Abstract
Farber disease is a rare lysosomal storage disorder (LSD) caused by a deficiency of acid ceramidase (AC) activity and subsequent accumulation of ceramide. Currently, there is no treatment for Farber disease beyond palliative care and most patients succumb to the disorder at a very young age. Previously, our group showed that gene therapy using oncoretroviral vectors (RV) could restore enzyme activity in Farber patient cells. The studies described here employ novel RV and lentiviral (LV) vectors that engineer co-expression of AC and a cell surface marking transgene product, human CD25 (huCD25). Transduction of Farber patient fibroblasts and B cells with these vectors resulted in overexpression of AC and led to a 90% and 50% reduction in the accumulation of ceramide, respectively. Vectors were also evaluated in human hematopoietic stem/progenitor cells (HSPCs) and by direct in vivo delivery in mouse models. In a xenotransplantation model using NOD/SCID mice, we found that transduced CD34+ cells could repopulate irradiated recipient animals, as measured by CD25 expression. When virus was injected intravenously into mice, soluble CD25 was detected in the plasma and increased AC activity was present in the liver up to 14 weeks post-injection. These findings suggest that vector and transgene expression can persist long-term and offer the potential of a lasting cure. To our knowledge, this is the first report of in vivo testing of direct gene therapy strategies for Farber disease.
Keywords: Farber lipogranulomatosis, lysosomal storage disease, gene therapy, hematopoietic stem cell transplantation
INTRODUCTION
Lysosomal storage disorders (LSDs) are a group of over 40 distinct metabolic conditions resulting from deficient activity of enzymes involved in macromolecule digestion. While individually their prevalence is low, as a group they can occur at high frequencies (up to 1 in 7,700) in some populations [1, 2, 3]. Farber disease is a rare, autosomally inherited LSD caused by mutation of the gene (ASAH1) encoding N-acylsphingosine deacylase (acid ceramidase, AC; EC 3.5.1.23), a protein that catabolizes the hydrolysis of ceramide into sphingosine and free fatty acids [4, 5]. While the phenotype of the disease varies, most patients present with a characteristic triad of symptoms: subcutaneous granulomas, a hoarse cry, and painful swollen joints [4]. In the classic and most severe type of Farber disease, patients also show progressive neurological deterioration and patients typically die by the age of two [4].
Treatment consists primarily of palliative care such as corticosteroids for the pain, tracheostomy to relieve respiratory difficulties, and surgery to remove the granulomas [4, 6]. Allogeneic bone marrow transplantation (BMT) has been attempted for some Farber patients based on the reasoning that a population of cells with normal enzyme activity could ameliorate the effects of the deficient enzyme [7, 8, 9, 10]. In transplants of two mildly-affected Farber patients (Type 2/3), granulomatous infiltrations were reduced, the hoarseness disappeared, and joint mobility improved [9]. However, earlier transplants on patients with the more common Type 1 Farber disease had limited success. In these studies, BMT lessened the peripheral symptoms but there was no improvement in neurological function and patients died soon after transplant [7, 11].
Farber disease is an attractive target for gene therapy since it is caused by a single gene defect, and the cDNA of AC has been cloned [12]. In addition, the enzyme is well-characterized [13, 14, 15]. Previous gene therapy studies from our laboratory directed towards this disorder have shown that the enzymatic deficiency in immortalized Farber patient cells could be corrected by transduction with an oncoretrovirus (RV) that engineers overexpression of human AC. That study showed that enzyme secreted from transduced cells could be endocytosed by non-transduced cells through the mannose-6-phosphate receptor pathway and restore enzyme activity [16]. This phenomenon, known as ‘metabolic cooperativity’ or ‘cross-correction’ is important for gene therapy applications as it allows for a lower level of functionally transduced cells to have greater therapeutic effect.
There are a number of approaches that have been used to introduce therapeutic genes in vivo. Viral methods using vectors such as retroviruses offer the advantage of long-term gene transfer since the viral DNA can integrate into the host genome and can be transmitted to progeny cells [17]. Our lab and others have shown that transduction of bone marrow-derived cells with a viral vector engineered to overexpress a therapeutic transgene can potentially provide a systemic, circulating source of enzyme [18, 19, 20]. In addition, it has been shown that lentiviral vectors (LV) directly delivered to neonatal Fabry mice can lead to sustained transgene expression in multiple organs [21].
Here novel retroviral vectors were constructed to engineer expression of human AC and a cell surface marker, human CD25, which can be used to enrich and track transduced cells [22]. These studies demonstrated that AC expression mediated by transduction with these viral vectors can restore enzyme activity in Farber patient cells. They also showed the potential efficacy of using these vectors in vivo in both a hematopoietic stem transplantation model and as a direct viral delivery agent to neonatal mice. These studies suggest that the use of viral vectors that engineer over-expression of AC is a potentially curative treatment for Farber disease.
MATERIALS AND METHODS
Vector Construction
The oncoretroviral vector (RV) employed in this study was made by subcloning the AC cDNA from pG1-ACER [16] into the cloning shuttle vector pSV/IRES/huCD25 and subsequently subcloning the AC/IRES/huCD25 sequence into the pUMFG backbone [18] as follows: mutagenesis was performed using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene, Cedar Creek, TX, USA) to remove an Nco I site from position 1634 of the pG1-ACER vector (primers: 5’- GGT GCA GTT CCC TGG TAC ACC ATA AAT C - 3’; 5’- GAT TTA TGG TGT ACC AGG GAA CTG CAC C - 3’). Both pG1-ACER and pSV/IRES/huCD25 were digested with Sal I and Nco I (New England Biolabs (NEB), Ipswich, MA, USA). The 1239 bp AC fragment was then ligated into the digested pSV/IRES/huCD25 vector to yield the new vector pSV/AC/IRES/huCD25. This vector was then digested with Nco I and Not I (NEB) to isolate the 2668 bp AC/IRES/huCD25 fragment that was then ligated into the pUMFG backbone to produce the vector pUMFG/AC/IRES/huCD25 (RV/AC/huCD25).
To construct the lentiviral vector (LV), the AC/IRES/huCD25 fragment from the pSV/AC/IRES/huCD25 shuttle vector was ligated into the pHR’ lentiviral backbone [23]. The shuttle vector was mutated to introduce a Bam HI site at position 644 by site-directed mutagenesis (Stratagene) (primers: 5’ - ACT CAC TAT AGG GAT CCG CCA TGG CGG GC - 3’; 5’ - GCC CGC CAT GGC GGA TCC CTA TAG TGA GT - 3’). Next, a Bam HI site was removed at position 1842 (5’ - AGG TTG GTG AGG GCG AAT CCC CCG GGC TGC - 3’; 5’ - CGA GCC CGG GGG ATT CGC CCT CAC CAA CCT - 3’). The pSV/AC/IRES/huCD25 shuttle vector was digested with Bam HI and Dra I (NEB) to isolate the 2654 bp AC/IRES/huCD25 fragment. The lentiviral vector pHR’EF-GW-SIN (LV/enGFP; provided by Robert Hawley, American Red Cross, Rockville, MD) was prepared by digesting with Bam HI to remove the enhanced green florescent protein (enGFP) cDNA. The AC/IRES/huCD25 fragment was then ligated into the pHR backbone to produce the vector pHR’EF-AC-IRES-huCD25-W-SIN (LV/AC/huCD25).
Oncoretrovirus production
The pUMFG/AC/IRES/huCD25 vector was transfected into the FLYRD18 packaging cell line [24] by calcium phosphate-mediated transfection using 10 µg of the plasmid and 1 µg of the pGTN28 plasmid that carries the neomycin-resistance cDNA. Stable transfectants were selected using 0.8 mg/ml G418 (Sigma, Oakville, ON, Canada). These cells were stained with a phycoerythrin-conjugated anti-human CD25 antibody (BD Bioscience Canada, Mississauga, ON, Canada) and then sorted by flow cytometry to derive an enriched pool of producer cells and a single cell-derived clone. Viral titer was determined by transduction of HeLa cells with serial dilutions of supernatant from producer cells, followed by measurement of downstream huCD25 expression by flow cytometry. The clone with the highest titer, clone #17, was then used to produce viral supernatant for all experiments that employed the oncoretroviral vector RV/AC/huCD25, except where otherwise indicated. The viral titer of supernatant collected from this clone was typically ~4 × 106 IU/ml.
Lentivirus production
Recombinant LV/AC/huCD25 virus was produced by transient transfection of 293T cells with three plasmids: pMD.G (VSV-g envelope), pCMV ΔR8.91 (packaging) and either LV/AC/huCD25 or LV/enGFP. Transfections of 293T cells were performed using calcium phosphate as previously described [21]. Viral supernatants were concentrated 300-fold by centrifugation at 50,000 × g for 2 h at 4°C. Viral titer was determined as previously described [21] and was typically in the range of 1–3 × 108 IP/ml after concentration.
Viral transduction of Farber cells
Immortalized Farber patient skin fibroblasts and B cells [16] were maintained in DMEM and RMPI 1640 (both Sigma), respectively, supplemented with 10% fetal calf serum (PAA Laboratories, Rexdale, ON, Canada), 2 mM L-glutamine, 1% sodium pyruvate, and 1% penicillin-streptomycin (all Sigma). Using RV/AC/huCD25, Farber fibroblasts were transduced three times with supernatant from an enriched pool of producer cells (MOI of 20) while B cells were transduced once with supernatant from clone #17 (MOI of 8). B cell transductions were performed in fibronectin-coated (5 µg/cm2; Roche, Mississauga, ON, Canada) plates. For experiments employing the LV vector, Farber fibroblasts were transduced once with unconcentrated virus at an MOI of ~1, while the B cells were transduced once with concentrated virus at an MOI of 50. All transductions were performed in the presence of 8 µg/ml protamine sulfate (Sigma). The transduced B cell pools were then enriched by magnetic-activated cell sorting (MACS) using magnetic beads conjugated to the anti-CD25 antibody and MS+ columns (both from Miltenyi Biotech, Auburn, CA, USA).
Ceramide turnover studies and AC activity assays
AC activity in intact cells was evaluated by studying lysosomal ceramide degradation as previously described [25]. Briefly, transduced cells, non-transduced (NT) Farber cells, and normal cells were pulsed with [3H-ceramide]-sphingomyelin (labelled on both the sphingosine and fatty acid moieties). The substrate is first cleaved by acidic sphingomyelinase to generate labelled ceramide. In the presence of AC, ceramide is degraded to sphingosine and fatty acid, which are then immediately incorporated into glycerolipids (e.g., phosphatidylcholine, phosphatidylethanolamine, cholesteryl-esters and triacylglycerols) and sphingolipids (eg. sphingomyelin and GM3) as described previously [26, 27, 28]. Lipids were extracted and resolved by analytical thin layer chromatography (TLC) 48 h after pulsing. The distribution of the radioactivity on the plate was analyzed using a Berthold LB 2832 radiochromatoscan. Additionally, ceramide and other lipid fractions were scraped off the plate for direct liquid scintillation counting. The percentage of radioactive ceramide out of total labelled ceramide metabolites (except sphingomyelin) was calculated from the radioactivity of each fraction.
In mice treated by direct virus injection, AC activity was assessed using a modified, fluorescence-based HPLC method. Organs were first homogenized in 0.25 M sucrose. Then, equal volumes of organ lysate and substrate buffer (0.2 M citrate/phosphate (pH 4.5), 0.2 mM Bodipy-labeled C5-ceramide (Molecular Probes, Burlington, ON), 0.5% sodium taurocholate (Sigma), 0.2% Igepal CA-630 (Sigma), 0.1% BSA, 0.3 M NaCl) were co-incubated for 6 h at 37°C. Samples were then evaluated as previously described [29].
Ceramide quantitation
Intracellular ceramide levels were measured using E. coli diacylglycerol (DAG) kinase and [γ32P]ATP as described [16]. Briefly, lipids were extracted from cell lysates by the Folch method [30] and subjected to mild alkaline hydrolysis using NaOH for 2 h at 37°C. The solution was then neutralized and lipids extracted using chloroform/methanol (2:1, v/v). Lipids were then incubated with sn-l,2-diacylglycerol kinase and [γ32P]ATP as described [31]. Lipids were resolved by TLC as above. Ceramide-[32P]-1-phosphate was quantified by scraping the band from the TLC plate and analyzing the fraction by liquid scintillation counting.
Metabolic cooperativity studies
Non-transduced Farber patient fibroblasts were cultured for 48 h in filtered media harvested from the following cell lines: non-transduced Farber fibroblasts, RV- or LV-transduced Farber fibroblasts, and normal fibroblasts. Cells were then pulsed with [3H-ceramide]-sphingomyelin for 24 h and the enzyme activity in each cell population was determined as described above (see AC activity assay).
Western blot
Fibroblasts were grown for 24 h in serum-free medium, then washed with PBS and harvested. Cell pellets were resuspended in 0.1 ml of ice-cold PBS containing 10% glycerol, 1% Triton X-100, 1 mM NaVO4, 10 mM NaF and a protease inhibitor cocktail (Roche, Mannheim, Germany), and further lysed by brief sonication. Samples were centrifuged at 10,000 g for 10 min. Supernatants were collected and protein content was determined by the Bradford method (BioRad, Munich, Germany). The culture medium was collected and concentrated 8x using Nanosep 10K centrifugal devices (Pall Gelman Laboratory, Ann Arbor Michigan, USA). Proteins (50 µg of cell lysates, or 20 µl of concentrated media) were separated on a 12.5 % polyacrylamide gel. After transfer onto a nitrocellulose membrane, immunoblotting was performed using a rabbit polyclonal anti-acid ceramidase antibody (a kind gift from Dr. K. Sandhoff, Bonn, Germany).
Infection of HSPCs
CD34+ cells were obtained from AllCells, LLC (Berkeley, CA). Cells were pre-stimulated for 12 h in StemSpan SFEM medium (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with recombinant human SCF, Flt3-L, thrombopoietin (all at 50 ng/ml), and IL-6 (20 ng/ml). All cytokines were obtained from R&D Systems Inc. (Minneapolis, MN, USA). Cells were then transduced with virus in the presence of cytokines and 8 µg/ml protamine sulfate. Transductions were performed in 6-well plates coated with RetroNectin CH296 (10 µg/cm2; Takara Shuzo, Otsu, Japan) in a total volume of 2 ml/well at a concentration of 0.5 × 106 cells/ml. For parallel comparison of infections on different sources of HSPCs, cells were infected with LV/AC/huCD25 at an MOI of 40. The cord blood-derived cells used for in vivo transplantation were infected with LV/AC/huCD25 or LV/enGFP at an MOI of 2 and 10 respectively. Cells were then washed twice in PBS and either re-cultured in StemSpan supplemented with cytokines, plated into Methocult™ H4434 (Stem Cell Technologies), or resuspended in PBS for transplantation.
Human cord blood transplantation in NOD/SCID mice
Mice were obtained from Taconic (New York, NY, USA) and were bred and maintained at the UHN Animal Resource Centre. Animal experimentation protocols were approved by the UHN Animal Care Committee. Mice were irradiated at 350 cGy and injected with 200 µg of anti-CD122 antibody into the intraperitoneal cavity 24 h prior to transplantation [32]. The anti-CD122 monoclonal antibody was used to clear any residual natural killer cells in vivo [33] and was purified from the TM-β1 hybridoma cell line (a gift from Dr T. Tanaka, Osaka University Medical Center, Osaka, Japan), using the High Trap Protein G Column (GE Healthcare). Transduced CD34+ cells were then injected into the animals via the tail vein (1.65 × 106 and 2.4 × 106 cells/mouse for AC and enGFP groups respectively). Six weeks later, peripheral blood (PB) was collected and the mice were sacrificed. Bone marrow (BM) was flushed from the tibia and femur of both hind legs and splenocytes were harvested by crushing the spleen through a nylon mesh. Engraftment of human cells was assessed by flow cytometry of living nucleated cells from the PB, BM and spleen; dead cells were excluded by staining with 7-amino-actinomycin D (Sigma). Cells were stained with antibodies against human CD45 (BD Bioscience Canada) and functional transgene expression was assessed by flow cytometry for either huCD25 or enGFP.
Assessment of vector positive cells
Cells were taken from methylcellulose colonies by aspiration using a micropipettor. Cells were lysed in 10 µl of lysis buffer (50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.1% gelatin, 0.45% Tween-20, 0.45% Nonidet-P40, 125 µg/mL proteinase K) and incubated at 55 °C for 2–16 h. 1 µl of lysate was then used for PCR using the primers specific for the viral vector (ACnest-FP: 5' - gaaacttacctgcgggactg - 3' and ACnest-RP: 5' - acaccggccttattccaag - 3') or for the human GAPDH gene (huGAPDH-FP: 5' - accgtcaaggctgagaaacgg - 3' and huGAPDH-RP: 5' - acgtactcagcgccagcatc - 3'). Cycling conditions were as follows: 94 °C for 45 s, 63°C for 30 s, 72 °C for 15 s.
Delivery of LV to neonatal mice
One- to two-day-old C57BL/6 mice (The Jackson Laboratories, Bar Harbor, MI) were injected via the superficial temporal vein with either LV/enGFP (6.65 × 107 IP/5,000 pg p24/mouse) or LV/AC/huCD25 (4.75 × 107 IP/10,000 pg p24/mouse) in a volume of 100 µl of sterile PBS. Mice were analyzed at weeks 7, 10 and 14 for the expression of soluble human CD25 (sCD25) in the plasma. Plasma was isolated from mouse PB samples by centrifugation at 16,000 × g for 20 min. The level of sCD25 was measured by a direct ELISA using the BD OptEIA™ Human IL-2 sRα ELISA Set (BD Bioscience Canada) as per the manufacturer’s instructions. Each sample was measured in triplicate. Mice were sacrificed at 14 weeks post-virus injection, organs were harvested and AC activity was measured as above.
RESULTS
RV- and LV-transduced Farber patient cells express human CD25 and have restored AC activity
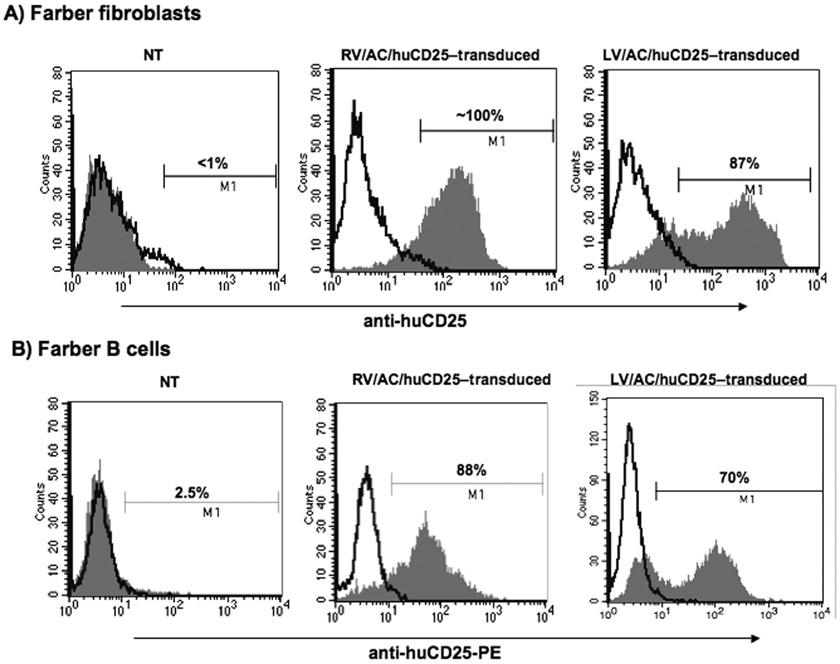
We first tested the efficacy of the novel recombinant viral vectors to transduce immortalized Farber patient fibroblasts and B cells. No huCD25 expression could be detected on non-transduced (NT) Farber patient fibroblasts, however, cells transduced with the recombinant RV and LV were ~100% and 87% positive for huCD25, respectively (Fig. 1a) and stably expressed the marker over time. Non-transduced B cells showed low levels of huCD25 expression (2.5%) but once transduced with RV/AC/huCD25, approximately 26% of the cells were positive for huCD25 (data not shown). Sorting of this pool enabled enrichment of the huCD25-expressing population to ~88% (Fig. 1b). Similar results were obtained when cells were transduced with LV/AC/huCD25 (Fig. 1b).
FIG. 1. huCD25 expression on transduced, immortalized Farber patient cells.
Farber patient fibroblasts (A) and B cells (B) were transduced with either the oncoretrovirus (RV) or lentivirus (LV) engineered to express both human AC and CD25. Cells were stained with anti-huCD25-PE antibody and analyzed by flow cytometry. NT: non-transduced.
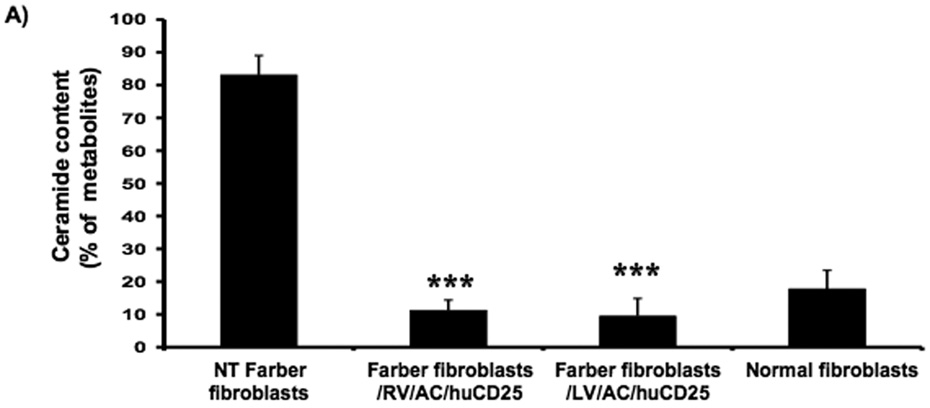
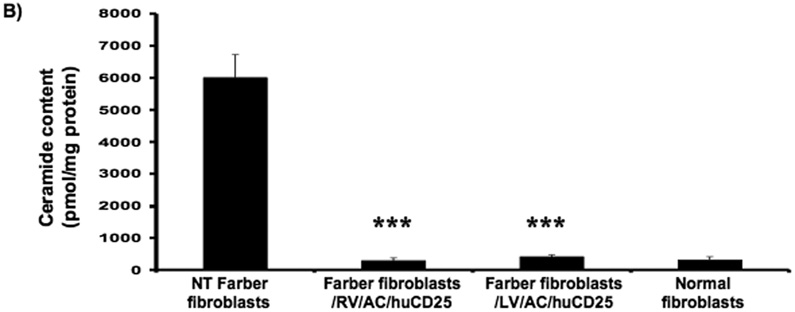
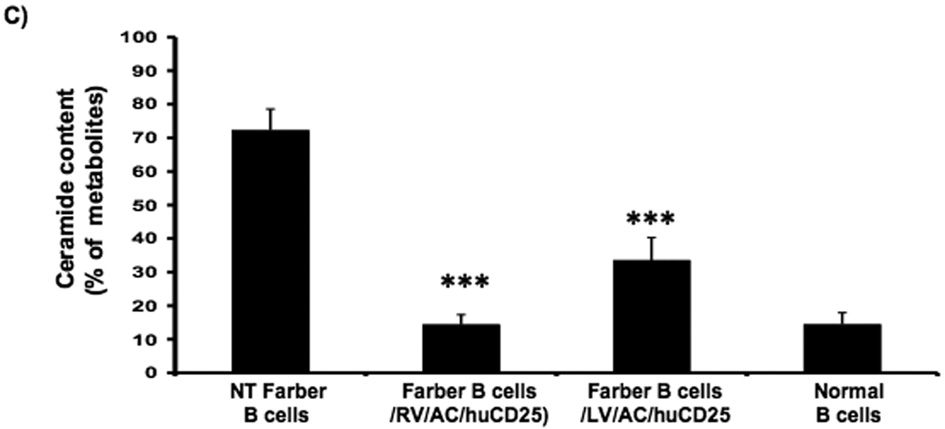
In order to determine if functional AC is expressed after transduction, ceramide degradation was then measured. Cells were pulsed with [3H-ceramide]-sphingomyelin and 48 h later, lipids were isolated from the cells and analyzed by TLC. In non-transduced Farber fibroblasts, ceramide comprised ~83% of the sphingomyelin metabolites while normal fibroblasts contain only 18%. Those values reflect previous data [16, 27, 34]. Transduction with the recombinant RV and LV reduced the ceramide fraction to 11% and 10%, respectively (Fig. 2a). Similar results were seen in the Farber B cells; ceramide levels were reduced from 72% to 14% and 33% in RV/AC/CD25- and LV/AC/CD25-transduced pools, respectively (Fig. 2c).
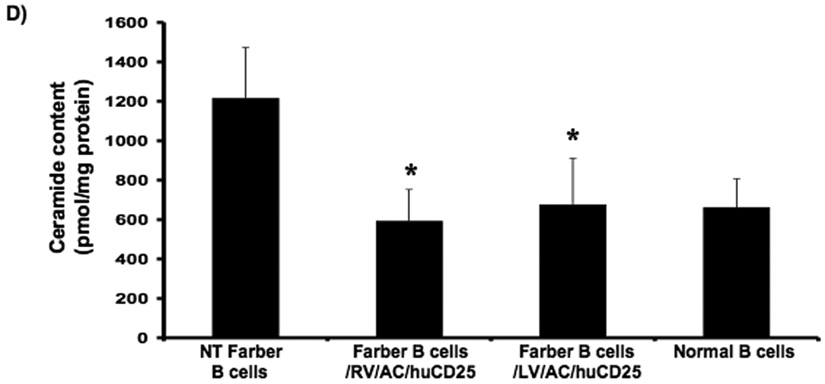
FIG. 2. Lysosomal ceramide turnover and ceramide content in transduced Farber patient cells.
Immortalized Farber patient cells were transduced with either oncoretrovirus (RV) or lentivirus (LV) encoding human AC and huCD25. Non-transduced and transduced Farber patient cells were pulsed with [3H-ceramide]-sphingomyelin for 48 h. Lipids were isolated, and then separated by TLC. The AC activity of fibroblasts (A) and B cells (C) are shown. To determine ceramide content, lipid extracts were incubated with E. coli diacylglycerol kinase and [γ32P]ATP. Radioactive ceramide 1-phosphate was isolated by TLC and quantified by liquid scintillation analysis for both fibroblast (B) and B cell (D) extracts. Error bars represent SD; measurements are averages of at least three separate experiments. * p < 0.05, *** p < 0.001, for groups indicated versus non-transduced (NT) controls.
In another assay, intracellular ceramide levels were also measured in transduced and control cells using E. coli diacylglycerol (DAG) kinase and [γ32P]ATP. DAG phosphorylates ceramide to produce ceramide-1-phosphate. In the presence of [γ32P]ATP, the ceramide-1-phosphate is radiolabelled and can be quantified by liquid scintillation counting. As seen in Figure 2b, while non-transduced Farber fibroblasts store large amounts of ceramide, there was ~90% reduction in ceramide storage in RV- and LV-transduced fibroblasts. Transduction of Farber B cells with both vectors resulted in ~50% reduction of ceramide storage (Fig. 2d). These results collectively demonstrate that transduction with the recombinant retroviral vectors results in restoration of the AC activity in Farber patient cells.
Metabolic cooperativity occurs between AC-transduced and NT Farber patient fibroblasts
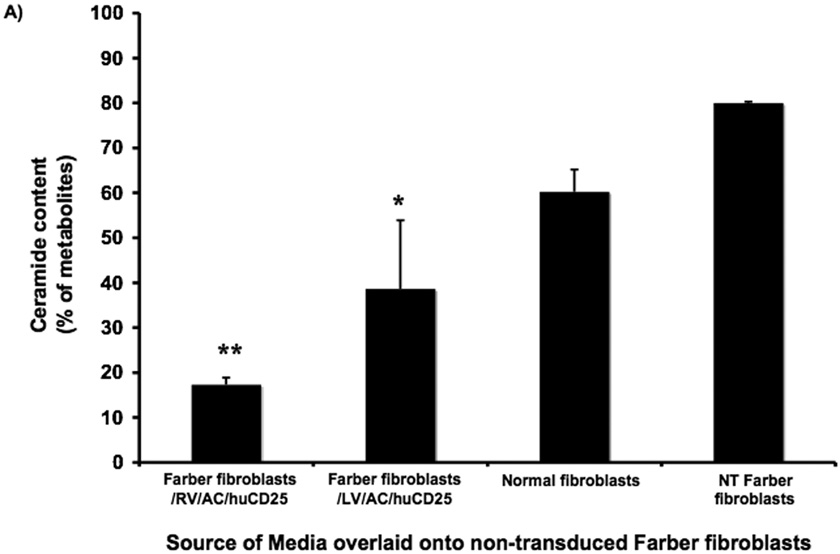
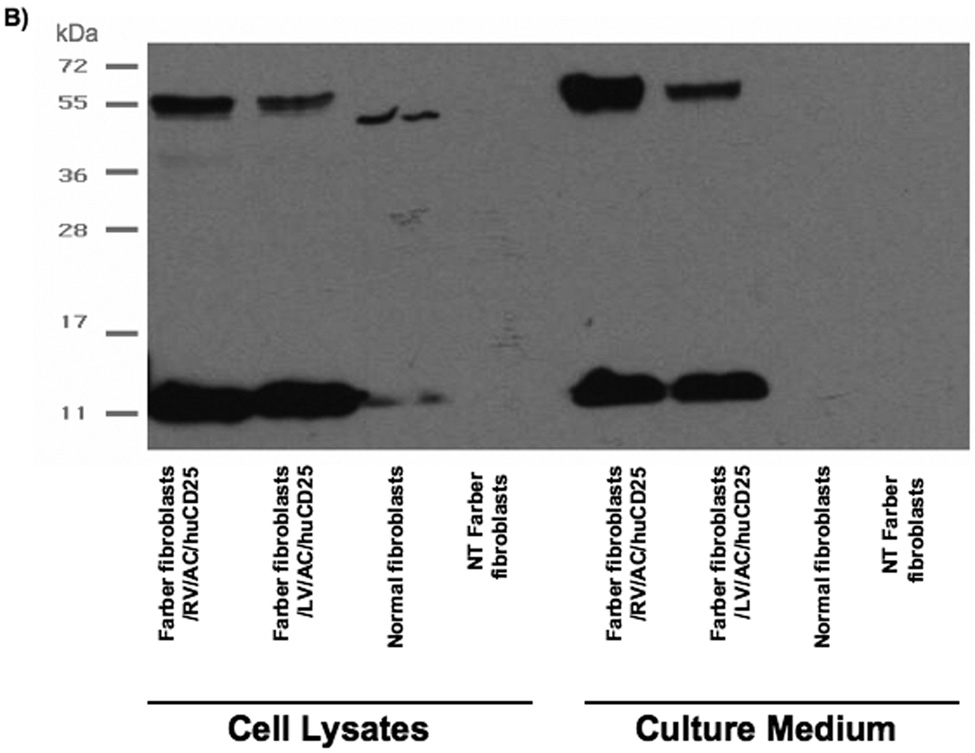
We next tested the ability of AC derived from therapeutically transduced cells to be taken up by non-transduced cells. This phenomenon of metabolic cooperativity is an important concept in gene therapy, especially as directed towards LSDs, since it enables a smaller number of transduced cells to have wide therapeutic effect. Non-transduced Farber fibroblasts were cultured for 48 h in conditioned media harvested from the following cell lines: non-transduced Farber fibroblasts, Farber fibroblasts transduced with RV and LV, and normal fibroblasts. Cells were then pulsed with [3H-ceramide]-sphingomyelin and the ceramide content in each cell population was determined. While media from normal fibroblasts reduced ceramide levels from 80% to only 60%, incubation with media from the RV and LV-transduced cells reduced ceramide content to approximately 20% and 40% of sphingomyelin metabolites, respectively (Fig. 3a), a level that is comparable to that observed in normal fibroblasts (see Fig. 2a). To confirm the secretion of AC into the cell supernatant, Western blot analysis was performed on both cell lysates and culture medium of the fibroblasts. It was found that both RV- and LV-transduced fibroblasts over-express and secrete a large amount of AC into the culture medium (Fig. 3b). However, although transduced cells contained proteolytically processed AC (as indicated by the presence of the 13 kDa alpha-subunit), RV-transduced fibroblasts produced more AC precursor (55 kDa form) than the LV-transduced cells. The secreted AC was likely taken up by non-transduced fibroblasts, enabling restoration of enzymatic activity as demonstrated in Fig. 3a.
FIG. 3. Metabolic cooperativity demonstrated by uptake of secreted AC by non-transduced Farber fibroblasts.
(A) Non-transduced (NT) Farber fibroblasts were overlaid with media harvested from the indicated cells and incubated for 48 h. The cells were then pulsed with [3H-ceramide]-sphingomyelin for 24 h and lipids analyzed by TLC. Error bars represent SD; measurements are averages of three independent experiments. * p < 0.05, ** p < 0.01, for groups indicated versus the non-transduced (NT) control. (B) Normal, NT and transduced (RV/AC/huCD25 or LV/AC/huCD25) Farber fibroblasts were grown in serum-free culture medium. After 24 h, both cells and media were collected. Proteins were separated by PAGE and immunoblotted with an anti-acid ceramidase antibody.
Transplantation of LV/AC/huCD25-transduced CD34+ cells can re-populate recipient animals
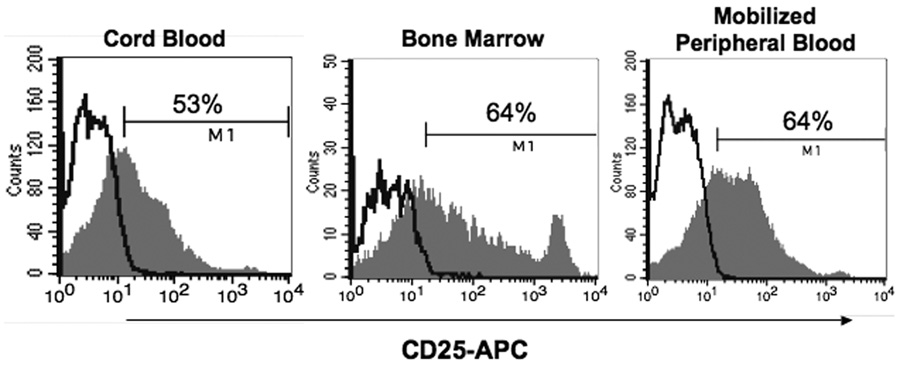
We then wanted to assess the effect of transduction and AC-overexpression on the engraftment and repopulation potential of human hematopoietic cells, as would be used in a patient. CD34+ cells derived from human umbilical cord blood, BM and mobilized peripheral blood (mPB) were transduced with LV/AC/huCD25 (MOI ~40) since these are all potential sources of HSPCs that can be used for transplantation. Following transduction, CD25 expression was assessed by flow cytometry and it was found that all populations were transduced with similar efficiency (Fig. 4). Transduced cells were seeded in methylcellulose to determine their ability to generate multiple lineages of hematopoietic colonies. It was found that all populations generated colonies derived from erythroid, granulocyte and macrophage precursors within normal proportions and with similar frequency to their non-transduced counterparts (data not shown). When granulocyte/macrophage colonies were assessed by PCR to determine the presence of vector, it was found that vector-positive colonies were more abundant in the cord blood-derived population (14/46; 30%) compared to the cells derived from the bone marrow (5/70; 7%) and mPB (0/52; 0%). This is likely due to the higher percentage of more primitive HSPCs found in cord blood [35, 36]. As such, CD34+ cells derived from human umbilical cord blood were used for in vivo transplants.
Fig. 4. Infection of human HSPCs from multiple sources.
CD34+ cells from human umbilical cord blood, bone marrow and mobilized peripheral blood were transduced with LV/AC/huCD25. CD25 expression was assessed by flow cytometry using a PE-conjugated anti-CD25 antibody.
CD34+ cells were transduced with either LV/AC/huCD25 or LV/enGFP and transplanted into sub-lethally irradiated NOD/SCID recipient mice. These animals are immuno-deficient and can thus accept xenografts [37]. Six weeks post-transplantation, BM, splenocytes, and PB were harvested. Human cell engraftment was assessed by flow cytometry to detect the human panhematopoietic marker CD45. It was found that, on average, human chimerism in the BM was 65% and 49% for the LV/AC/huCD25 and LV/enGFP-transduced groups, respectively (Table 1). Further, in mice transplanted with LV/AC/huCD25, it was found that ~0.41% of the human cells expressed the downstream marking transgene huCD25. Expression of this surrogate marker has been shown to be correlated with functional transgene expression [20, 21]. It is also important to note that this marker allows an evaluation of transgene expression even in enzymatically normal cells in which changes above background AC activity may be hard to detect. In addition, analysis of the BM harvested from transplanted mice by flow cytometry and by colony-forming assays showed the presence of both lymphoid and myeloid cells (data not shown). Therefore, it appears that CD34+ cells transduced with an LV that engineers overexpression of AC are able to reconstitute an irradiated recipient and can give rise to all lineages of hematopoietic cells, suggesting that the repopulation ability of these cells is not impaired following transduction with our viral vector.
Table 1.
Engraftment of LV/enGFP- or LV/AC/huCD25-transduced human CD34+ cells into NOD/SCID recipients
| Bone marrow | Spleen | Peripheral Blood | ||||
|---|---|---|---|---|---|---|
| Mouse | ||||||
| CD45+ | enGFP+ | CD45+ | enGFP+ | CD45+ | GFP+ | |
| 1 | 33% | 8% | 8% | 0.60% | 11% | 3% |
| 2 | 67% | 12% | 13% | 1.45% | 11% | 2.6% |
| 3 | 46% | 9% | 16% | 4% | 8% | 1.6% |
| Mouse | Bone marrow | Spleen | Peripheral Blood | |||
| CD45+ | CD25+ | CD45+ | CD25+ | CD45+ | CD25+ | |
| 1 | 75% | 0.46% | 33% | - | 18% | - |
| 2 | 56% | 0.21% | N/A | - | 12% | - |
| 3 | 63% | 0.50% | 30% | - | 6% | - |
| 4 | 67% | 0.47% | 19% | - | 13% | - |
Cord blood-derived CD34+ cells were transduced with either LV/enGFP (top panel) or LV/AC/huCD25 (bottom panel) and transplanted into sub-lethally irradiated NOD/SCID recipient mice. Six weeks post-transplantation, bone marrow, peripheral blood and splenocytes were harvested and analyzed for expression of CD45 and either enGFP or human CD25 by flow cytometry. Abbreviations: enGFP, enhanced green fluorescent protein; N/A, data not available.
Neonatal LV delivery engineers persistent expression of downstream marking transgene
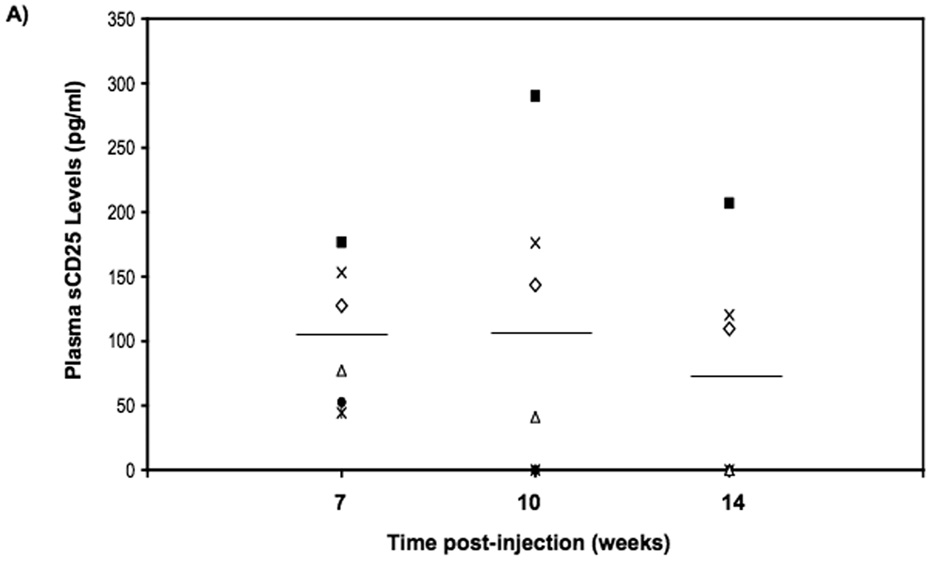
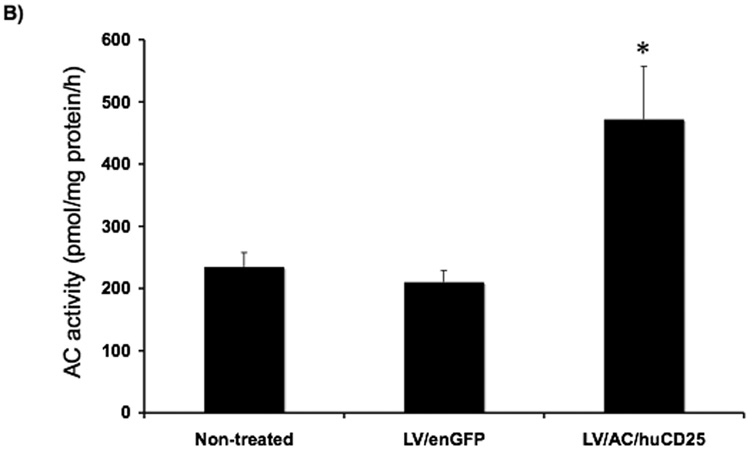
Treatment of neonatal recipients offers several advantages that include exploiting an incompletely formed blood-brain barrier and an immature immune system, as well as the possibility of administering treatment before irreversible organ and neurological damage has occurred as a result of the disease. In proof-of-principle experiments, one- to three-day-old C57BL/6 mice were treated with either LV/AC/huCD25 or LV/enGFP. No adverse effects were seen in treated animals and mice developed normally. PB was harvested at weeks 7, 10 and 14 16 post-viral delivery and plasma levels of sCD25 were measured by ELISA. As shown in Fig. 5a, at 7 weeks post-treatment all mice treated with LV/AC/huCD25 showed high levels of sCD25 in the plasma and three of six mice showed persistent levels of sCD25 up to 14 weeks post-viral delivery. As expected, untreated mice and animals treated with LV/enGFP showed no detectable levels of sCD25. In addition, it was found that the livers of mice treated with LV/AC/huCD25 showed increased AC activity over both non-treated mice and mice treated with LV/enGFP (Fig. 5b). This proof-of-principle experiment shows the potential utility of treating Farber disease shortly after birth, a time that may be critical to preventing irreversible organ and neurological damage.
FIG. 5. Transgene expression following direct LV delivery to neonatal mice.
(A) One- to three-day-old neonatal animals were injected with LV/AC/huCD25 or LV/enGFP via the temporal vein. Plasma was collected from the PB at weeks 7, 10 and 14 post-viral delivery. The levels of sCD25 were measured by ELISA. Results are presented for each LV/AC/huCD25-treated mouse in the study. LV/enGFP and untreated mice showed no detectable levels of sCD25 (data not shown). (B) At 14 weeks post-viral delivery, mice were sacrificed and AC activity was measured in the organs. Shown are the results of liver enzyme activity. Values are represented as means ± SEM. For LV/AC/huCD25-treated and non-treated mice, n = 6; for LV/enGFP-treated mice, n = 7. Other organs showed no significant increase in AC activity over normal background levels (data not shown). * p < 0.05.
DISCUSSION
Current treatment for Farber disease consists mainly of symptomatic supportive care since enzyme replacement therapy is not available as it is for some other LSDs [38]. While BMT can relieve some of the symptoms of the disease, it is only available to patients with matched donors and it does not relieve the progressive neurological deterioration that is seen in the majority of patients affected with this disease [4, 7, 9]. Therefore, the prognosis for Farber patients remains poor and the development of novel treatments remains important.
Virus-mediated gene therapy offers the potential for a one-time curative treatment since integrating vectors such as retroviruses have the ability to persist long-term in transduced cells and their progeny. We have previously shown in vitro that infection of Farber patient cells with an RV engineering expression of AC could restore enzymatic activity [16]. Here we show similar in vitro results using novel recombinant RV and LV that we developed and expand those results to the testing of our viral vectors in vivo. As a complete deletion of the AC gene is embryonic lethal [39] and the heterozygous animals are not widely available, we developed surrogate models for in vivo testing of gene therapy strategies for the treatment of Farber disease. To our knowledge, this is the first report of such studies.
The recombinant RV used in these experiments is based on a viral backbone that has been shown to result in higher transgene expression [40] than the pG1-ACER vector we previously developed [16]. The virus is also pseudotyped with a more clinically-relevant envelope that is not inactivated by human sera [24]. Although not yet as widely used in humans as RVs are, a recombinant LV was developed since its ability to transduce more slowly-dividing cell populations and the broad tropism offered by the VSV-g envelope make it a useful vehicle to target cells such as hematopoietic stem/progenitor cells (HSPCs) and certain neural cells [41]. In both vectors, we also included the huCD25 cell surface marker that we have used in previous studies to enrich for and track transduced cells [21, 22]. In addition, in recent studies we have shown that huCD25 could be used as a target antigen to eliminate therapeutically transduced cells in the event of insertional mutagenesis and the development of leukemia [42].
To confirm that the viral vectors constructed could infect cells and produce functional enzyme, we transduced Farber patient B cells and fibroblasts. We could detect high levels of huCD25 expression from transduced cells and were able to use the huCD25 marker to enrich the pool of transduced B cells. Measurement of AC activity showed that transduced cells had significantly increased enzyme activity and decreased ceramide storage following transduction. We also showed that functional enzyme was secreted and could be taken up and utilized by non-transduced cells. Previous studies have shown that this uptake is mediated by the mannose-6-phosphate receptor and can be blocked by co-incubation with mannose-6-phosphate [43]. Western blot analysis of cell lysates and culture medium from the fibroblasts used in this experiment further demonstrated the benefit of engineering cells to over-express the therapeutic protein. Transduced cells secrete a large excess of AC while secretion from normal fibroblasts is minimal. This is likely to lead to greater therapeutic benefit in vivo compared to transplants that utilize cells from normal donors.
The transduction of HSPCs is an attractive option for treating a number of LSDs since these cells can provide a circulating source of the therapeutic factor. Moreover, HSPCs self-renew and cells of the hematopoietic lineage can migrate from the circulation into tissues throughout the body, including the liver, kidney, spleen, heart and lungs [8, 20]. It has also been shown that transplanted HSPCs can repopulate the brain as resident microglial turnover [44]. The ability to target multiple organs is important for Farber disease since along the spectrum of the disorder, multiple tissues are affected by granulomatous infiltrations including the joints, liver, spleen, lymphoid tissue, lungs and brain [4, 45].
In order to test the effect of transduction on the engraftment of human cells, we used the NOD/SCID xenotransplantation model [46] that is commonly used to assess human cell engraftment. We used CD34+ hematopoietic cells since they are a prime target for treatment of patients with Farber disease. We transduced CD34+ cells with LV/AC/huCD25 and transplanted them into irradiated NOD/SCID mice. We achieved high levels of human cell engraftment as assessed by measurement of CD45 expression and found that engrafted cells expressed the huCD25 marking gene in the bone marrow.
We also investigated the effect of delivering an LV expressing AC and huCD25 directly to neonatal animals. At the neonatal stage, the blood-brain barrier is not fully formed and the immune system is not fully developed [47]. These physiological properties may contribute to the efficacy of treatment since delivery to the brain will be increased and tolerance to the transgene can be induced. In addition, when treatment is administered before symptoms appear, it may prevent irreversible neurological damage from occurring. In this aspect of the study, we injected normal neonatal mice with LV/AC/huCD25 and found that up to 14 weeks post-injection, sCD25 was detected in the plasma, suggesting the persistence of vector and long-term transgene expression. Non-treated mice did not have any detectable levels of sCD25 (data not shown). Therapeutic transgene expression was further evidenced by the increased AC activity found in the livers of mice treated with LV/AC/huCD25 as compared to wild-type mice.
The results of these studies represent significant progress towards the development of gene therapy strategies for Farber disease. Therapeutic vectors can be delivered in a number of ways. The use of transduced HSPCs can provide a circulating source of enzyme and the progeny can differentiate into cells that reside throughout the body, such as microglia in the brain [48]. Neonatal delivery of virus via the temporal vein also been shown to result in transduction of numerous cell types. It is hypothesized that this treatment strategy may result in better transduction in the brain than HSPC transplantation and thus have a greater effect on the neurological effects of the disease. Further, improved delivery to the brain could possibly be achieved by using vascular endothelial growth factor to further permeabilize the blood brain barrier as previously reported [49]. However, this approach is limited by the requirement of early diagnosis e.g., after prenatal diagnosis or, when available, newborn screening for LSDs. Studies are also underway in our laboratory that will examine the safety of using the recombinant LV we have developed here in a non-human primate model.
ACKNOWLEDGEMENTS
These studies were supported in part by grants from the National Institutes of Health (R21 777606496) to JAM and from the Vaincre les Maladies Lysosomales (VML) to TL and JAM. SR was funded by the Canadian Institutes of Health Research Training Program in Regenerative Medicine, and CBG by a fellowship from La Caixa Foundation. We would like to thank Gillian Sleep and Renee Head for technical assistance with in vivo experiments and members of the Medin lab for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- 1.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcao A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sa Miranda MC. Prevalence of lysosomal storage diseases in Portugal. Eur. J. Hum. Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 3.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 4.Moser HW. Acid Ceramidase Deficiency: Farber Lipogranulomatosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3573–3588. [Google Scholar]
- 5.Bar J, Linke T, Ferlinz K, Neumann U, Schuchman EH, Sandhoff K. Molecular analysis of acid ceramidase deficiency in patients with Farber disease. Hum. Mutat. 2001;17:199–209. doi: 10.1002/humu.5. [DOI] [PubMed] [Google Scholar]
- 6.Haraoka G, Muraoka M, Yoshioka N, Wakami S, Hayashi I. First case of surgical treatment of Farber's disease. Ann. Plast. Surg. 1997;39:405–410. doi: 10.1097/00000637-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Yeager AM, Uhas KA, Coles CD, Davis PC, Krause WL, Moser HW. Bone marrow transplantation for infantile ceramidase deficiency (Farber disease) Bone Marrow Transplant. 2000;26:357–363. doi: 10.1038/sj.bmt.1702489. [DOI] [PubMed] [Google Scholar]
- 8.Malatack JJ, Consolini DM, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr. Neurol. 2003;29:391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Vormoor J, Ehlert K, Groll AH, Koch HG, Frosch M, Roth J. Successful hematopoietic stem cell transplantation in Farber disease. J. Pediatr. 2004;144:132–134. doi: 10.1016/j.jpeds.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 10.Ehlert K, Roth J, Frosch M, Fehse N, Zander N, Vormoor J. Farber's disease without central nervous system involvement: bone-marrow transplantation provides a promising new approach. Ann. Rheum. Dis. 2006;65:1665–1666. doi: 10.1136/ard.2005.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souillet G, Guibaud P, Fensom AH, Maire I, Zabot MT. Outcome of Displacement Bone Marrow Transplantation in Farber's Disease: A Report of a Case. In: Hobbs JR, editor. Correction of Certain Genetic Diseases by Transplantation. London: COGENT; 1989. pp. 137–141. [Google Scholar]
- 12.Li CM, Park JH, He X, Levy B, Chen F, Arai K, Adler DA, Disteche CM, Koch J, Sandhoff K, Schuchman EH. The human acid ceramidase gene (ASAH): structure, chromosomal location, mutation analysis, and expression. Genomics. 1999;62:223–231. doi: 10.1006/geno.1999.5940. [DOI] [PubMed] [Google Scholar]
- 13.Ferlinz K, Kopal G, Bernardo K, Linke T, Bar J, Breiden B, Neumann U, Lang F, Schuchman EH, Sandhoff K. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J. Biol. Chem. 2001;276:35352–35360. doi: 10.1074/jbc.M103066200. [DOI] [PubMed] [Google Scholar]
- 14.Okino N, He X, Gatt S, Sandhoff K, Ito M, Schuchman EH. The reverse activity of human acid ceramidase. J. Biol. Chem. 2003;278:29948–29953. doi: 10.1074/jbc.M303310200. [DOI] [PubMed] [Google Scholar]
- 15.He X, Okino N, Dhami R, Dagan A, Gatt S, Schulze H, Sandhoff K, Schuchman EH. Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J. Biol. Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- 16.Medin JA, Takenaka T, Carpentier S, Garcia V, Basile JP, Segui B, Andrieu-Abadie N, Auge N, Salvayre R, Levade T. Retrovirus-mediated correction of the metabolic defect in cultured Farber disease cells. Hum. Gene Ther. 1999;10:1321–1329. doi: 10.1089/10430349950018003. [DOI] [PubMed] [Google Scholar]
- 17.Medin JA, Karlsson S. Viral vectors for gene therapy of hematopoietic cells. Immunotechnology. 1997;3:3–19. doi: 10.1016/s1380-2933(96)00059-0. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka T, Qin G, Brady RO, Medin JA. Circulating alpha-galactosidase A derived from transduced bone marrow cells: relevance for corrective gene transfer for Fabry disease. Hum. Gene Ther. 1999;10:1931–1939. doi: 10.1089/10430349950017293. [DOI] [PubMed] [Google Scholar]
- 19.Jin HK, Schuchman EH. Ex vivo gene therapy using bone marrow-derived cells: combined effects of intracerebral and intravenous transplantation in a mouse model of Niemann-Pick disease. Mol. Ther. 2003;8:876–885. doi: 10.1016/j.ymthe.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimitsu M, Higuchi K, Ramsubir S, Nonaka T, Rasaiah VI, Siatskas C, Liang SB, Murray GJ, Brady RO, Medin JA. Efficient correction of Fabry mice and patient cells mediated by lentiviral transduction of hematopoietic stem/progenitor cells. Gene Ther. 2007;14:256–265. doi: 10.1038/sj.gt.3302839. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimitsu M, Sato T, Tao K, Walia JS, Rasaiah VI, Sleep GT, Murray GJ, Poeppl AG, Underwood J, West L, Brady RO, Medin JA. Bioluminescent imaging of a marking transgene and correction of Fabry mice by neonatal injection of recombinant lentiviral vectors. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16909–16914. doi: 10.1073/pnas.0407572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin G, Takenaka T, Telsch K, Kelley L, Howard T, Levade T, Deans R, Howard BH, Malech HL, Brady RO, Medin JA. Preselective gene therapy for Fabry disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3428–3433. doi: 10.1073/pnas.061020598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 24.Cosset FL, Takeuchi Y, Battini JL, Weiss RA, Collins MK. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levade T, Leruth M, Graber D, Moisand A, Vermeersch S, Salvayre R, Courtoy PJ. In situ assay of acid sphingomyelinase and ceramidase based on LDL-mediated lysosomal targeting of ceramide-labeled sphingomyelin. J. Lipid Res. 1996;37:2525–2538. [PubMed] [Google Scholar]
- 26.Levade T, Tempesta MC, Salvayre R. The in situ degradation of ceramide, a potential lipid mediator, is not completely impaired in Farber disease. FEBS Lett. 1993;329:306–312. doi: 10.1016/0014-5793(93)80243-n. [DOI] [PubMed] [Google Scholar]
- 27.Levade T, Moser HW, Fensom AH, Harzer K, Moser AB, Salvayre R. Neurodegenerative course in ceramidase deficiency (Farber disease) correlates with the residual lysosomal ceramide turnover in cultured living patient cells. J. Neurol. Sci. 1995;134:108–114. doi: 10.1016/0022-510x(95)00231-0. [DOI] [PubMed] [Google Scholar]
- 28.Chatelut M, Leruth M, Harzer K, Dagan A, Marchesini S, Gatt S, Salvayre R, Courtoy P, Levade T. Natural ceramide is unable to escape the lysosome, in contrast to a fluorescent analogue. FEBS Lett. 1998;426:102–106. doi: 10.1016/s0014-5793(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 29.He X, Li CM, Park JH, Dagan A, Gatt S, Schuchman EH. A fluorescence-based high-performance liquid chromatographic assay to determine acid ceramidase activity. Anal. Biochem. 1999;274:264–269. doi: 10.1006/abio.1999.4284. [DOI] [PubMed] [Google Scholar]
- 30.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 31.Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal. Biochem. 2001;298:141–150. doi: 10.1006/abio.2001.5342. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259–1261. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long-term elimination of natural killer cells in vivo by an anti-interleukin 2 receptor beta chain monoclonal antibody in mice. J. Exp. Med. 1993;178:1103–1107. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatelut M, Harzer K, Christomanou H, Feunteun J, Pieraggi MT, Paton BC, Kishimoto Y, O'Brien JS, Basile JP, Thiers JC, Salvayre R, Levade T. Model SV40-transformed fibroblast lines for metabolic studies of human prosaposin and acid ceramidase deficiencies. Clin. Chim. Acta. 1997;262:61–76. doi: 10.1016/s0009-8981(97)06527-3. [DOI] [PubMed] [Google Scholar]
- 35.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 36.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 37.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 38.Brady RO. Enzyme replacement for lysosomal diseases. Annu. Rev. Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 39.Li CM, Park JH, Simonaro CM, He X, Gordon RE, Friedman AH, Ehleiter D, Paris F, Manova K, Hepbildikler S, Fuks Z, Sandhoff K, Kolesnick R, Schuchman EH. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics. 2002;79:218–224. doi: 10.1006/geno.2002.6686. [DOI] [PubMed] [Google Scholar]
- 40.Krall WJ, Skelton DC, Yu XJ, Riviere I, Lehn P, Mulligan RC, Kohn DB. Increased levels of spliced RNA account for augmented expression from the MFG retroviral vector in hematopoietic cells. Gene Ther. 1996;3:37–48. [PubMed] [Google Scholar]
- 41.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsubir S, Yoshimitsu M, Medin JA. Anti-CD25 targeted killing of bicistronically transduced cells: a novel safety mechanism against retroviral genotoxicity. Mol. Ther. 2007;15:1174–1181. doi: 10.1038/sj.mt.6300147. [DOI] [PubMed] [Google Scholar]
- 43.Medin JA, Brandt JE, Rozler E, Nelson M, Bartholomew A, Li C, Turian J, Chute J, Chung T, Hoffman R. Ex vivo expansion and genetic marking of primitive human and baboon hematopoietic cells. Ann. N. Y. Acad. Sci. 1999;872:233–240. doi: 10.1111/j.1749-6632.1999.tb08468.x. discussion 240-2. [DOI] [PubMed] [Google Scholar]
- 44.Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J. Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonarakis SE, Valle D, Moser HW, Moser A, Qualman SJ, Zinkham WH. Phenotypic variability in siblings with Farber disease. J. Pediatr. 1984;104:406–409. doi: 10.1016/s0022-3476(84)81106-3. [DOI] [PubMed] [Google Scholar]
- 46.Lapidot T, Fajerman Y, Kollet O. Immune-deficient SCID and NOD/SCID mice models as functional assays for studying normal and malignant human hematopoiesis. J. Mol. Med. 1997;75:664–673. doi: 10.1007/s001090050150. [DOI] [PubMed] [Google Scholar]
- 47.Sarzotti M. Immunologic tolerance. Curr. Opin. Hematol. 1997;4:48–52. doi: 10.1097/00062752-199704010-00008. [DOI] [PubMed] [Google Scholar]
- 48.Krivit W, Sung JH, Shapiro EG, Lockman LA. Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant. 1995;4:385–392. doi: 10.1177/096368979500400409. [DOI] [PubMed] [Google Scholar]
- 49.Young PP, Fantz CR, Sands MS. VEGF disrupts the neonatal blood-brain barrier and increases life span after non-ablative BMT in a murine model of congenital neurodegeneration caused by a lysosomal enzyme deficiency. Exp. Neurol. 2004;188:104–114. doi: 10.1016/j.expneurol.2004.03.007. [DOI] [PubMed] [Google Scholar]