Abstract
It has been established that both domain-specific (e.g. spatial) as well as domain-general (general intelligence) factors influence human cognition. However, the separation of these processes has rarely been attempted in studies using laboratory animals. Previously, we have found that the performances of outbred mice across a wide range of learning tasks correlate in such a way that a single factor can explain 30– 44% of the variance between animals. This general learning factor is in some ways qualitatively and quantitatively analogous to general intelligence in humans. The complete structure of cognition in mice, however, has not been explored due to the limited sample sizes of our previous analyses. Here we report a combined analysis from 241 CD-1 mice tested in five primary learning tasks, and a subset of mice tested in two additional learning tasks. At least two (possibly three) of the seven learning tasks placed explicit demands on spatial and/or hippocampus-dependent processing abilities. Consistent with previous findings, we report a robust general factor influencing learning in mice that accounted for 38% of the variance across tasks. In addition, a domain-specific factor was found to account for performance on that subset of tasks that shared a dependence on hippocampal and/or spatial processing. These results provide further evidence for a general learning/cognitive factor in genetically heterogeneous mice. Furthermore (and similar to human cognitive performance), these results suggest a hierarchical structure to cognitive processes in this genetically heterogeneous species.
Keywords: Intelligence, Working Memory, Learning, Cognitive Abilities, Hippocampus, Spatial Learning, Mice
For much of the history of animal studies of learning and memory, research has focused primarily on the processes and mechanisms that regulate single domains of learning (e.g., spatial abilities or Pavlovian conditioning). While this tactic has been successful in delineating the neuroanatomical substrates of certain forms of learning (e.g., Christian & Thompson; 2003; Eichenbaum, 2000; Phelps & LeDoux, 2005; White & McDonald, 2002) it has left unexplored those aspects of learning that are common across all domains. Conversely, studies of humans have focused extensively on the mechanisms that underlie general influences that impinge on all cognitive abilities (i.e., general intelligence). However, such studies are constrained by certain practical and ethical considerations that do not similarly limit studies of laboratory animals. Therefore, a synthesis of these two approaches would be of great virtue in beginning to elucidate the structure of and mechanisms underlying general intelligence.
Previously, we have reported the existence of a general learning factor in genetically heterogeneous mice, and this factor is in many ways analogous to general intelligence in humans (Matzel et al., 2003, 2006; Kolata et al., 2005, 2007). Specifically, we found that when genetically heterogeneous mice are assessed on a battery of 5–8 learning tasks (e.g., Lashley III maze, passive avoidance, spatial water maze, odor discrimination, fear conditioning) designed to tax different sensory/motor, information processing and motivational systems, as much as 44% of the variance in performance across the tasks can be explained by a single factor. Furthermore, it seems this factor is independent of stress reactivity and sensory / motor abilities (Matzel et al., 2006; Grossman et al., in press). It therefore appears from these results that while any individual learning task draws on specific domains of abilities (e.g. spatial) there also exists some learning-specific common factor that influences performance on all tasks.
Our previous studies have suggested that the mechanisms underlying general learning abilities in mice are similar to those implicated in general intelligence in humans (Kolata et al., 2005, 2007). For instance, human studies have indicated that working memory capacity covaries with general intelligence (Colom, Rbello, Palacios, Juan-Espinosa, & Kyllonen, 2004; Conway, Kane, & Engle, 2003; Conway, Cowan, Bunting, Therriault, & Scott, 2002; Engle, Tuholski, Laughlin, & Conway, 1999). Similarly, we reported finding a substantial positive correlation between the aggregate performance of mice in a battery of learning tasks and their ability to withstand competing demands on working memory (Kolata et al., 2005). Furthermore, we found that one aspect of working memory, selective attention, was most strongly predictive of general learning abilities (Kolata et al., 2007). Taken together, our previous work suggests a hierarchical structure of cognitive abilities in mice with domain general abilities, such as working memory, influencing domain specific abilities, such as spatial ability. However, the sample sizes of our previous factor analyses (number of subjects ≤ 56) precluded a direct test of this hypothesis.
In the present paper, we report a combined data analysis from 241 mice tested in a learning battery designed to assess a range of cognitive processes. Of those mice, subsets were also tested in two additional tasks (spatial win-stay and reinforced alternation), both of which tax spatial abilities and which have been asserted to engage the hippocampus (O’Keefe & Speakman, 1987; Stevens & Cowey, 1973; Woods, Dudchenko, Robitsek, & Eichenbaum, 2000). It was therefore hypothesized that these two tasks should share a domain specific factor with the spatial water maze, which is also commonly thought to be hippocampal dependant (Moser, Moser, Forrest, Andersen, & Morris, 1995). Using such a large subject pool allows for both a verification of previous findings based on smaller factor analyses as well the assessment of the possibility that general cognitive abilities have a hierarchical influence on domain-specific abilities.
Materials and Methods
Subjects
CD-1 exhibit considerable behavioral variability, and thus are particularly well suited for studies of individual differences. These mice are an outbred strain that was derived in 1926 from an original colony of non-inbred Swiss mice consisting of 2 males and 6 females. Estimates of genetic variation in this line have indicated that despite over 50 years of breeding they are very similar to wild mouse populations (Rice & O’Brien, 1980). For this study, 241 male, CD-1 mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). The mice arrived in our laboratory between 66–80 days of age, and ranged from 22–35 grams at the start of testing. Testing began when the mice were 90–110 days of age, an age which corresponds with young adulthood. The mice were housed individually in clear shoebox cages in a temperature and humidity controlled colony room and were maintained on a 12 h light/dark cycle. In order to minimize any effect of individual differences in stress reactivity to handling, prior to the start of the experiment all of the animals were handled for 90 sec/day, five days/week over a period of two weeks prior to the start of behavioral testing.
Behavioral Training and Testing
To quantify individual differences in learning among mice, a variant of the procedures previously reported was used (Matzel et al., 2003). All animals were tested in a series of five independent learning tasks (Lashley III maze, passive avoidance, spatial water maze, odor discrimination, and fear conditioning) that place unique sensory, motor, motivational, and information processing demands on the animals. Briefly, performance in the Lashley III maze depends on animals’ use of largely non-spatial search strategies (egocentric navigation) to locate a food reward. Passive avoidance is an operant conditioning paradigm in which the animals must learn to be passive in order to avoid aversive light and noise stimulation. The spatial water maze encourages the animals to integrate spatial information to efficiently escape from a pool of water. Odor discrimination is a task in which animals must discriminate and use a target odor to guide their search for food. Lastly, fear conditioning (assessed by suppression of ongoing behavior) is a conditioning test in which the animals learn to associate a white noise with a shock. In addition to these tasks, a subset of 98 animals received additional training in a spatial win-stay task. Of those 98 animals, 78 were also trained on a reinforced alternation task. In order to minimize the potential transfer of learning between tasks, the order in which animals were exposed to each task was arranged so that tasks that draw on similar information processing demands, motor requirements, or motivational systems were segregated (to the extent possible) in the test sequence.
The samples used for this analysis were drawn from both previously published studies (Matzel et al., 2003 [n=56]; Matzel et al., 2006 [n=43]; Kolata et al., 2005 [n=21]; Kolata et al., 2007 [n=27]) as well as from unpublished studies (n=94) conducted over a period of four years. With minor exceptions, the procedures were nominally identical across replications (any differences between replications are noted).
Lashley III Maze (LM)
This maze consists of a start box, three interconnected alleys and a goal box. Previous studies have shown that over successive trials the latency to find the goal box decreased as well as the number of wrong turns. When extra-maze cues are minimized (as was the case here), the animals tend to use egocentric methods (e.g., “turn right, turn left”) to locate the goal box. This differentiates this task from explicitly spatial tasks (see below), wherein the animals rely on extramze spatial cues to guide navigation.
The Lashley III maze was originally designed for use in rats, therefore in the present study it was reduced in size to roughly ½ scale for use with mice (60 cm long × 27 cm wide × 15 cm high). It was constructed from black Plexiglas and located in a dimly lit room (10 Lux at the floor of the maze). A 3 cm diameter white circle was located in the center of the goal box, and a 45 mg Bio-serv food pellet (dustless rodent grain) was placed in the cup to motivate the animal’s behavior.
Food-deprived animals were acclimated and trained on two successive days. Prior to acclimation they were exposed to three pellets of the reinforcer in their home cage. On the acclimation day, each mouse was confined in each of the first three alleys of the maze for 4 min and the final alley (wherein the goal box was located) for 6 min. Three food pellets were placed in goal box during this acclimation training. At the end of each period, the animal was physically removed from the alley and placed in the next alley. On the training day, the animals were placed in the start box and allowed to freely navigate the maze during and retrieve the single food pellet located in the goal box. During each trial, the number of errors (wrong turns or re-tracing a path) was recorded. Upon retrieving and consuming the food pellet, the animal was returned to its home cage for a 25 min inter-trial interval (ITI) during which time the maze was cleaned. The animals completed five trials during training, and all trials occurred within a single day.
Passive Avoidance (PA)
In this assay, animals learn to suppress their exploratory tendency in order to avoid aversive stimuli. The animals are placed on a platform and when they step down they are administered aversive stimuli, in this case a bright light, noise and vibration.
A chamber with a white grid floor 16 × 12 cm (l × w) which is illuminated by a dim red light was used for both acclimation and testing. An enclosed platform (70 × 45 × 45 cm, l × w × h) constructed of black Plexiglas and elevated 5 cm above the grid floor was located at the back of the chamber. It had only one opening facing the grid floor which allows the animal to step down onto the floor. The exit from the platform can be blocked remotely by a clear Plexiglas guillotine-style door. When an animal leaves the platform and makes contact with the grid floor it initiates the aversive stimuli.
During training, animals were placed on the platform with the door closed, confining them in the enclosure. After 5 min., the door was opened and the latency of the animal to leave the platform and make contact with the floor was recorded. After they make contact, the aversive stimuli were initiated and the door was lowered, exposing them to the stimuli for 4 sec. after which they were allowed access to the enclosure again. They were then confined on the platform for 5 min. after which the door opened and their latency to walk onto the grid floor was recorded for a second time. For the purpose of ranking the animals, the ratio of the step down latency post exposure to the aversive stimuli to step down latency prior to that exposure served as the index of learning. The use of this ratio allows for an assessment of learning independent of exploratory tendencies (which can influence step-down latencies).
Spatial Water Maze (WM)
This task requires the animals to locate a submerged platform in a pool of opaque water. Absent distinct intra-maze cues, animals’ performance in this maze is highly dependent on the integration of extra-maze spatial cues. The animals are motivated by their aversion to water. The latency and the path length to locate the platform decrease over successive trials even though the animals enter the pool from different locations.
A round pool (140 cm diameter, 56 cm deep) was filled to within 20 cm of the top with water that was clouded with a nontoxic, water soluble black paint. A hidden 14 cm diameter black platform was located in a fixed position 1 cm below the surface of the water. The pool was enclosed by a ceiling high black curtain on which five different light patterns (which served as spatial cues) were fixed at various positions.
On the day prior to training, each animal was confined to the platform for 360 sec. by a clear Plexiglas cylinder that fits around the platform. On the next two training days, the animals were started from one of three positions for each trial such that no two subsequent trials start from the same position. The animal was said to have successfully located the platform when it placed all four paws on the platform and remained for 5 sec. After locating the platform or swimming for 90 sec., the animals were left or placed on the platform for 10 sec. They were then removed for 10 min. and placed in a holding box before the start of the next trial. Each animal completed 10 total trials (6 on the first training day, 4 on the second). The latency to find the platform was recorded for each trial.
Associative Fear Conditioning (FC)
Animals receive a tone (CS) paired with a mild foot shock (US). To measure the conditioned fear responses, lick rates of water-deprived animals were assessed prior to and during the presentation of the white noise CS. Mice will not consume water during the period of immobility induced by the CS. Thus, like the common measure of “freezing” (i.e., immobility), lick suppression is thought to reflect “fear”. However, lick suppression is more easily quantifiable than is freezing.
Two distinct experimental chambers were used (a training context and a testing context) to avoid interactions between the training context and the CS at the time of testing. Such interactions could include freezing to the training context independent of the CS. Each 32 × 32 × 28 cm box (l × w × h) was contained within a sound and light-attenuating chamber. The training box (the box where the animals received CS-US pairings) was brightly lit with clear Plexiglas walls, no lick tube, and had a stainless still grid floor (5 mm spacing). A 0.6 mA constant current footshock could be delivered through the floor. The test chamber was dimly lit and the walls were covered with an opaque pattern of alternating black and white vertical stripes (in order to differentiate it from the training context) and the floor was formed in a grid of stainless 1.5 mm rods arranged in 8 mm squares. A water lick tube protruded through a small hole in one wall of the box.
Water deprived animals were acclimated to the training and testing contexts by placing each animal in both boxes for 20 min. on the day before training. Training on the subsequent day occurred in a single 20 min. session during which the animals received two white noise-shock pairings after 7 min. and after 14 min. They were then returned to their home cages for 60 min. after which they were re-acclimated to the test chamber for 20 min. The following day (test day) the animals were placed in the testing chamber and after completion of 25 licks, the CS was presented and stayed on until the animal completed an additional 25 licks or timed out at 600 sec. The ratio of the time to complete 25 licks prior to the CS presentation to the time to complete 25 licks during the CS presentation served as an index of learning.
Odor Discrimination (OD)
Rodents are adept at using odor to guide their reinforced behavior. This task is modified from one used by Sara, Roullet, & Przybyslawski (1999) but scaled down for use with mice. In this task, mice navigate through a field using unique odors to guide them. The animals learn to choose the food cup that contains the target smell when given three choices. The food cup locations are rearranged on each trial but the accessible food is always marked by the target odor (in this case mint).
The odor discrimination chamber consisted of a black Plexiglas 60 cm square field with 30 cm high walls located in a dimly lit room with good ventilation. One of three aluminum food cups was placed in three corners. Only the target cup had the food accessible. The other two cups had food located in a covered hole drilled into the side with a ventilation hole allowing the mice to smell the food but not access it. One 30 mg portion of chocolate flavored puffed rice acted as a reinforcer and was placed in a depression on top of the target cup. A cotton tipped laboratory swab (2 cm long) was loaded before each trial with 25 µl of lemon, mint or almond flavored extract and extended vertically from the back corner of each cup. Mint was designated the target odor. In some replications, in lieu of aluminum food cups, movable 30 cm × 15 cm walls (h × l) with attached food cups and slots for the cotton swaps were used. The animals had to go behind the movable walls in order to reach the food cups and hence find the rewards.
Each animal had one day of acclimation and one day of testing. The night prior to the acclimation day, food was removed from each animal’s home cage. The next day each mouse was placed in the box for 20 min. without the cups. At the end of the day each animal received three pieces of the reinforcer in their home cage. On the training day each animal received four trials in which they were placed in the corner of the training chamber which did not contain a food cup. On the first trial an additional reinforcer was placed on the edge of the target cup (mint). At the end of each trial the food cups and the starting location were rearranged but mint always remained as the target odor. For each trial both latency to locate the food and number of errors are recorded (where an error is making contact with or sniffing within 2 cm of an incorrect food cup). On replications which used removable walls with attached food cups instead of aluminum food cups, an error was counted when an animal went behind the movable wall. For purpose of this analysis, the average errors across trials 2 and 3 served to index learning.
Spatial Win-Stay (SWS)
In this task, animals must learn on successive trials to consistently enter a specific goal arm of a four arm maze in order to obtain food, despite randomly alternating starting positions on each trial between the remaining three arms.
An elevated maze with four open arms, constructed of black Plexiglas, was used for this task. For some of the replications the maze was a ‘+’ shaped maze with four arms measuring 8 × 40 cm (w × l). While for other replications the maze was an eight arm radial maze, each measuring 8 × 40 (w × l). Four of the arms were blocked, leaving only four arms the mice could enter. In both of the mazes, a 4 mm diameter food cup was located 2 cm from the end of each arm. A 14 mg Bio-serv pellet (dustless rodent grain) was located in every arm, but was accessible to the animal only in the arm designated as the goal arm. The maze was located in a room surrounded by numerous visually distinct objects.
The animals were food deprived two days before the start of training. On the first day of deprivation, the animals were given three sample reinforcers in their home cage. On the first day of training, the animals were confined in each of the three starting arms for 60 sec. as well as in the goal arm, with two reinforcers present, for 90 sec. This was done to acclimate the animals to the arms but not to allow them to learn the relationship between the arms. On the second day of training, the animals were given 10 training trials. At the start of each trial the animal was placed at the end of one of the three starting arms for 10 sec. and then allowed to freely explore the maze until it located and consumed the food in the goal arm or until it timed out at 90 sec. If the animal timed out, it was placed in the goal arm with the food and allowed to consume the reward. Following consumption of the reward, the animal was placed in the next randomly selected start arm for the start of the next trial. While each animal experienced a randomly chosen sequence of start arms during training, all of the animals were trained on the same sequence. On each trial, both the number of incorrect arm entries (errors) and the latency to locate the food were recorded. For purpose of data analysis only the number of errors was used.
Reinforced Alternation (RA)
In this task, animals learn to alternate between entering one of two arms that intersected to form the top of a “T”. On each trial a food reinforcer was present in the end of one arm. The location of the reinforcer shifted to the alternate arm after each successful retrieval of food. In order to perform efficiently in such a task the animals had to alternate choices on successive trials (win-shift) in order to minimize the amount of effort it required to locate the food.
The apparatus consisted of a start arm (7.5 cm wide × 17 cm long) that intersected at the middle of an alley that forms the top of a “T” (92 cm long × 6 cm wide). The entire maze was enclosed in a 5 cm high wall. The initial segment of the start arm (the start box) was segregated by a guillotine door that was remotely operated by the experimenter. At the entry of each choice arm there was another experimenter-operated door. On the wall of the right arm there were vertical white strips an inch apart. While on the wall of the left arm there were horizontal stripes. These were used to help the animal discriminate between the arms.
Training was conducted over three consecutive days. On Day 1, animals were acclimated to the maze and experienced four forced choices. On the first exposure, the animal was held in the start box for 30 sec, after which it was allowed to traverse the maze. The door into the left arm was closed, forcing the animal to choose the right arm. A 14 mg Bio-Serv pellet (dustless rodent grain) was located in the food cup in the right arm. After consuming the food, the animal was returned to the start box for a 20 sec. inter-trial interval (ITI). On the second exposure, this procedure was repeated, but the right door was locked and the left door open. After a 20 sec. ITI, this sequence was repeated for two additional exposures.
On days 2 and 3, animals were trained. On all training trials, both choice doors were open. On trial 1, a reinforcer was available in both food cups and the animal could make a free choice. On the second trial, reinforcement was available in the arm not entered on the first trial. After an animal chose the correct arm, the location of the reinforcer alternated on the following trial. If an incorrect choice was made, the animal was allowed to correct its mistake and locate the food in the other arm. In either case, after the reinforcer was consumed, the animal was placed back in the start box to begin a 20 sec. ITI. Animals were given a 12 trials a day on each of two successive days.
Analysis
In all learning tasks, the animals’ performance was assessed during the acquisition phase of learning (i.e., prior to reaching their stable, asymptotic level of performance). Thus the dependent measure for each task was analogous to the animals’ rate of learning on that task, and these measures of each individual’s performance could be ranked relative to other animals in the sample. We have consistently found that only the performance during acquisition of the various tasks correlate with each other (e.g., Figure 1). To quantify an animal’s performance in tasks in which there were multiple training/test trials, performance during trials that fell within the acquisition phase were averaged. In tasks in which there was only one test trial (i.e. fear conditioning and passive avoidance), training parameters were used that were previously determined to result in sub-asymptotic responding by most animals. To minimize the impact of any variations across the multiple replications that contributed to this study, for purposes of factor analysis, animals’ performance on each task was converted to a z score, thus mitigating slight variations in training parameters that might impact acquisition rates.
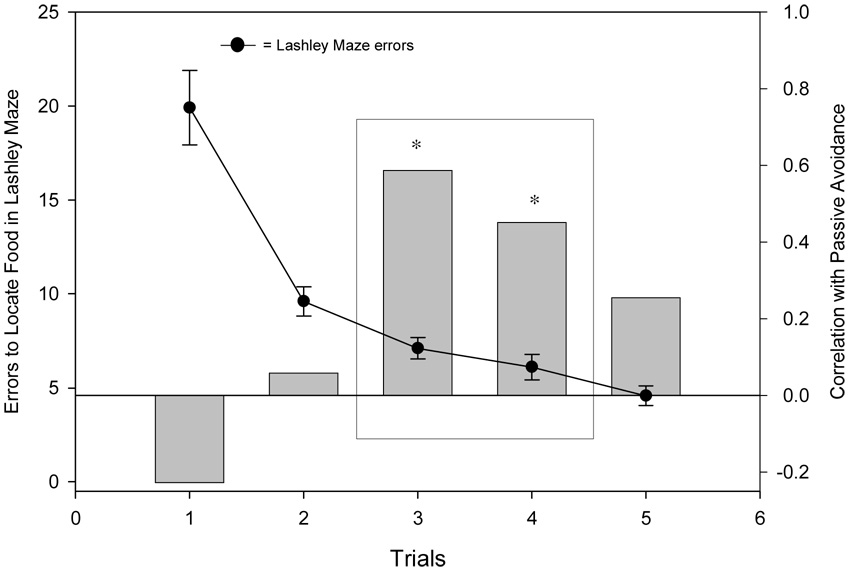
Figure 1.
The acquisition data in the Lashley maze for a subset of 36 animals is shown on the left y-axis. A trial-by-trial Pearson’s correlation between the performance in the Lashley maze and learning in passive avoidance is shown on the right y-axis (* p<.05). As can be seen only the trials that fall within the main part of the acquisition curve correlate with learning in passive avoidance. The rectangle indicates the two trials that were averaged for purpose of analysis.
Results
The correlation matrix containing the performances of the 241 mice in each of five learning tasks revealed a positive manifold, with all of the tasks correlating such that an animal’s level of performance on one task was indicative of its performance on other tasks in the battery (Table 1a). Of the 10 unique correlations in the matrix, 8 were significant at the .01 level and one was significant at the .05 level (Pearson’s r). Figure 2a shows sample data from three mice (a fast learner, an average learner, and a poor learner). As can be seen, there is a high correspondence between performances across tasks. A similar positive manifold is seen when spatial win-stay (Table 1b, n = 98) and reinforced alternation (Table 1c, n = 78) are added. Both of these additional tasks display similarly positive correlations with the other learning tasks. In previous studies (Matzel et al., 2003, Kolata et al., 2005, Kolata et al., 2007) sample sizes (generally fewer than 56 subjects) have limited the number of significant correlations reported. A power analysis assuming a significance level of .05 and a power of .80 indicated that a sample size greater than 120 would be required to find a significant correlation of .22 (average correlation in the correlation matrix).
Table 1.
(a) A correlation matrix of all the tasks in the primary (five task) learning battery (n=241). LM = Lashley III maze, PA = passive avoidance, WM = spatial water maze, OD = odor discrimination, FC = fear conditioning. (b) Correlation matrix with spatial win-stay (SWS) added, n = 98 (c) Correlation matrix with reinforced alternation (RA) added, n = 78.
| A | |||||
|---|---|---|---|---|---|
| (n = 241) | |||||
| LM | PA | WM | FC | OD | |
| LM | 1.00 | ||||
| PA | 0.45** | 1.00 | |||
| WM | 0.23** | 0.22** | 1.00 | ||
| FC | 0.15* | 0.22** | 0.08 | 1.00 | |
| OD | 0.19** | 0.23** | 0.17** | 0.23** | 1.00 |
| B | ||||||
|---|---|---|---|---|---|---|
| (n = 98) | ||||||
| LM | PA | WM | FC | OD | SWS | |
| LM | 1.00 | |||||
| PA | 0.25* | 1.00 | ||||
| WM | 0.16 | 0.21* | 1.00 | |||
| FC | 0.16 | 0.23* | 0.06 | 1.00 | ||
| OD | 0.14 | 0.20 | 0.15 | 0.16 | 1.00 | |
| SWS | 0.07 | 0.17 | 0.25* | 0.15 | 0.18 | 1.00 |
| C | |||||||
|---|---|---|---|---|---|---|---|
| (n = 78) | |||||||
| LM | PA | WM | FC | OD | SWS | RA | |
| LM | 1.00 | ||||||
| PA | 0.26* | 1.00 | |||||
| WM | 0.15 | 0.21 | 1.00 | ||||
| FC | 0.14 | 0.15 | 0.06 | 1.00 | |||
| OD | 0.18 | 0.19 | 0.16 | 0.10 | 1.00 | ||
| SWS | 0.01 | 0.22 | 0.25* | 0.18 | 0.16 | 1.00 | |
| RA | 0.22 | 0.31** | 0.33** | 0.13 | 0.20 | 0.22 | 1.00 |
p < .05
p<.01
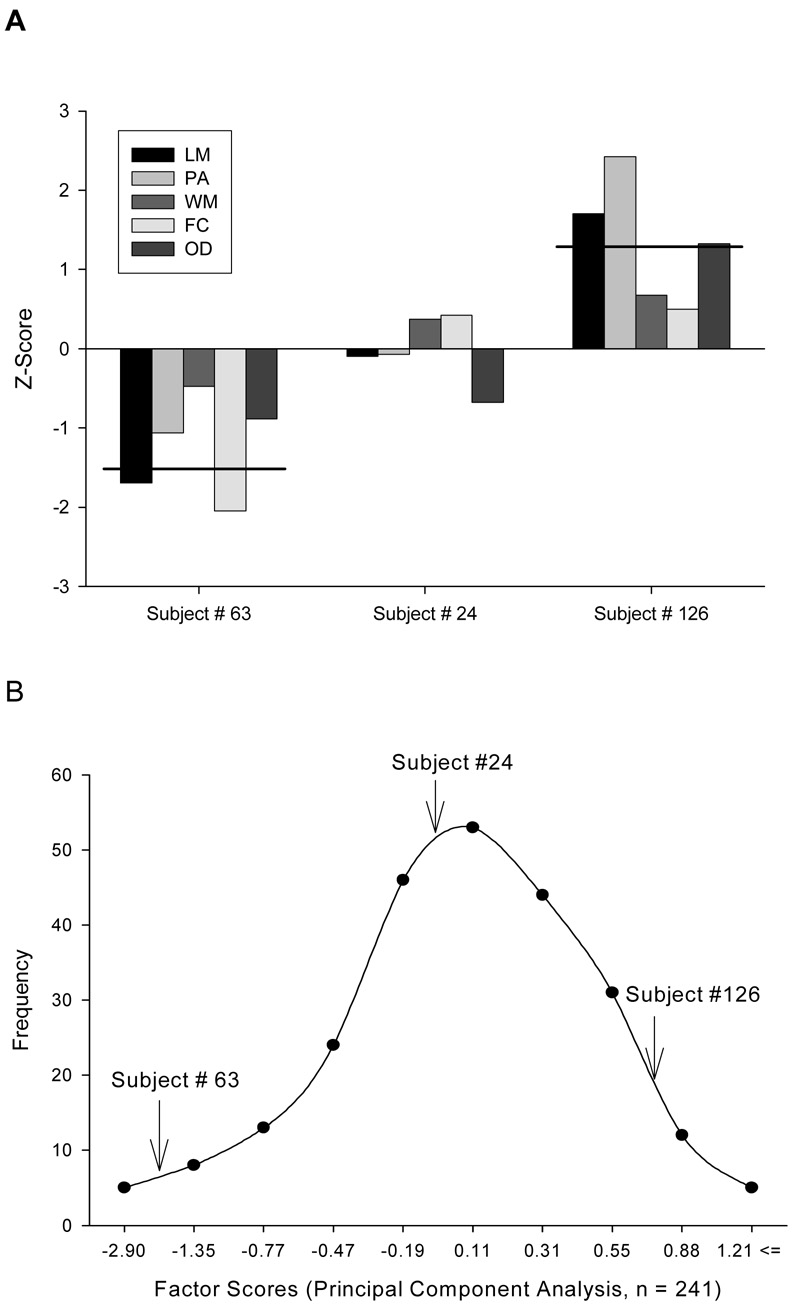
Figure 2.
(a) Sample data from three animals that were run through the learning battery (LM = Lashley maze, PA = passive avoidance, WM = spatial water maze, OD = odor discrimination, FC = fear conditioning). Values indicate the z-scores of the individual animal’s learning rate. Performances across the different tasks are highly related such that one could designate a good learner (subject # 63), an average learner (subject # 24) and a poor learner (subject #126). The solid lines indicate the average score across trials. (b) Normal distribution of factor scores extracted from a principal component factor analysis performed on the learning data from 241 mice tested on the five learning tasks (Shapiro-Wilk W = .99, p = n.s.). Arrows indicate where the three sample animals (subject # 63, subject #24, and subject # 126) fall in the distribution.
An unrotated principal component factor analysis of the performance data on the primary (five task) learning battery revealed a single factor that accounted for 38% of the total variance (eigenvalue = 1.90, n = 241, Table 2a). This value is comparable to previous factor analyses done with much smaller subject pools (Matzel et al., 2003, Kolata et al., 2005). The factor scores extracted from the primary factor were normally distributed (Shapiro-Wilk Test, W(241) = .99, n.s.; Figure 2b). Unlike previous findings, no additional factors with eigenvalues greater then 1 were extracted. Due to the absence of secondary factors, no factor rotations were possible on this data set. Similarly, a principal component factor analysis on the data set which included the two additional tasks (reinforced alternation and spatial win-stay) revealed a primary factor that accounted for 30% of the variance across tasks and on which all of the learning tasks loaded consistently (Table 2b). A secondary factor (not shown) with an eigenvalue of 1.02 was also found. This factor explained only 15% of the variance and had loadings that were not easily interpretable. In contrast, a Varimax normalized rotated principal component factor analysis on this data set revealed a primary factor as well as a secondary factor which explained roughly equal variance (eigenvalues = 1.67 and 1.48 respectively, n = 78, Table 2c). The three tasks that were a priori hypothesized to reflect a common domain (water maze, spatial win-stay and reinforced alternation) loaded consistently on this secondary factor, while the other tasks loaded negligibly and inconsistently on this factor.
Table 2.
(a) An unrotated principal component factor analysis of the primary learning battery reveals only one factor (general learning abilities) explaining 38% of the total variance. (b) An unrotated principal component factor analysis of the learning battery including spatial win-stay and reinforced alternation reveals a primary factor (general learning abilities) explaining 30% of the total variance on which all the tasks load. (c) A Varimax normalized rotated principal component factor analysis of the learning battery which includes spatial win-stay and reinforced alternation. In addition to the primary factor, a secondary factor in which water maze, spatial win-stay and reinforced alternation load is extracted. These three tasks share a common dependence on the hippocampus.
| A | |
|---|---|
| Unrotated Principal Component Factor Analysis | |
|
Factor 1 (General Learning Ability) |
|
| Lashley Maze | 0.71 |
| Passive Avoidance | 0.75 |
| Water Maze | 0.51 |
| Fear Conditioning | 0.50 |
| Odor Discrimination | 0.57 |
| Eigenvalue | 1.90 |
| Proportion of Total Variance | 0.38 |
| B | |
|---|---|
| Unrotated Principal Component Factor Analysis | |
|
Factor 1 (General Learning Ability) |
|
| Lashley Maze | 0.49 |
| Passive Avoidance | 0.65 |
| Water Maze | 0.59 |
| Fear Conditioning | 0.38 |
| Odor Discrimination | 0.50 |
| Reinforced Alternation | 0.68 |
| Spatial Win-Stay | 0.52 |
| Eigenvalue | 2.12 |
| Proportion of Total Variance | 0.30 |
| C | ||
|---|---|---|
| Varimax Rotated Principal Component Factor Analysis | ||
|
Factor 1 (General Learning Ability) |
Factor 2 (Hippocampal-Dependant) |
|
| Lashley Maze | 0.82 | −0.22 |
| Passive Avoidance | 0.59 | 0.30 |
| Water Maze | 0.25 | 0.62 |
| Fear Conditioning | 0.33 | 0.20 |
| Odor Discrimination | 0.48 | 0.20 |
| Reinforced Alternation | 0.49 | 0.47 |
| Spatial Win-Stay | 0.00 | 0.81 |
| Eigenvalue | 1.67 | 1.48 |
| Proportion of Total Variance | 0.24 | 0.21 |
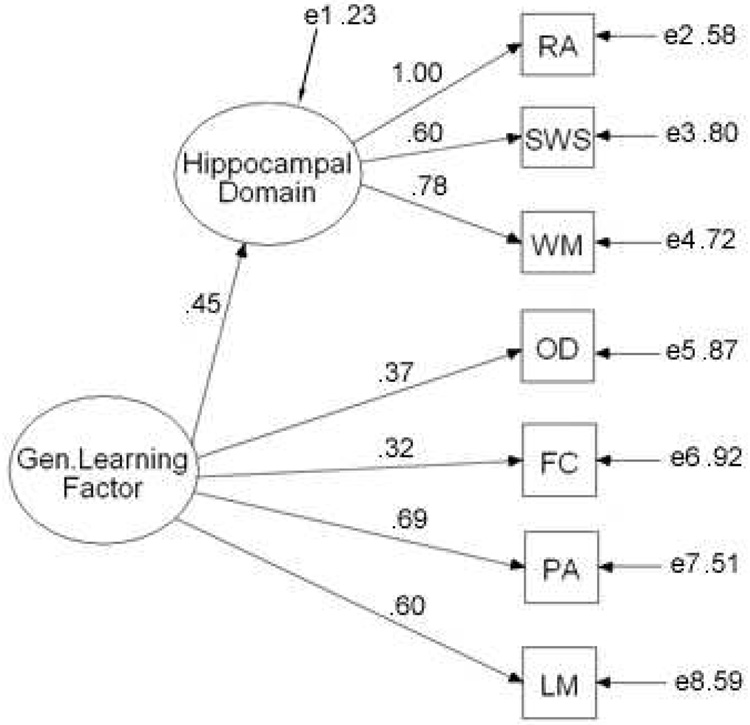
In addition to principal component factor analyses, a confirmatory factor analysis was performed to assess the feasibility of a factor structure consistent with a hierarchical organization of cognitive abilities. Two latent factors were postulated: a first-order hippocampal-dependant factor which would underlie performance in the water maze, win-stay, and reinforced alternation tasks, as well as one second-order factor (general learning ability) which would influence all learning tasks. The confirmatory factor analysis was performed using AMOS 5.0 which uses maximum likelihood estimation to derive factor loadings. The model is presented in Figure 3 (χ2 (13) = 9.9, p = .70). There is no standard estimate of goodness of fit for confirmatory factor analysis, but the root-mean squared error of approximation (RMSEA) value of 0.00 (90% confidence interval = 0.00 – 0.05) suggests that this is an almost perfect description of the data (Brown and Cudek, 1993). Similarly, the comparative fit index (CFI) value of 1.0 suggests that this model is a good fit (Bentler, 1990). No other domain specific factors were tested because there is no good theoretical reason to suggest a common source of variance other than the general learning factor for any of the other learning tasks. (The lack of additional domains reflects how the initial tasks were chosen, i.e., to specifically isolate general learning abilities.) However, while the hierarchical model presented here is a good fit for the data, it was not substantially different from a model (not illustrated) that contained only one general factor and no domain-specific latent factors. A Bayes Information Criterion (BIC) value for the hierarchical model did not differ by more then 10 (a standard threshold for confirming differences between models) from the BIC value obtained for the un-nested model.
Figure 3.
A confirmatory factor analysis (AMOS 5.0) testing the feasibility of a model of learning abilities in mice with one first-order domain-specific factor (hippocampal factor) as well as one second-order general factor (gen. learning factor) influencing acquisition rates on seven learning tasks (RA = reinforced alternation, SWS = spatial win-stay, WM = water maze, OD = odor discrimination, FC = fear conditioning, PA = passive avoidance, and LM = Lashley III maze). Residual values (e1–e8) represent variance not explained by the factors. Loadings on the first-order factor (hippocampal domain) are set relative to reinforced alternation. Tests of goodness-of-fit suggest this model is a good fit for the observed data (χ2 (13) = 9.9, p = .70; RMSEA = 0.00 (0.00 – 0.05); CFI = 1.00). Standardized parameters are shown.
Discussion
Individual differences in human cognitive abilities, specifically intelligence, have been the subject of intense research for well over a century (Jensen, 1998). However, attempts to address similar questions in animals have for the most part been infrequent and unsystematic, despite their potential to add to our understanding of cognition (Plomin, 2001). Previous studies from our laboratory have suggested the existence of a general learning factor in mice, which influences learning rates across many different types of tasks that impinge on various information-processing and motivational systems (Matzel, et al., 2003, Matzel et al., 2006, Kolata et al., 2005). This factor has been interpreted as being analogous to intelligence in humans (Blinkhorn, 2003). Similarly other laboratories have reported results consistent with these findings (Locurto & Caitlin, 1998; Locurto, Fortin, & Sullivan, 2003; Galsworthy, Paya-Cano, Monleó, & Plomin, 2002; Galsworthy et al, 2005).
While human studies of general intelligence do not assess learning per se, studies have shown that the rate of acquisition of a skill is positively correlated with IQ test performance (Ackerman, 1987, 2005; Jensen, 1987). Fitts and Posner (1967) described learning as a three stage process (cognitive, associative and autonomous). It is performance during the initial cognitive stage that most highly correlates with general intelligence test performance (Ackerman, 2005). During this stage, performance is said to be a “controlled” process that requires sustained effort and which is detrimentally affected by extraneous attentional demands. Similarly, in our cognitive battery using mice, learning was assessed during its initial acquisition phase, wherein animals exhibit considerable individual differences. Therefore, there appears to be a close conceptual correspondence between the cognitive phase of human learning, which correlates with intelligence, and our general learning factor in mice.
While previous work from our laboratory has shown the existence of a general learning factor in mice, the size of the samples used in these studies has limited the types of analyses done to explore the nature of this factor. Therefore, in the present study, we report a combined analysis from 241 mice tested on five learning tasks as well as from a subset of animals that was tested on two additional learning tasks.
Consistent with previous findings (Matzel et al., 2003; Matzel et al., 2005; Kolata et al., 2005; Kolata et al. 2007) analysis of the performance of the 241 outbred mice tested on the primary (five task) learning battery revealed a correlation matrix with consistently positive correlations. However, in previous reports we observed only sporadic significant correlations between tasks. In contrast, here we found that nearly all of the correlations between tasks were significant. If the previously observed positive, though non-significant, correlations were due to chance, then one would expect the correlations to approach zero as the sample size increased. Thus the present results suggest that the previous reports of a positive manifold were robust despite the limited sample sizes.
From the correlation matrix, a principal component factor analysis extracted a single factor explaining 38% of the variance across the five primary learning tasks. The size of this factor is comparable to previous findings. Unlike previous studies, no secondary factors were detected. At first this may seem counter-intuitive since a general influence on learning should not be the only factor that influences learning rates. However, the tasks chosen in the primary learning battery of five tasks were explicitly chosen to be as different as possible (impinging on different information-processing, motivational, motor and emotional systems). This contrasts with human cognitive batteries designed to measure intelligence, where tasks often cluster around different domains (e.g. verbal, spatial). This was done in order to maximize the likelihood of isolating a general factor. Therefore, in light of the nature of the tasks in the battery, it follows that the only factor that the tasks share in common is the most general factor. In previous studies, secondary factors were occasionally observed, but were often difficult to interpret, suggesting that they were not extracting anything meaningful about the nature of the animals’ cognition and were instead most likely due to chance. This is consistent with the relatively small amount of variance (typically less then 15%) accounted for by these factors (Matzel et al., 2003; Matzel et al., 2005; Kolata et al., 2005; Kolata et al., 2007).
A subset of the animals reported here was also tested on two additional tasks (spatial win-stay and reinforced alternation) as well as the five tasks in the primary learning battery. It was hypothesized that these two tasks plus the spatial water maze would share a single domain-specific factor because of their common dependence on the hippocampus (O’Keefe & Speakman, 1987; Woods et al., 2000; Moser et al., 1995). As hypothesized, a rotated principal component factor analysis extracted a second factor on which these three tasks loaded most prominently. In addition, a confirmatory factor analysis revealed that the hypothesized domain specific factor was a good fit for the data. This result suggests that, as in human cognition, there is a hierarchical structure to factors influencing learning in mice, whereby a general factor (g) influences domain-specific factors and performance on individual tasks. However, while the model presented here is suggestive of a hierarchical structure of cognitive abilities in mice, it should still be taken as a hypothesis because the sample size (78) is relatively small and the hierarchical model was not a substantially better fit of the data than was a model containing only a general learning factor. Further work will be necessary to clarify the exact nature of the latent factors.
The exact nature of the putative domain-specific factor reported here cannot be fully known. It seems most likely that that the three tasks cluster together due to their shared dependence on the hippocampus. Nevertheless, it is also possible that they all share a spatial component. Indeed several studies have extracted a domain-specific factor from the performance of mice on a set of explicitly spatial tasks (Locurto et al., 1998; Locurto et al., 2003). While the water-maze and win-stay tasks employed here are both explicitly spatial, reinforced alternation, as tested here, is not necessarily a spatial task. However, it is conceivable that the mice learned a spatial strategy to solve the task (e.g., go to the opposite spatial location last visited to locate food). The hippocampus, especially the dorsal hippocampus, is commonly thought of as being necessary for efficient performance on spatial tasks (Moser et al., 1995) and has been reported to impact performance in reinforced alternation (Stevens & Cowey, 1973; Woods et al., 2000). Therefore, even if the tasks are all spatial in nature, a more parsimonious explanation of the domain-specific factor might still be their common reliance on the hippocampus. Without further manipulations, such as lesion studies, it is not possible to fully elucidate the nature of this factor. However, it is known that these three tasks share more in common than any other group of tasks in the battery, hence the hypothesized latent factor.
An animal model of general cognitive abilities would allow for a further elucidation of the possible neural and genetic determinants of general intelligence using techniques not possible in human studies. The results reported here offer further support to the idea that the structure of cognitive abilities in both animals and humans share numerous similarities.
Acknowledgements
This work was supported by grants from the National Institute of Aging (AG 00298) and the Busch Foundation to L.D.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman PL. Individual differences in skill learning: An integration of psychometric and information processing perspectives. Psychological Bulletin. 1987;102:3–27. [Google Scholar]
- Ackerman PL. Ability determinants of individual differences in skilled performance. In: Sternberg RJ, Pretz JE, editors. Cognition and Intelligence: Identifying the Mechanisms of the Mind. Cambridge: Cambridge University Press; 2005. pp. 142–159. [Google Scholar]
- Blinkhorn S. Neuroscience: Of mice and mentality. Nature. 2003;24:1004–1005. doi: 10.1038/4241004a. [DOI] [PubMed] [Google Scholar]
- Brown MW, Cudeck R. Alternative ways of assessing model fit. Sociological Methods and Research. 1992;21:230–258. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning and Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Colom R, Rbello I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Conway AR, Cowan N, Bunting M, Therriault DJ, Scott RB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–183. [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cognitive Science. 2003;7:547–522. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Posner MI. Human Performance. Belmont, CA: Brooks/Cole; 1967. [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleó S, Plomin R. Evidence for general cognitive abilities (g) in heterogeneous stock mice and an analysis of potential confounds. Genes, Brain and Behavior. 2002;1:88–95. doi: 10.1034/j.1601-183x.2002.10204.x. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleó S, Gregoryan G, Fernandes C, Schwalkwyk, Plomin R. Assessing reliability, heritability, and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behavior Genetics. 2005;10:675–692. doi: 10.1007/s10519-005-3423-9. [DOI] [PubMed] [Google Scholar]
- Grossman HC, Hale G, Light K, Kolata S, Townsend D, Goldfarb Y, Kusnecov A, Matzel LD. Pharmacological modulation of stress reactivity dissociates general learning ability from the propensity for exploration. Behavioral Neuroscience. 2007 doi: 10.1037/0735-7044.121.5.949. in press. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The relationship between learning and intelligence. Learning and individual differences. 1989;1:37–62. [Google Scholar]
- Jensen AR. The g factor: The science of mental ability (human evolution, behavior, and intelligence) New York: Praeger; 1998. [Google Scholar]
- Kolata S, Light K, Townsend DA, Hale G, Grossman HC, Matzel LD. Variations in working memory capacity predict individual differences in general learning abilities among genetically diverse mice. Neurobiology of Learning and Memory. 2005;84:241–246. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Kolata S, Light K, Grossman H, Hale G, Matzel LD. Selective Attention is a primary determinant of the relationship between working memory and general learning ability in outbred mice. Learning and Memory. 2007;14:22–28. doi: 10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCurto C, Caitlin S. Individual differences and a spatial learning factor in two strains of mice (Mus musculus) Journal of Comparative Psychology. 1998;112:344–352. [Google Scholar]
- LoCurto C, Fortin E, Sullivan R. The structure of individual differences in heterogeneous stock mice across problem types and motivational systems. Genes, Brain and Behavior. 2003;2:40–55. doi: 10.1034/j.1601-183x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a “general” learning ability in mice. Journal of Neuroscience. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality and physical attributes. Neurobiology of Learning and Memory. 2006;82:228–240. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Moser M, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. PNAS. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keafe JO, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Experimental Brain Research. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotional processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Plomin R. The genetics of g in human and mouse. Nature Reviews Neuroscience. 2001;2:136–141. doi: 10.1038/35053584. [DOI] [PubMed] [Google Scholar]
- Rice MC, O’Brien SJ. Genetic variance of laboratory outbred Swiss mice. Nature. 1980;283:157–161. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association:β-adrenergic receptor involvement in late phase. Learning and Memory. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Research. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Woods ER, Dudchenko R, Robitsek J, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]