Inc Available from: http://www.cellsignal.com/products/4857.html. [homepage on the Internet]. Danvers, MA; c1999−2008 [updated 2008 Jun 12; cited 2008 Jul 16]. Product Pathways – Translational Control; [about 2 screens].
- 19.Bejarano PA, Mousavi F. Incidence and significance of cytoplasmic thyroid transcription factor-1 immunoreactivity. Arch Pathol Lab Med. 2003;127:193–5. doi: 10.5858/2003-127-193-IASOCT. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 21.Abraham RT. Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell. 2002;111:9–12. doi: 10.1016/s0092-8674(02)01009-7. [DOI] [PubMed] [Google Scholar]
- 22.Jacinto E, Hall MN. Tor signaling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–26. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 23.Volarevic S, Thomas G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog Nucleic Acid Res Mol Biol. 2001;65:101–27. doi: 10.1016/s0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- 24.Conde E, Angulo B, Tang M, et al. Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res. 2006;12:710–7. doi: 10.1158/1078-0432.CCR-05-1362. [DOI] [PubMed] [Google Scholar]
- 25.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–67. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 26.Tampellini M, Longo M, Cappia S, et al. Co-expression of EGF receptor, TGFalpha and S6 kinase is significantly associated with colorectal carcinomas with distant metastases at diagnosis. Virchows Arch. 2007;450:321–8. doi: 10.1007/s00428-007-0370-2. [DOI] [PubMed] [Google Scholar]
- 27.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12:3935–41. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 28.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705–19. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 29.Morabia A, Wynder EL. Relation of bronchioloalveolar carcinoma to tobacco. Bmj. 1992;304:541–3. doi: 10.1136/bmj.304.6826.541. [DOI] [PMC free article] [PubMed] [Google Scholar]