Abstract
In this study, denitrification linked to the oxidation of arsenite (As(III)) to arsenate (As(V)) was shown to be a widespread microbial activity in anaerobic sludge and sediment samples that were not previously exposed to arsenic contamination. When incubated with 0.5 mM As(III) and 10 mM NO3−, the anoxic oxidation of As(III) commenced within a few days, achieving specific activities of up to 1.24 mmol As(V) formed g−1 volatile suspended solids d−1 due to growth (doubling times of 0.74 to 1.4 d). The anoxic oxidation of As(III) was partially to completely inhibited by 1.5 and 5 mM As(III), respectively. Inhibition was minimized by adding As(III) adsorbed onto activated aluminum (AA). The oxidation of As(III) was shown to be linked to the complete denitrification of NO3− to N2 by demonstrating a significantly enhanced production of N2 beyond the background endogenous production as a result of adding As(III)-AA to the cultures. The N2 production corresponded closely the expected stoichiometry of the reaction, 2.5 mol As(III) mol−1 N2-N. The oxidation of As(III) linked to the use of common occuring nitrate as an electron acceptor may be an important missing link in the biogeochemical cycling of arsenic.
Keywords: Chemolithotrophic, Arsenic, Arsenate, Nitrate, Anaerobic, Biotransformation
1. Introduction
The occurrence of arsenic in drinking water is a global public health concern. Epidemiological studies have provided compelling evidence for a link between the chronic exposure to arsenic in drinking water and several types of cancer and other medical disorders (ATSDR, 2007). The evidence has prompted various environmental agencies to lower the maximum contaminant level in drinking water from 50 to 10 µg L−1 (USEPA, 2001; WHO, 1993). The main anthropogenic sources of arsenic to the environment are from liberation of arsenic by mining (Roussel et al., 2000), use of arsenical pesticides (Bednar et al., 2002), and treatment of preserved wood with arsenic (Katz and Salem, 2005). However, the natural release of arsenic to groundwater from the weathering of arsenic bearing rocks and sediments is the most important source from a global public health perspective (Smedley and Kinniburgh, 2002).
Microorganisms play a key role in the speciation and mobility of arsenic in the environment (Oremland and Stolz, 2003). In anaerobic environments, a biodiverse group of dissimilatory arsenate reducing bacteria utilize arsenate (As(V)) as a terminal electron acceptor and reduce it to arsenite (As(III)). As(V) reduction to As(III) is also microbially catalyzed by the ars operon as a detoxification mechanism which pumps arsenic out of the cell (Silver and Phung, 2005). Compared to As(V), As(III) is less strongly adsorbed by some common-occurring soil oxides such as aluminum oxyhydroxides (Hering and Dixit, 2005). Release of arsenic in anaerobic conditions can also result form the dissimilatory reductive dissolution of iron oxyhydroxides (Straub et al., 2001), which are important sorbents for As(V) and As(III) (Dixit and Hering, 2003). The combined result of the microbial reduction of arsenic and iron is the enhanced mobility of arsenic in groundwater, posing a threat of arsenic contamination in ground water (Anawar et al., 2006; Smedley and Kinniburgh, 2002). On the other hand in aerobic environments, As(III) is oxidized to As(V) by a large variety of heterotrophic and autotrophic As(III) oxidizing bacteria (Inskeep et al., 2007). Additionally, Fe(II) is oxidized by both chemical and biological activity in the presence of dissolved oxygen (Moses and Herman, 1989; Rentz et al., 2007). The iron oxyhydroxides formed from the reaction strongly adsorb the inorganic arsenic species and remove them from the liquid phase (Katsoylannis and Zouboulis, 2006). Thus oxidizing conditions provided in aerobic environments lower the risk of arsenic mobility and groundwater contamination.
The maximum solubility of dissolved oxygen (DO) in water equilibrated with air is approximately 9 mg L−1. The modest inventory of DO in groundwater may be consumed by organic matter, sulfides and other reducing compounds in the subsurface preventing the oxidation of As(III) and Fe(II) by aerobic microorganisms. However, recently it has come to light that an alternative oxidant can be utilized by anaerobic microorganisms to gain energy from As(III) (Oremland et al., 2002) and Fe(II) oxidation (Straub et al., 2001). Nitrate is an example of an ecologically significant alternative oxidant that can support these anoxic oxidation processes. Nitrate is very soluble and therefore can potentially occur in subsurface water at concentrations far exceeding the electron accepting capacity of DO. The first indications that the anoxic oxidation of As(III) occurs in the environment comes from a field study evaluating the speciation arsenic in relation to nitrate profiles in anoxic lake water columns (Senn and Hemond, 2002). At depths where nitrate was depleted, As(III) was present, but at shallower depths where nitrate was present, As(V) was the dominant species. Additionally, arsenic was attenuated by injection of nitrate into contaminated groundwater in Bangladesh (Harvey et al., 2002) but this was potentially attributable to anoxic biological oxidation of Fe(II) to oxyhydroxides, which adsorbed the arsenic.
An anoxic chemolithoautotrophic haloalkaliphilic arsenite oxidizing bacterium, Alkalilimnicola ehrlichi strain MLHE-1, was isolated from ansoda lake in California (Mono Lake), which was capable of linking the oxidation of As(V) to As(III) to partial denitrification of nitrate to nitrite as shown in equation 1 (Hoeft et al., 2007; Oremland et al., 2002).
| [eq 1] |
Subsequently, two anoxic chemolithoautotrophic strains were isolated from arsenic polluted industrial soil (Rhine et al., 2006). These included Azoarcus strain DAO1 and strain DAO10 which was most closely related to Sinorhizobium. These two strains metabolized high concentrations of As(III) (5 mM) and the metabolism was linked to the complete denitrification of nitrate to dinitrogen (N2) gas (equation 2) as was implied from the stoichiometry between As(V) formed and nitrate consumed as well as the successful amplification of the nitrous oxide gene, nosZ.
| [eq 2] |
In this study, we report on the anoxic biological oxidation of As(III) by denitrifying microorganisms in sludges and sediments with no prior exposure to arsenic. The study demonstrates that the biological capacity to link As(III) oxidation to denitrification is widespread in anaerobic environmental samples. However the activity is sensitive to high concentrations of As(III). The production of N2 gas linked to As(III) oxidation was demonstrated by direct measurements.
2. Material and Methods
2.1. Microorganisms
Sludge and sediment samples obtained from different locations were used as inocula in the batch bioassays. Aerobic activated sludge (RAS) and anaerobically digested sewage sludge (ADS) was obtained from a local municipal wastewater treatment plant (Ina Road, Tucson, Arizona). Methanogenic granular sludge (biofilms pellets) samples were obtained from industrial upward-flow anaerobic sludge blanket (UASB) treatment plants treating recycled paper wastewater (EGS) (Industriewater, Eerbeek, The Netherlands) and alcohol distillery wastewater (NGS) (Nedalco, Bergen op Zoom, The Netherlands). Chemolithotrophic denitrifying granular sludge from was obtained from a laboratory-scale thiosulfate-oxidizing denitrifying enrichment bioreactor (TDE) (Chemical and Environmental Engineering Department, University of Arizona). Duck pond sediments were obtained at the Agua Caliente Park (DPS) (Tucson, Arizona). Additional sediments were also collected from Pinal Creek (PCS) (Arizona) and from a Winogradsky column (WCS) (Chemical and Environmental Engineering Department, University of Arizona) originally inoculated with a mixture of cattle manure lagoon sludge mixed with creek sediments obtained in Patagonia, Arizona. The total suspended solids (TSS) contents of the sludge and sediment samples were 6.32 ± 0.27, 17.64 ± 1.12, 79.2 ± 0.7; 74.48 ± 4.01, 16.65 ± 0.21 and 1.63 ± 0.09 % wet weight basis; and the volatile suspended solids (VSS) content were 5.96 ± 0.28, 12.91 ± 0.97, 0.72 ± 0.01, 0.67 ± 0.07, 10.03 ± 0.1 and 1.12 ± 0.07% wet weight basis for NGS, EGS, DPS, PCS, ADS and RAS, respectively. The granular sludge samples were washed and sieved before use to remove fines. The inocula were stored under nitrogen gas at 4°C.
2.2. Basal medium
The standard basal medium was prepared using ultra pure water (Milli-Q system; Millipore) and contained the following compounds (mg/l): NH4HCO3 (3.16); NaHCO3 (672); CaCl2 (10), MgSO4.7H2O (40); K2HPO4 (300); KH2PO4.2H2O (800); and 0.2 ml/l of a trace element solution containing (in mg/l): FeC13.4 H20 (2000); CoCl2. 6 H20 (2000); MnCl2 4 H20 (500); AlCl3 6 H20 (90); CuCl2.2H20 (30); ZnCl2 (50); H3BO3 (50); (NH4)6Mo7O24.4 H2O (50); Na2SeO3.5 H2O (100); NiCl2.6 H20 (50); EDTA (1000); resazurin (200); HCl 36% (1 ml).
2.3. Batch bioassay
Batch bioassays were performed in shaken flasks, which were incubated in a dark climate-controlled room at 30±2°C. Serum flasks (160 ml) were supplied with 120 ml of a basal mineral medium (pH 7.0–7.2) containing bicarbonate as the only C source, as described above. The medium was also supplemented with arsenite (As(III)) as electron donor (concentration indicated in Tables and Figures of each experiment) and nitrate as the electron acceptor, typically 10 mM, unless otherwise specified. Flasks for anoxic assays were sealed with butyl rubber stoppers, and then the medium and headspace were purged with N2/CO2 (80/20, v/v) for 20 min to exclude oxygen from the assay. Various controls (e.g. abiotic controls, killed sludge controls, controls without electron acceptor, etc) were applied based on the requirements of each experiment. Abiotic controls were prepared without adding microbial inoculum. Killed sludge controls were prepared by autoclaving the flasks added with inoculum for 20 min at 121°C, the content was allowed to cool down overnight, and the cycle was repeat two more times and then sealed aseptically. Controls lacking As(III) were included to measure endogenous consumption of nitrate and correct the net nitrate reduction linked to As(III) oxidation. All assays were conducted in triplicate. Liquid samples were analyzed for the concentration of electron acceptor and biotransformation products (NO3− and NO2−) and arsenic species (As(III) and As(V)). Granular sludge and sediment inoculum was added to the assays at 10–12 wet weight g L−1 medium; RAS and ADS inoculum was added to the assays at 6% (v/v).
End products of denitrification were measured by monitoring gaseous nitrogen species in the headspace of bioassays flushed with He/CO2 (80/20, v/v) in lieu of N2/CO2 as described above. Gaseous nitrogen in As(III) spiked samples (3.5 mM) compared to endogenous controls. Microbial reduction of NO3− coupled to As(III) oxidation was assessed in shaken batch bioassays inoculated with NGS and DPS. The NO3− concentration utilized was 4 and 5 mM for the experiments with NGS and DPS, respectively. Some samples were supplied 50 g l−1 of either activated aluminum (AA-400G, Alcan Bauxite and Alumina) or TiO2 (The Dow Chemical company) adsorbents to lower the effective aqueous concentration of As(III) so as to minimize its toxicity. Headspace samples were analyzed periodically for N2 and N2O with a pressure lock gas tight syringe (1710RN, 100 µl (22s/2″/2), Hamilton company) to confirm denitrification. Liquid samples were analyzed for NO3− and NO2−. Flushed headspace controls incubated with just water were monitored to ensure background levels of N2 were low.
2.4. Analytical methods
All liquid samples were centrifuged (10 min., 10,000 rpm) or membrane filtered (0.45 µm) immediately after sampling and stored in polypropylene vials. Samples for arsenic analysis were then stored at −20°C. As(III) and As(V) were analyzed by ion chromatography–inductively coupled plasma–mass spectroscopy (HPLC-ICP-MS). The system consists of an HPLC (Agilent 1100) and an ICP-MS (Agilent 7500a) with a Babington nebulizer as the detector. The operating parameters were as follows: Rf power 1500 watts, plasma gas flow 15 l/min, carrier flow 1.2 l/min, arsenic was measured at 75 m/z and terbium (IS) measured at m/z 159. The injection volume was 10 µl. The detection limit for the various arsenic species was 0.1 µg/l. The total concentration of arsenic in liquid samples was determined using an ASX500 auto sampler (CETAC Technologies, Omaha, NE) and an Agilent 7500a ICP-MS. The analytical system was operated at a Rf power of 1500 W, a plasma gas flow of 151 min−1 and a carrier gas flow of 1.21 min−1. The acquisition parameters used were: arsenic measured at m/z 75; terbium (IS) measured at m/z 159; 3 points per peak; 1.5 s dwell time for As, 1.5 s dwell time for Tb; number of repetitions =7.
The total arsenic content and arsenic speciation in solid matrices (i.e., activated alumina, biomass) were measured following extraction of samples with 25 ml of NaOH (2.0 M) in anaerobic tubes (under nitrogen) immersed in a shaking water bath at the temperature of 90±2°C for 12 h. All liquid samples were adjusted to pH 6–9 with HNO3 (2.5 M) and membrane filtered (0.45 µm) immediately after sampling and stored in polypropylene vials at −20°C.
All liquid samples were centrifuged or membrane filtered (0.45 µm) immediately after sampling and stored in polypropylene vials at 4°C. Nitrate, nitrite and were analyzed by suppressed conductivity ion chromatography using a Dionex 500 system (Sunnyvale, CA, USA) fitted with a Dionex IonPac AS11 analytical column (4 × 250 mm) a AG16 guard column (4 mm × 40 mm). During each injection the eluent (KOH) used was 20 mM for 20 min. All liquid samples were centrifuged or membrane filtered (0.45 µm) immediately after sampling and stored in polypropylene vials. Samples for arsenic analysis were then stored at −20°C until the analysis was performed in order to reduce changes in arsenic speciation. Other filtered samples were stored at 4°C.
N2 and N2O were analyzed using a Hewlett Packard 5890 Series II gas chromatograph fitted with a Carboxen™ 1010 Plot column (30 m × 0.32 mm) and a thermal conductivity detector. The temperature of the column, the injector port and the detector were 220, 110 and 100, respectively. Helium was used as the carrier gas and the injection volume was 100 µl.
Other analytical determinations (e.g., pH, TSS, VSS, etc.) were conducted according to Standard Methods (APHA, 1999).
3. Results
3.1. Screening
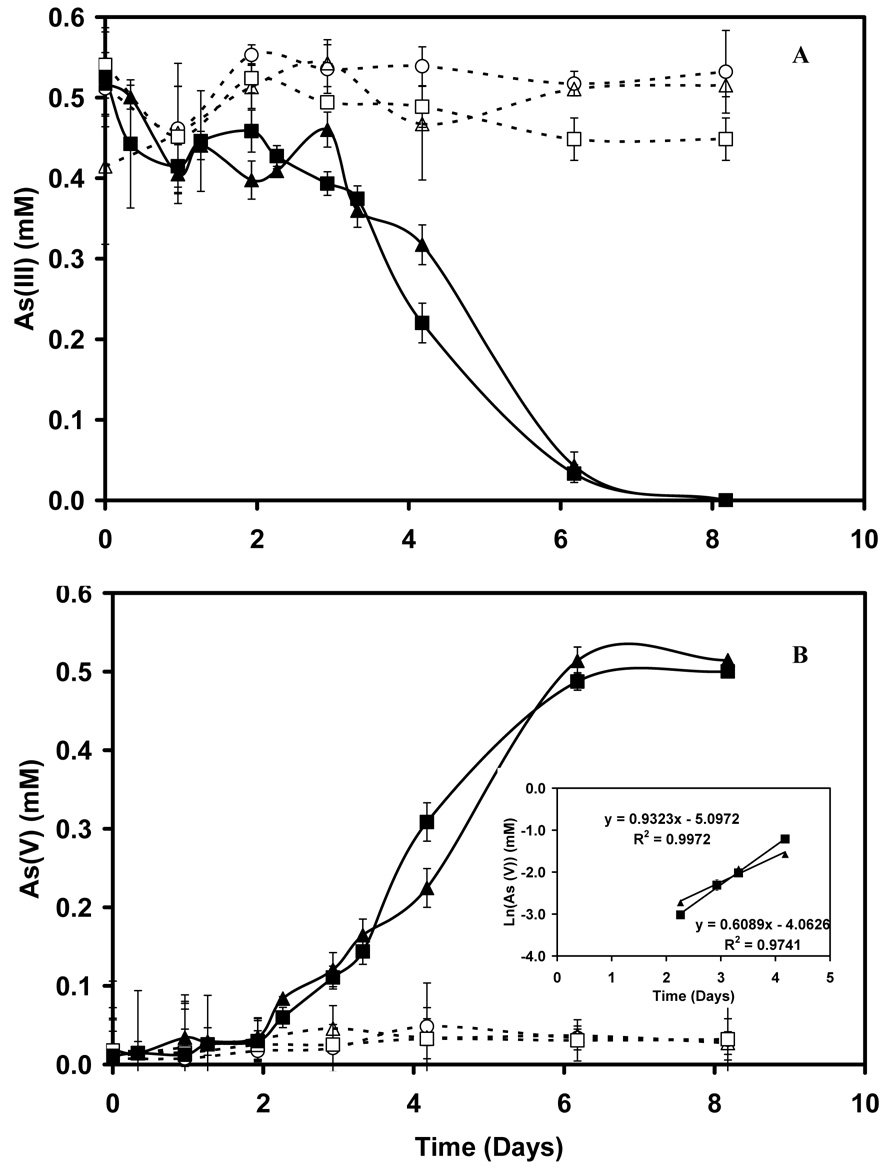
Sediments and sludge samples from environments not known to be contaminated with arsenic were incubated with As(III) (0.5 mM) either in the presence and absence of NO3− (10 mM) and were incubated in the absence of elemental oxygen. An example time course from these incubations is provided in Figure 1 for DPS and NGS. In treatments incubated with NO3−, As(V) formation started after approximately 2 days and was concomitant with the disappearance of As(III). After approximately one week the reaction was complete. The reactions were dependent on the presence of NO3− as evidenced by the lack of any significant conversion in incubations lacking NO3−. Likewise, the reactions were also dependent on being inoculated, since no reaction occurred in bottles receiving the medium with As(III) and NO3− but lacking sediment or sludge.
Figure 1.
The removal of As(III) (Panel A) and formation of As(V) (Panel B) by NGS and DPS under denitrifying condition, NGS and DPS with NO3− (■) and (▲),NGS without NO3− (□) and (△), abiotic (○). Insert shows natural logarithm plot of data during exponential growth phase.
The results for all the sludge and sediments samples screened are shown in Table 1. Six out of the eight inocula tested displayed microbial activity towards the anoxic oxidation of As(III). Five out six of the positive samples, showed a dependency on the presence of NO3− for the anoxic oxidation of As(III). One of the positive samples, PCS, had this activity both in the presence and absence of added NO3− which may have been due to high levels of oxidized manganese species known to occur in that sediment (Lind and Hem, 1993). The average molar yield of nitrate linked As(III) oxidation was 0.943±0.040 mol As(V) formed per mol AS(III) consumed and the value for the PCS sediment was similar.
Table 1.
Summary of microbial As(III) oxidation under denitrifying conditions
| Inoculum | As(V) formation‡ | Time† | ||
|---|---|---|---|---|
| Number | Sources | With NO3− | Without NO3− | (Days) |
| 1 | NGS | 0.423±0.004 | — | 6 |
| 2 | EGS | — | — | — |
| 3 | ADS | 0.425±0.003 | — | 13 |
| 4 | RAS | — | — | — |
| 5 | TDE | 0.416±0.002 | — | 10 |
| 6 | DPS | 0.415±0.003 | — | 6 |
| 7 | WCS | 0.413±0.001 | — | 5 |
| 8 | PCS | 0.295±0.020 | 0.298±0.014 | >14+ |
Time to oxidize 80% of 0.5 mM As(III) to As(V)
Conversion of As(III) to As(V): “Average ± STDE” for “conversion ≥ 80%”; “—“ for “conversion ≤ 5%”
As(V) formation =conc. of As(V) (at the end of the experiment, d10 or d14) minus conc. of As(V) (at day 0)
In PCS, at day 14 only 60% of As(III) was converted to As(V)
3.2. Kinetics
The experiments conducted with NGS, DPS and ADS were monitored with sufficient sampling times to obtain kinetic data. The growth rates and maximum specific activity normalized to volatile suspended solids (VSS) in the sludge or sediment sample are summarized in Table 2. The growth rate, estimated from the slope of the natural logarithm of As(V) formation versus time, indicated doubling times of around one day. The maximum specific activities ranged from 0.16 to 1.2 mmol As(V) formed g−1 VSS d−1
Table 2.
Summary of kinetics† of microbial As(III) oxidation (0.5 mM) under denitrifying conditions
| Inoculum sources | Doubling time (days)* | Highest specific activity |
|---|---|---|
| (mmol As (V)/g VSSadded • d) | ||
| NGS | 0.744±0.027 | 1.243±0.045 |
| ADS | 1.041±0.028 | 0.160±0.004 |
| DPS | 1.145±0.106 | 0.609±0.060 |
Estimated from As(V) formation data.
The coefficient of determination (R2) was 0.9972, 0.9804 and 0.9741 for the Ln(ΔAs(V)) versus time plots of NGS, ADS and DPS
3.3. Toxicity
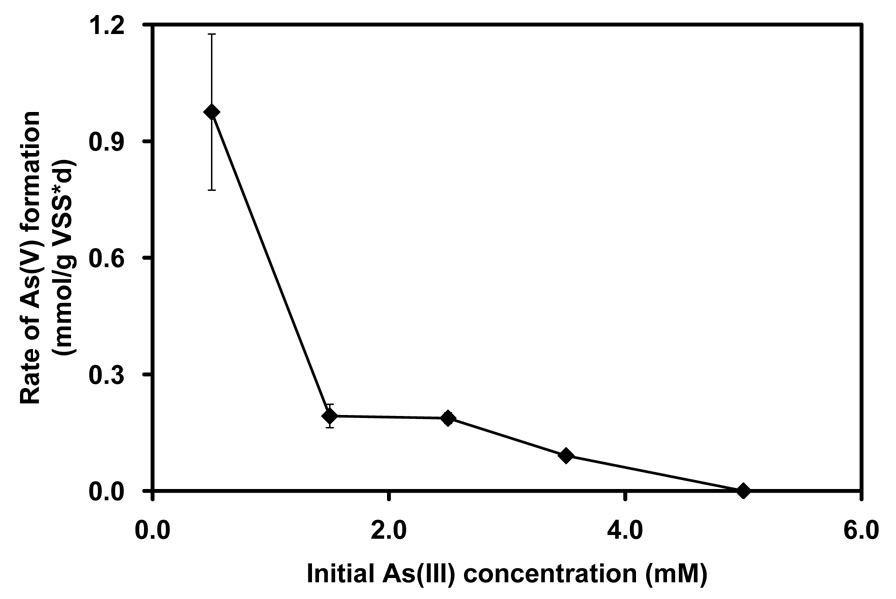
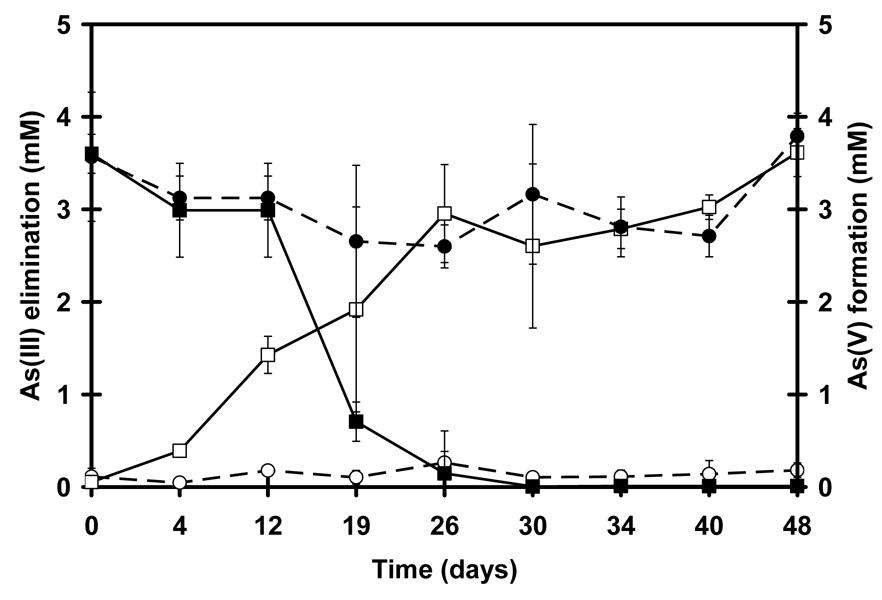
The anoxic oxidation of As(III) was inhibited by increasing the As(III) concentrations as illustrated in Figure 2. Compared to the kinetic parameters observed at 0.5 mM As(III), the maximum specific activities were inhibited by 50% at 1.24 mM As(III) and by 90% at 3.5 mM As(III) in assays utilizing NGS as inoculum. For the most part, no activity was observed at 5 mM As(III) or higher. Albeit, that inhibition was severe, As(III) could be completely biologically transformed at 3.5 mM by anoxic oxidation over long incubation times (Figure 3).
Figure 2.
Substrate toxicity as shown by a decrease in the rate of As(V) formation (◆) from the oxidation of As(III) at different initial As(III) concentrations.
Figure 3.
Elimination of As(III) and formation of As(V) by NGS under denitrifying condition, As(III) removal (■), As(V) formation (□), abiotic control for As(III) (●) and As(V) (○).
3.4. Consumption of NO3− and formation of N2 linked to As(III) oxidation
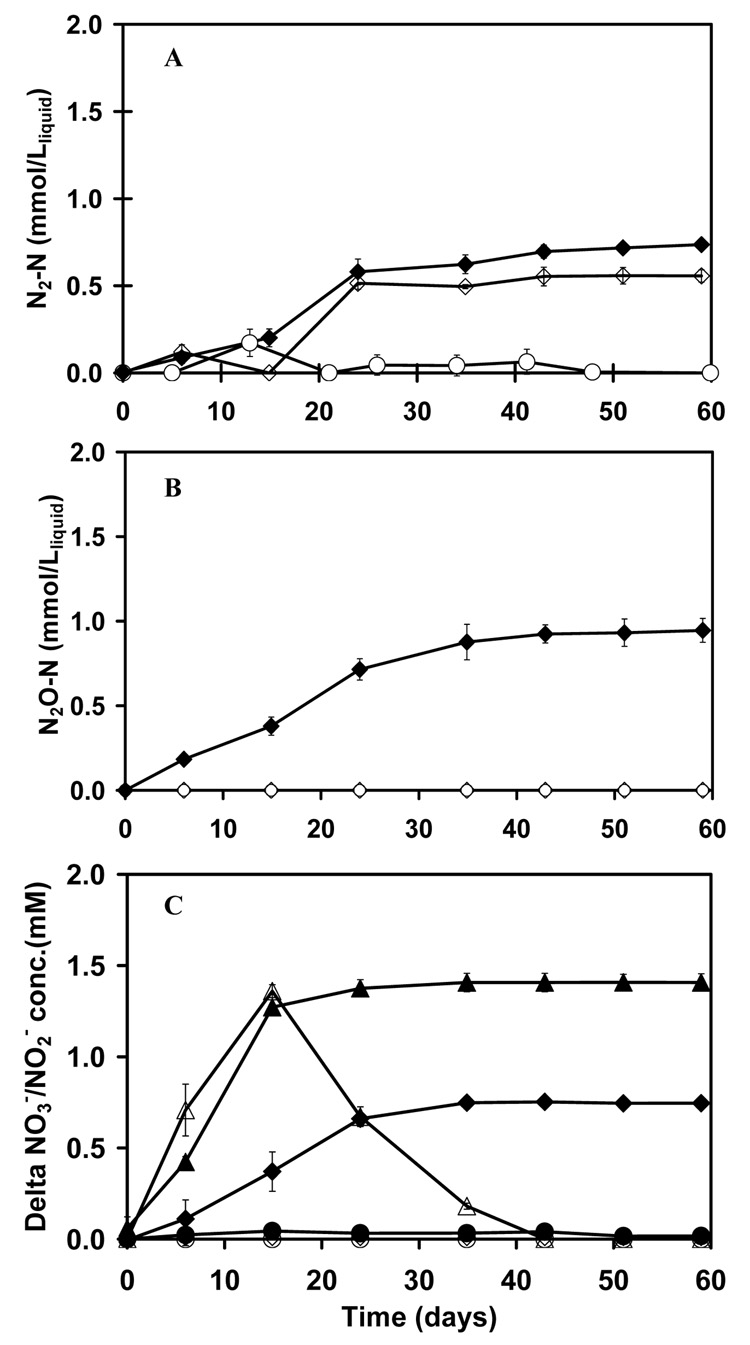
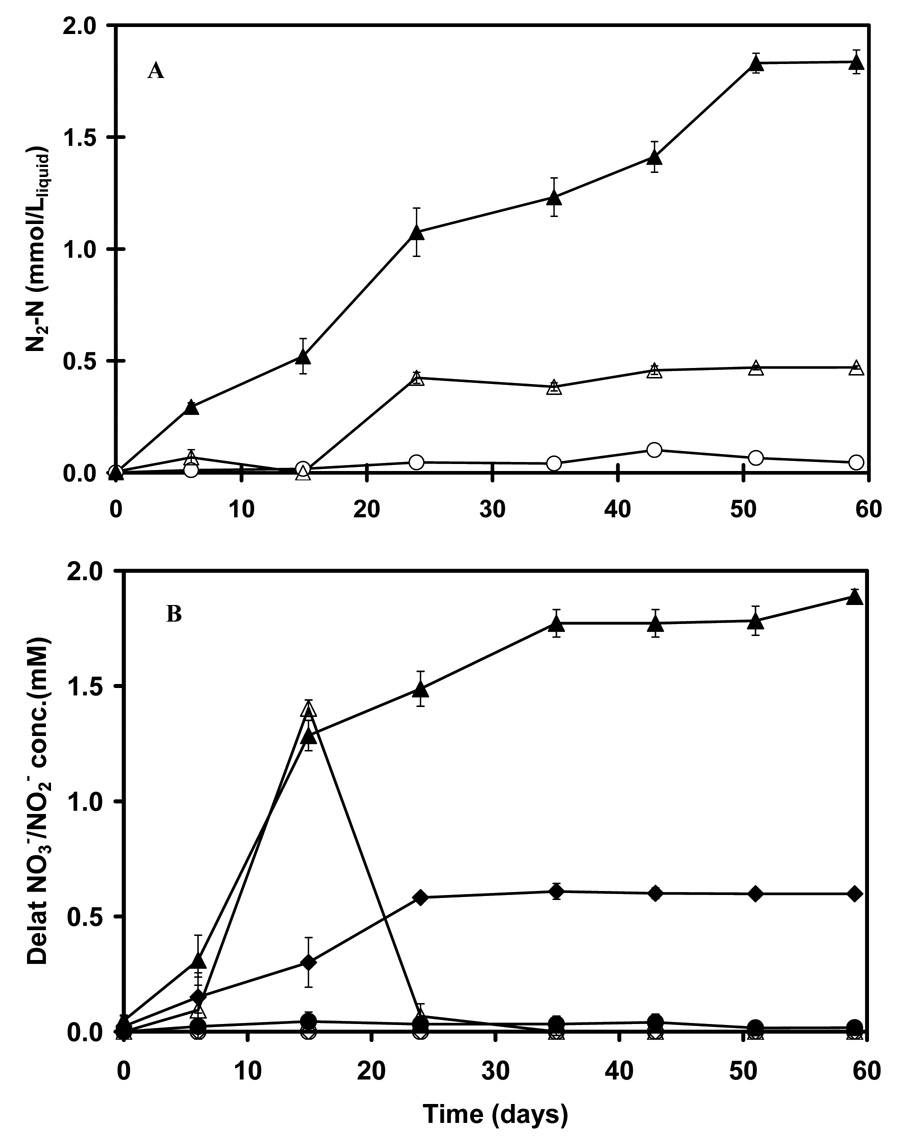
In environmental samples of sludges and sediments, the measurement of NO3− - consumption and N2 - formation linked to As(III) oxidation is a difficult task. The inhibition of As(III) limits the concentration that can be used, such that the electron equivalents provided by As(III) are relatively small compared to the endogenous substrates in the environmental sample. Therefore a distinction between denitrification attributable to As(III) oxidation and that which is attributable to endogenous substrate utilization is infeasible. A distinction can potentially be made if a higher supply of As(III) is introduced without causing inhibition. In this study, this was achieved by using weak adsorbents of As(III), activated alumina (AA) and TiO2 to slowly release As(III) over the course of the bioassay. By including 50.0 g dwt L−1 liquid of AA or TiO2, together with As(III), supplied at a rate of 3.5 mmol L−1 liquid; the equilibrium concentration of As(III) was 0.65 and 0.63 mM, respectively. Based on the NO3−, NO2−, N2O and N2 data (Figure 4 and Figure 5), the adsorbents dramatically decreased the toxicity of As(III) to denitrification. When 3.5 mM As(III) was introduced directly, the inhibited denitrification resulted in a major accumulation of N2O (0.95±0.07 or 1.62±0.12 mmol N2O-N L−1 liquid in different experiments) instead of complete denitrification to N2, as well as a longer period of NO2− accumulation. The results suggest that As(III) is most inhibitory towards the last step of the denitrification process. When sorbents were utilized, the NO3− consumption was more rapid, there was a shorter period of NO2− accumulation and the denitrification was complete to N2. The molar ratios of As(III) consumption and As(V) formation to the net NO3− consumption and N2-N formation are provided in Table 3. The molar ratios of As(III) removal to NO3− consumption and N2-N formation from the adsorbent amended DPS and NGS cultures were close to the theoretical ratio of 2.5 expected for As(III) oxidation linked to complete denitrification to N2.
Figure 4.
Denitrification at a high initial As(III) aqueous concentratio (3.5 mM). The formation of N2 (panel A) and N2O (panel B) by NGS. Legend for panels A and B: full treatment with As(III) and NO3− (◆); endogenous control with only NO3− added (◇); and the abiotic control with As(III) and NO3− (○). Panel C shows the consumption of NO3− and the temporal accumulation of NO2−. Legend for panel C: NO3− consumption in the full treatment with As(III) and NO3− (▲); NO3− consumption in the endogenous substrate control with only NO3− added (◆); NO3− consumption in the abiotic control with As(III) and NO3− (●); NO2− accumulation in the full treatment with As(III) and NO3− (△); NO2− accumulation in the endogenous substrate control with only NO3− added (◇); NO2− accumulation in the abiotic control with As(III) and NO3− (○).
Figure 5.
Denitrification with As(III) (3.5 mM) adsorbed to activated aluminum. The formation of N2-N (panel A) by NGS. Legend for panel A: full treatment with As(III) and NO3− (▲); endogenous control with only NO3− added (△); and the abiotic control As(III) and NO3− (○); Panel B shows the consumption of NO3− and the temporal accumulation of NO2−. Legend for panel B: NO3− consumption in the full treatment with As(III) and NO3− (▲); NO3− consumption in the endogenous control with only NO3− added (◆); NO3− consumption in the abiotic control with As(III) and NO3− (●); NO2− accumulation in the full treatment with As(III) and NO3− (△); NO2− accumulation in the endogenous control with only NO3− added (◇); NO2− accumulation in the abiotic control (○) with As(III) and NO3−. No N2O was observed in any treatment.
Table 3.
Summary of sorbed As(III) oxidation linked to denitrification
| Parameters | NGS | DPS | ||||
|---|---|---|---|---|---|---|
| AA | AA | TiO2 | ||||
| As(III)+NO3− | NO3− only | As(III)+NO3− | NO3− only | As(III)+NO3− | NO3− only | |
| As(III) fed (mM) | 3.52±0.08 | — | 3.55±0.06 | — | 3.55±0.06 | — |
| NO3− consumed (mM) | 1.84±0.01 | 0.58±0.03 | 3.37±0.01 | 1.94±0.10 | 2.72±0.14 | 1.13±0.07 |
| Corrected† NO3− consumed (mM) | 1.27±0.04 | — | 1.43±0.10 | — | 1.59±0.14 | — |
| N2-N formed (mmol/Lliquid) | 1.84±0.05 | 0.47±0.01 | 3.21±0.03 | 1.60±0.17 | 2.53±0.13 | 0.98±0.09 |
| Corrected N2-N formed (mmol/Lliquid) | 1.37±0.06 | — | 1.60±0.15 | — | 1.55±0.19 | — |
| Corrected N2-N formed/corrected NO3− consumed | 1.09±0.07 | — | 1.12±0.13 | — | 0.97±0.04 | — |
| Mol As(III) fed/corrected mol NO3− consumed | 2.78±0.12 | — | 2.48±0.14 | — | 2.24±0.21 | — |
| Mol As(III) fed/corrected mol N2-N formed | 2.57±0.07 | — | 2.23±0.23 | — | 2.32±0.31 | — |
Corrected for the endogenous nitrate consumption or N2-N formation determined in the nitrate treatment lacking added As(III).
4. Discussion
4.1. Evidence of bioconversion
In this study, the capacity of microorganisms form diverse anaerobic samples of sludges and sediments to utilize nitrate as an electron acceptor for the oxidation of As(III) was demonstrated. The biological nature of the reaction is inferred from the lack of any conversion in uninoculated samples, heat killed samples and the inhibition of the reaction at high As(III) concentrations. Likewise, kinetic measurements indicate an exponential increment in the conversion rate with time, which is consistent with microbial growth.
The biological conversion of As(III) to As(V) in the absence of O2, was dependent on the presence of NO3−. No As(III) oxidation occurred in the inoculated incubations without NO3− addition, except for one sediment sample known to contain manganese oxides. The presence of As(III), supplemented in a non-inhibitory fashion, greatly enhanced N2 production beyond the background endogenous production, suggesting its role as an electron donor to the microbial reaction. The stoichiometry of the As(III) conversion to As(V) in relation to NO3− conversion to N2 clearly suggests As(III) oxidation was linked to the complete denitrification to N2 as indicated in equation 2. The experimentally measured ratios approximated 2.5 mol arsenic metabolized per mol NO3− converted as is expected from the equation. While the microbial activity in this study was demonstrated in pristine samples, there is at least one reported precedent from arsenic contaminated soils for the enrichment and isolation of two denitrifying bacterial strains capable of oxidizing As(III) at the expense of NO3− reduction (Rhine et al., 2005). Likewise, a partial denitrifying bacterium capable of oxidizing As(III) was isolated form a soda lake (Oremland et al., 2002).
4.2. The occurrence of anoxic As(III) oxidizing bacteria
Microorganisms capable of gaining energy from the oxidation of inorganic chemicals are widespread in the environment, including anoxic environments. There are ample examples of chemolithotrophic denitrification utilizing H2 (Lee and Rittmann, 2002), Fe(II) (Straub et al., 2001), S0 (Sierra-Alvarez et al., 2007), H2S (Cardoso et al., 2006) and U(IV) (Beller, 2005) as electron donors among others. Nitrate is also a common alternative electron acceptor found in groundwater and lakes that could potentially support these reactions (Nolan et al., 1997; Senn and Hemond, 2002). Energy for microbial growth can also be gained from the anoxic oxidation of As(III), The standard reduction potential (Eo) for the redox couple As(V)/As(III) is 0.139 V (Madigan and Martinko, 2006); while that for NO3−/N2 is 0.747 V, which equates to a standard change of Gibb’s free energy (ΔGo’) for As(III) oxidation linked to complete denitrification (eq. 2) of −117.3 kJ/mol As(III).
With respect to arsenic, the environmental samples used in this study can be regarded as pristine samples. The spring that feeds the pond from where DPS was collected has a measured arsenic concentration of 67 nM (RECON, 2002). The effluent from the wastewater treatment plant where ADS was collected has a measured arsenic concentration below the detection limit of 52 nM (PAG, 2002). NGS was collected from wastewater originating from a distillery utilizing sugar beet molasses as a feedstock. Sugar beet molasses is reported to have an arsenic concentration of 2.4 µmol kg−1 (Skrbic and Durisic-Mladenovic, 2005), the actual concentration in the wastewater would be lower due to dilution of the feedstock into the process water. TDE was an enrichment culture fed with medium prepared with milli-Q water to which no arsenic was added. Nonetheless, microbial activity towards anoxic oxidation of As(III) developed rapidly in these inoculum samples. The microbial community had a moderate growth rate with doubling times ranging from 0.74 to 1.34 d. Once the conditions of As(III) and NO3− were provided, responsible organisms grew and established a large enough population to account for significant As(III) activity in the microcosms, ranging from 0.16 to 1.24 mmol arsenic g−1 VSS d−1. The presence of the anoxic As(III) oxidizers in the anaerobic samples lacking arsenic is probably due to the metabolic versatility of organisms responsible for the reaction. As an example, one of the previously reported anoxic As(III)-oxidizing isolates, Azoarcus sp. strain DAO1 (Rhine et al., 2006), is from a genus well known for denitrification utilizing diverse substrates. The substrate spectrum includes simple organic acids, amino acids, sugars, aromatic acids, phenolics and aromatic hydrocarbons (Rabus, 2005; Reinhold-Hurek and Hurek, 2006; Rhine et al., 2006). Aside from these heterotrophic carbon sources, the CO2 fixing gene for ribulose-1,5′-bisphosphate carboxylase/oxygenase was identified in Azoarcus sp. strain DAO1, indicating the capability for autotrophic metabolism (Rhine et al., 2006).
4.3. As(III) substrate inhibition
The previous attempts to isolate anoxic As(III)-oxidizing bacteria have been from sites containing high concentrations of arsenic. For example, the soda lake from where Alkalilimnicola ehrlichi strain MLHE-1 was isolated contains 0.2 mM arsenic (Oremland et al., 2002). Thus investigators utilized high concentrations of arsenic for the enrichment and isolation. The initial enrichments leading to the isolation of Azoarcus sp. strain DAO1 and Alkalilimnicola ehrlichi strain MLHE-1 contained 5 mM As(III) (Oremland et al., 2002; Rhine et al., 2006). Such high concentrations of As(III) are highly toxic to microorganisms (Stasinakis et al., 2003). The 50% inhibitory concentrations of As(III) to methanogenic activity are reported to be as low as 15 µM (Sierra-Alvarez et al., 2004). A concentration of 5 mM As(III) was lethal to the As(III) oxidizing microbial communities described in this study and concentrations from 1 to 3.5 mM caused partial inhibition compared to kinetic rates observed at 0.5 mM. Therefore, the previous studies selected for anoxic As(III)-oxidizing microorganisms with remarkable resistance to As(III). A common resistance mechanism is afforded by the ars operon which pumps arsenic out of cells (Silver and Phung, 2005). By inhibiting microorganisms with low As(III) resistance, the previous studies potentially overlooked the biodiversity of anoxic As(III) oxidation. The environmentally relevant concentrations in the subsurface are typically well below the toxicity range to microorganisms (Cullen and Reimer, 1989; Smedley and Kinniburgh, 2002) and therefore anoxic As(III) oxidizers that would thrive at low As(III) concentrations are highly relevant to the speciation and mobility of arsenic in the environment.
4.4. Sorbed As(III) as a substrate for denitrification
Sediments contain oxyhydroxy oxides such as those of aluminum, which adsorbs As(III) (Lin and Wu, 2001) and attenuate the effective soluble arsenic concentration. In the presence of AA and TiO2, As(III) was adsorbed and the equilibrium soluble concentrations was approximately 6-fold lower than treatments without adsorbents, lowering the inhibitory impact of A(III). The adsorbed As(III) was effectively utilized as an electron donor for denitrification to N2 gas. In parallel incubations without adsorbents, N2O gas accumulated instead due to the severe toxicity of non-attenuated aqueous As(III) concentrations. In cultures with AA and TiO2, the quantity of N2 gas produced corresponded to the complete oxidation of As(III), including the adsorbed fraction. The complete oxidation was also evidenced by the recovery of As(V) when the AA was extracted at the end of the incubation. These results clearly indicate that adsorbed As(III) was bioavailable for oxidation by denitrifiers, most likely due to the reestablishment of the equilibrium upon consumption of aqueous As(III). This is consistent with previous observations that As(V) coprecipitated with aluminum hydroxide was bioavailable for bacterial dissimilatory As(V) reduction to As(III) (Zobrist et al., 2000). Likewise biological reduction has been found to be the main reason for the remobilization of As(V) adsorbed to AA and TiO2 when incubated with anaerobic mixed cultures (Jing et al., 2008; Sierra-Alvarez et al., 2005).
4.5. Environmental significance
The findings presented here demonstrate a widespread capacity in the environment for the anoxic microbial oxidation of As(III). The oxidation of As(III) linked to the use of ubiquitous nitrate as an electron acceptor may be an important missing link the biogeochemical cycling of arsenic between two common inorganic species, As(III) and As(V), where DO is absent. The concentrations of arsenic in the subsurface pore water are attenuated by adsorption to metal oxyhydroxides. The aqueous concentrations encountered are generally not inhibitory to microorganisms. Therefore the biodiversity of anoxic As(III) oxidizers may be greater than the few arsenic resistant strains reported previously. In this study, it was shown that tolerance to As(III) was not a prerequisite for anoxic As(III) oxidation by anaerobic microbial communities.
5. Conclusions
Microorganisms capable of linking anoxic As(III) oxidation to denitrification are widespread in anaerobic sediments and sludges. The use of ubiquitous nitrate as an electron acceptor may be an important missing link the biogeochemical cycling of arsenic between two common inorganic species, As(III) and As(V), where DO is absent
The doubling times for growth of the anoxic As(III) oxidizers range from 0.74 to 1.34 d.
The anoxic oxidation of As(III) linked to denitrification is inhibited by As(III) with 5 mM causing complete inhibition.
Dinitrogen gas is the end product of denitrification linked to As(III) oxidation if As(III) is not present at inhibiting concentrations, nitrous oxide accumulates a major product at inhibitory As(III) concentrations
As(III) weakly adsorbed to AA or TiO2 is available as an electron donor for denitrification.
The biodiversity of anoxic As(III) oxidizers is potentially greater than the few arsenic resistant strains reported previously. In this study, it was shown that tolerance to As(III) was not a prerequisite for anoxic As(III) oxidation by anaerobic microbial communities.
Acknowledgments
The work presented here was funded by a USGS, National Institute for Water Resources 104G grant (2005AZ114G), and by a grant of the NIEHS-supported Superfund Basic Research Program (NIH 5 P42 ES004940). The authors are grateful to Ron Oremland for reviewing the manuscript. The use of trade, product, or firm names in this report is for descriptive purposes only and does not constitute endorsement by the U.S. Geological Survey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anawar HM, Akai J, Yoshioka T, Konohira E, Lee JY, Fukuhara H, Alam MTK, Garcia-Sanchez A. Mobilization of arsenic in groundwater of Bangladesh: evidence from an incubation study. Environ. Geochem. Hlth. 2006;28(6):553–565. doi: 10.1007/s10653-006-9054-0. [DOI] [PubMed] [Google Scholar]
- APHA. Standard methods for the examination of water and wastewater. 20th edn. Washington D. C.: American Public Health Association; 1999. [Google Scholar]
- ATSDR. Toxicological profile for arsenic, p. 500, Agency for Toxic Substances and Disease Registry. Atlanta: U.S. Department of Health And Human Services; 2007. [PubMed] [Google Scholar]
- Bednar AJ, Garbarino JR, Ranville JF, Wildeman TR. Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern United States. J. Agr. Food Chem. 2002;50(25):7340–7344. doi: 10.1021/jf025672i. [DOI] [PubMed] [Google Scholar]
- Beller HR. Anaerobic, nitrate-dependent oxidation of U(IV) oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl. Environ. Microbiol. 2005;71(4):2170–2174. doi: 10.1128/AEM.71.4.2170-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RB, Sierra-Alvarez R, Rowlette P, Flores ER, Gomez J, Field JA. Sulfide oxidation under chemolithoautotrophic denitrifying conditions. Biotechnol. Bioeng. 2006;95(6):1148–1157. doi: 10.1002/bit.21084. [DOI] [PubMed] [Google Scholar]
- Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem. Rev. 1989;89:713–764. [Google Scholar]
- Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003;37(18):4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF. Arsenic mobility and groundwater extraction in Bangladesh. Science. 2002;298(5598):1602–1606. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- Hering JG, Dixit S. In: Contrasting sorption behavior of aresnic(III) and aresnic(V) in suspensions of iron and aluminum oxyhydroxides. O'Day PA, Vlassopoulos D, Meng X, Benning L, editors. Washington, DC: American Chemical Society; 2005. pp. 8–24. Advances in Arsenic Research. ACS Symposium Series No. 915. [Google Scholar]
- Hoeft SE, Blum JS, Stolz JF, Tabita FR, Witte B, King GM, Santini JM, Oremland RS. Alkalilimnicola ehrlichii sp nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int. J. Syst. Evol. Micr. 2007;57:504–512. doi: 10.1099/ijs.0.64576-0. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ. Microbiol. 2007;9(4):934–943. doi: 10.1111/j.1462-2920.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- Jing CY, Liu SQ, Meng XQ. Arsenic remobilization in water treatment adsorbents under reducing conditions: Part I. Incubation study. Sci Tot. Environ. 2008;389:188–194. doi: 10.1016/j.scitotenv.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Katsoyiannis IA, Zouboulis AI. Use of iron- and manganese-oxidizing bacteria for the combined removal of iron, manganese and arsenic from contaminated groundwater. Wat. Qual. Res. J. Can. 2006;41(2):117–129. [Google Scholar]
- Katz SA, Salem H. Chemistry and toxicology of building timbers pressure-treated with chromated copper arsenate: a review. J. Appl. Toxicol. 2005;25(1):1–7. doi: 10.1002/jat.1005. [DOI] [PubMed] [Google Scholar]
- Lee KC, Rittmann BE. Applying a novel autohydrogenotrophic hollow-fiber membrane biofilm reactor for denitrification of drinking water. Water Res. 2002;36(8):2040–2052. doi: 10.1016/s0043-1354(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Lin TF, Wu JK. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001;35(8):2049–2057. doi: 10.1016/s0043-1354(00)00467-x. [DOI] [PubMed] [Google Scholar]
- Lind CJ, Hem JD. Manganese Minerals and Associated Fine Particulates in the Streambed of Pinal Creek, Arizona, USA - A Mining-Related Acid Drainage Problem. Appl. Geochem. 1993;8(1):67–80. [Google Scholar]
- Madigan MT, Martinko JM. Brock Biology of Microorganisms. 11th Edition. Upper Saddle River, NJ: Pearson Prentice Hall; 2006. [Google Scholar]
- Moses CO, Herman JS. Homogeneous Oxidation-Kinetics of Aqueous Ferrous Iron at Circumneutral pH. J. Solution. Chem. 1989;18(8):705–725. [Google Scholar]
- Nolan BT, Ruddy BC, Hitt KJ, Helsel DR. Risk of nitrate in groundwaters of the United States - A national perspective. Environ. Sci. Technol. 1997;31(8):2229–2236. [Google Scholar]
- Oremland RS, Hoeft SE, Santini JA, Bano N, Hollibaugh RA, Hollibaugh JT. Anaerobic oxidation of arsenite in Mono Lake water and by facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 2002;68(10):4795–4802. doi: 10.1128/AEM.68.10.4795-4802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland RS, Stolz JF. The ecology of arsenic. Science. 2003;300(5621):939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- PAG. Water Quality in Pima County. Tucson: Pima Association of Governments; 2002. [Google Scholar]
- Rabus R. Functional genomics of an anaerobic aromatic-degrading denitrifying bacterium, strain EbN1. Appl. Microbiol. Biotechnol. 2005;68(5):580–587. doi: 10.1007/s00253-005-0030-x. [DOI] [PubMed] [Google Scholar]
- RECON. Agua Caliente Spring Aquatic Ecosystem Restoration Study. San Diego, California: Pima County, Arizona, Recon Consultants and U.S. Army Corps Of Engineers; 2002. [Google Scholar]
- Reinhold-Hurek B, Hurek T. The Genera Azoarcus, Azovibrio, Azospira and Azonexus. Prokaryotes. 2006;5:873–891. [Google Scholar]
- Rentz JA, Kraiya C, Luther GW, Emerson D. Control of ferrous iron oxidation within circumneutral microbial iron mats by cellular activity and autocatalysis. Environ. Sci. Technol. 2007;41(17):6084–6089. doi: 10.1021/es062203e. [DOI] [PubMed] [Google Scholar]
- Rhine ED, Garcia-Dominguez E, Phelps CD, Young LY. Environmental microbes can speciate and cycle arsenic. Environ. Sci. Technol. 2005;39(24):9569–9573. doi: 10.1021/es051047t. [DOI] [PubMed] [Google Scholar]
- Rhine ED, Phelps CD, Young LY. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ. Microbiol. 2006;8(5):899–908. doi: 10.1111/j.1462-2920.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- Roussel C, Bril H, Fernandez A. Arsenic speciation: Involvement in evaluation of environmental impact caused by mine wastes. J. Environ. Qual. 2000;29(1):182–188. [Google Scholar]
- Senn DB, Hemond HF. Nitrate controls on iron and arsenic in an urban lake. Science. 2002;296(5577):2373–2376. doi: 10.1126/science.1072402. [DOI] [PubMed] [Google Scholar]
- Sierra-Alvarez R, Beristain-Cardoso R, Salazar M, Gomez J, Razo-Flores E, Field JA. Chemolithotrophic denitrification with elemental sulfur for groundwater treatment. Water Res. 2007;41(6):1253–1262. doi: 10.1016/j.watres.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Sierra-Alvarez R, Cortinas I, Yenal U, Field JA. Methanogenic inhibition by arsenic compounds. Appl. Environ. Microbiol. 2004;70(9):5688–5691. doi: 10.1128/AEM.70.9.5688-5691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Alvarez R, Field JA, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. Anaerobic microbial mobilization and biotransformation of arsenate adsorbed onto activated alumina. Water Res. 2005;39(1):199–209. doi: 10.1016/j.watres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Silver S, Phung LT. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 2005;71(2):599–608. doi: 10.1128/AEM.71.2.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrbic B, Durisic-Mladenovic N. Toxic and essential trace elements in Serbian sugarbeet, molasses and white sugar. Zuckerindustrie. 2005;130(12):913–917. [Google Scholar]
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002;17(5):517–568. [Google Scholar]
- Stasinakis AS, Thomaidis NS, Giannes AS, Lekkas TD. Effect of arsenic and mercury speciation on inhibition of respiration rate in activated sludge systems. Environ. Sci. Poll. R. 2003;10(3):177–182. doi: 10.1065/espr2002.05.121. [DOI] [PubMed] [Google Scholar]
- Straub KL, Benz M, Schink B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001;34(3):181–186. doi: 10.1111/j.1574-6941.2001.tb00768.x. [DOI] [PubMed] [Google Scholar]
- USEPA. National Primary Drinking Water Regulations: Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring. Federal Register. 2001;66:6976–7066. [Google Scholar]
- WHO. Geneva: World Health Organization; Guidelines for drinking-water quality Volume 1: Recommendations. (2nd ed) 1993
- Zobrist J, Dowdle PR, Davis JA, Oremland RS. Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environ. Sci. Technol. 2000;34(22):4747–4753. [Google Scholar]