The siglec family of glycan binding proteins recognizes sialic acid containing glycans of glycoproteins and glycolipids as ligands. Most are differentially expressed on various white blood cells that mediate immune function, and one of them, myelin associated glycoprotein (MAG), is expressed on glial cells and functions in myelin-axon interactions1. Detailed oligosaccharide specificities toward its natural ligands have been sought to provide the type of information that will help understand their interactions with ligands in situ, and develop ligand based tools to help probe their functions in the cells that express them. In general, siglecs bind with low intrinsic affinity (0.1–1 mM) to their natural sialoside ligands, and while several exhibit unique specificities, most siglecs bind to sialosides containing the common Neu5Acα2–6Gal and/or Neu5Acα2–3Gal linkages2–4.
To investigate the roles of ligand binding in the functions of siglecs, high affinity probes are desired to compete with natural ligands and bind to siglecs on native cells. Several studies have documented the importance of sialic acid substituents (e.g. at the 9C and 5C positions on sialic acid) for modulating the affinity of siglec binding 5–9, and the utility of high affinity 9-substituted sialoside ligands as biological probes of Siglec-2 (CD22) 10,11. To facilitate development of such ligands for other siglecs, we have implemented a strategy of making a sialoside analog library compatible with glycan microarray technology12 to screen for substituents that increase affinity.
The synthesis of sialosides with 9-azido-Neu5Ac opens a route to the facile synthesis of 9-substituted sialic acids. Synthesis of the key azido-intermediates (C, D) with 9-azido-Neu5Acα2–3Gal and 9-azido-Neu5Acα2–6Gal termini was accomplished by enzymatic transfer of 9-azido-Neu5Ac from CMP-9-azido-Neu5Ac13,14 to an oligosaccharide precursor containing a 4-pentenoyl or CBZ protected 2-amino ethyl aglycone (Scheme 1). Portions (1–5 mg) of the azido-intermediates were reduced to the amine with PPh3, and acylated in excess with from a library of acylchlorides (see Table S1 in supporting information). After quenching of excess acylating reagents with water, glycans were deprotected15,16 to expose the amine (E, F) for printing onto amino-reactive N-hydroxy succinimide (NHS) activated glass slides (Schott Nexterion, Slide-H) 12. The 4-pentenoyl protecting group was advantageous in that it could be deprotected in situ with iodine (I2/H2O) and the products printed directly onto slides. In total, the library comprised 16 α2–3 and 28 α2–6 sialosides.
Scheme 1.
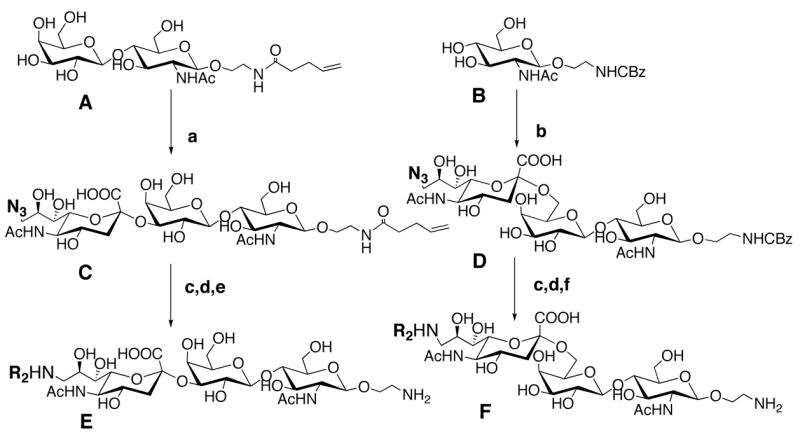
a) ST3Gal and CMP-9-azido-9-deoxy-Neu5Ac; b) GalT-GalE, , UDP-Gal, hST6Gal I, CMP-9-azido-9-deoxy-Neu5Ac; c) PPh3, MeOH:TFA:H2O (4:5:1),; d) acylation, R2COCl (R2=1–34, see Table S1) (2–4 eq), base (5 eq); e) I2(5 eq), MeOH:H2O (9:1); f) Pd/C, H2
To determine if the increased affinity of siglecs towards acyl substituents could be detected on a glycan array, we tested the binding of CD22 (Siglec-2) to an array of selected analogs of the α2–6 sialoside F. The six compounds included one with a 9-N-biphenylcarboxyl (BPC, 15) substituent previously shown to increase affinity for CD22 by 100 fold6,10. The glycans were printed at ten 2-fold dilutions (10 replicates each). Binding was assessed with a fluorescently labeled recombinant CD22-Fc chimera, an antibody like molecule containing two CD22 ligand binding domains, that has been demonstrated to bind to glycan microarrays 12. Binding of CD22 was sialic acid dependent (Figure 1), with binding observed to the natural Neu5Ac containing compound (c) and increased binding to each of the three 9-acyl-substituted compounds (b, d, f). Scanning the slide to measure relative fluorescence allowed comparison of binding levels at each printing concentration (Figure 1B). Relative to the natural sialoside (c), four analogs exhibited stronger binding of CD22, and the 9-BPC substituent (f) produced equivalent binding at 64–128 fold lower printing concentration.
Figure 1.
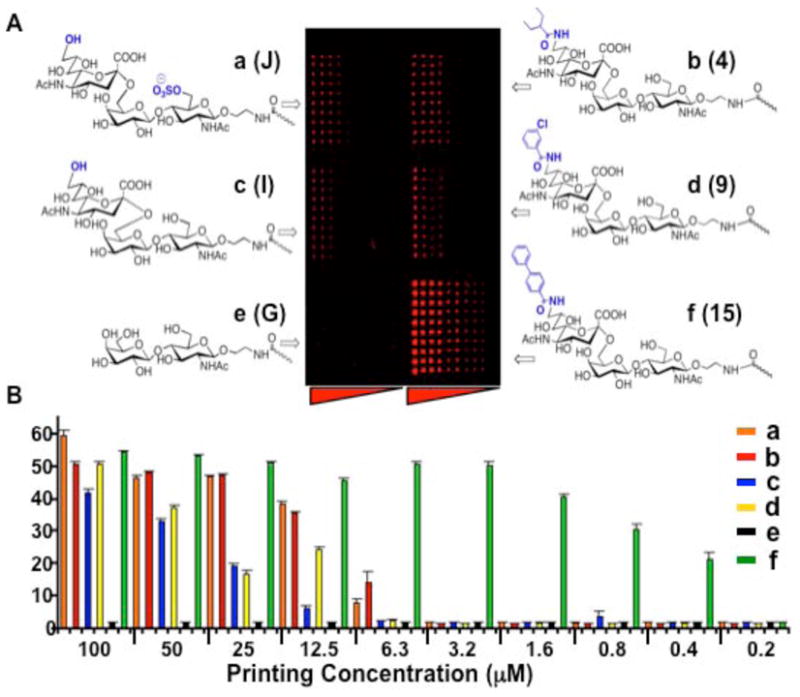
Binding of CD22-Fc chimera to sialoside analogs printed on a glycan array. Sialosides were printed with 10 replicates of 10 two-fold dilutions from 100-0.2 μM (left to right). 9-Acyl-substituents of F are indicated in parentheses (Table 1S). A Fluorescent image of the array labeled with a complex of CD22-Fc chimera and secondary FITC-labeled goat anti-human Fc antibody (see Figure 3). B Relative fluorescence intensity at each printing concentration is compared for the six compounds.
The quality of a glycan array containing the full library was assessed using the binding of the plant lectins, Maccia amurensis agglutinin (MAA) and Sambucus nigra agglutinin (SNA), specific for α2–3- and α2–6-sialosides, respectively. Fortuitously, binding of neither lectin is blocked by 9-acyl substituents, facilitating their use to assess the printing efficiency. Relative to the control compound with no substituent (NeuAc), all 9-acyl substituted E and F sialosides were recognized comparably by MAA and SNA, respectively (Figure 2).
Figure 2.
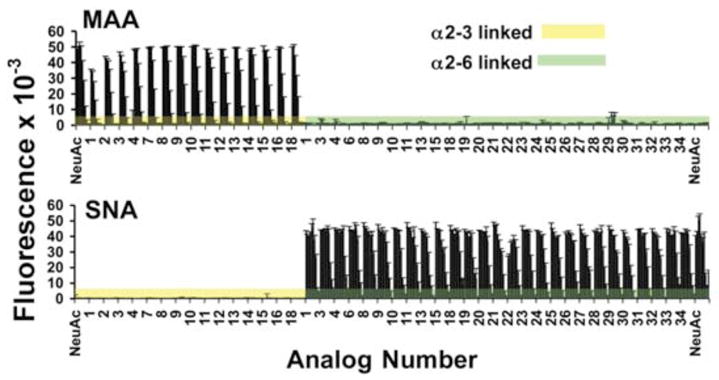
Binding of sialoside specific plant lectins to a sialoside analog glycan array. Biotinylated lectins, MAA (top) and SNA (bottom) specific for Neu5Acα2–3Gal or Neu5Acα2–6Gal linkages, respectively, were mixed with Alexa-fluor 488 labeled streptavidin, overlaid onto the printed analog array, washed and scanned for fluorescence (see Supplementary material). Acyl analogs 1–34 of E (yellow) and F (green) correspond to acyl groups listed in Table S1. Shown is relative fluorescence of lectins bound to each glycan printed at 5 serial dilutions starting at 100 μM. ‘NeuAc’ labeled lanes are sialosides with unsubstituted Neu5Ac in α2–3 (H) and α2–6 (I) linkage to N acetyl-lactosamine.
The full array was then used to assess the specificity of three siglecs, CD22 (Siglec-2), MAG (Siglec-4) and sialoadhesin (Siglec-1). In keeping with its specificity for α2–6 sialosides, CD22 bound only to sialosides in the F series (Figure S2). However, none of the N-acyl substituents exhibited an affinity equivalent to BPC (F15; see Figures 1 and S1).
Surprisingly, Fc chimeras of sialoadhesin and MAG did not bind to sialosides with unsubstituted NeuAc, but bound α2–3 (E) or α2–6 sialosides with selective 9-N-acyl substituents in repeated assays (Figure 3). Sialoadhesin exhibited increased affinities to a few bis-phenyl analogs of E (14, 15), and F (15, 30), with the equivalent binding of the bi-phenyl substituent 15 to both demonstrating that the affinity to the acyl substituent is dominant over the sialic acid to galactose linkage. MAG, bound to sialosides with other substituents including benzoyl- (7) and chloro-benzoyl (8–10) 9-substituted sialoside analogs of either α2–3 (E) or α2–6 (F) sialosides. The results are consistent with previous studies evaluating the affinity of MAG towards 9-N-acyl substituents on α-methyl-NeuAc8.
Figure 3.
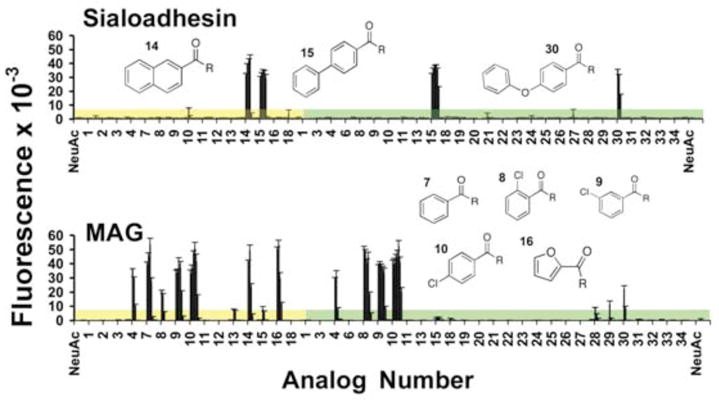
Binding of Siglec-Fc chimeras to the sialoside analog array. Mouse sialoadhesin-Fc chimera (top) and mouse MAG-Fc chimera (bottom) complexed with goat anti-human IgG-FITC (2:1; 50 μg/mL total protein) were overlayed onto the printed analog array, washed for Figure 2). Numbered acyl analogs attached to compound E (yellow) or F (green) are found in Table S1.
To confirm that the signals for MAG resulted from increased affinity, a selected series of related sialosides with 9-acyl substituents (7, 8, 10) of E and F, were re-synthesized and tested for binding by MAG and CD22(Figure S2). While CD22 bound only the α2–6 sialoside (F) series, MAG bound both, with preference for the α2–3 (E) series compounds, consistent with its preference for α2–3 sialosides 2,17. These compounds were also assessed for inhibitory potency in an ELISA competition assay10. Compounds 7E, 8E, and 10E gave IC50 values of 4.9, 9.7 and 2.2 μM, respectively, representing 50–200 fold enhancement of affinity over the unsubstituted α2–3 sialoside H (IC50 of 490 μM; data not shown).
The 9-substituted analog library for E was also prepared with a corresponding 5-benzolyloxy carbonyl (Cbz) substituent, this substituent exhibited no effect or decreased affinity. Nonetheless, this opens a route to 9- and 5- disubstituted sialosides (data not shown).
In summary, we demonstrate that a sialoside analog array allows for the synthesis of sialoside analogs in small quantity for subsequent screening for high affinity ligands of siglecs and other sialoside binding GBPs.
Supplementary Material
General procedures for preparations of sialoside analog library, a complete list of printed analogs, procedures for printing and analysis of GBPs and analytical data for MAA and SNA plant lectins.
Acknowledgments
The authors thank Dr. Paul Crocker for providing cell lines producing siglec-Fc chimeras of MAG and sialoadhisin, the Consortium for Functional Glycomics (GM62116) for the CD22-Fc chimera, Dr. Nicolai Bovin for compound J, and Ms. Anna Tran-Crie for help in preparing this manuscript. This research was supported by NIH grant GM060938.
References
- 1.Crocker PR, Paulson JC, Varki A. Nature Reviews Immunology. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 2.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. J Biol Chem. 2003;278:31007–19. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 3.Alphey MS, Attrill H, Crocker PR, van Aalten DMF. Journal of Biological Chemistry. 2003;278:3372–3377. doi: 10.1074/jbc.M210602200. [DOI] [PubMed] [Google Scholar]
- 4.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Journal of Biological Chemistry. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 5.Kelm S, Brossmer R, Isecke R, Gross HJ, Strenge K, Schauer R. European Journal of Biochemistry. 1998;255:663–672. doi: 10.1046/j.1432-1327.1998.2550663.x. [DOI] [PubMed] [Google Scholar]
- 6.Zaccai NR, Maenaka K, Maenaka T, Crocker PR, Brossmer R, Kelm S, Jones EY. Structure. 2003;11:557–567. doi: 10.1016/s0969-2126(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 7.Schnaar RL, Collins BE, Wright LP, Kiso M, Tropak MB, Roder JC, Crocker PR. Sphingolipids as Signaling Modulators in the Nervous System. 1998;845:92–105. doi: 10.1111/j.1749-6632.1998.tb09664.x. [DOI] [PubMed] [Google Scholar]
- 8.Shelke SV, Gao GP, Mesch S, Gathje H, Kelm S, Schwardt O, Ernst B. Bioorg Med Chem. 2007;15:4951–65. doi: 10.1016/j.bmc.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Oetke C, Brossmer R, Mantey LR, Hinderlich S, Isecke R, Reutter W, Keppler OT, Pawlita M. J Biol Chem. 2002;277:6688–95. doi: 10.1074/jbc.M109973200. [DOI] [PubMed] [Google Scholar]
- 10.Collins BE, Blixt O, Han SF, Duong B, Li HY, Nathan JK, Bovin N, Paulson JC. Journal of Immunology. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- 11.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. J Exp Med. 2002;195:1207–13. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Proc Natl AcadSci U S A. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blixt O, Paulson JC. Adv Synth Catal. 2003;345:687–690. [Google Scholar]
- 14.Han S, Collins BE, Bengtson P, Paulson JC. Nat Chem Biol. 2005;1:93–7. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 15.Madsen R, Roberts C, Fraserreid B. Journal of Organic Chemistry. 1995;60:7920–7926. [Google Scholar]
- 16.Barstrom M, Bengtsson M, Blixt O, Norberg T. Carbohydrate Research. 2000;328:525–531. doi: 10.1016/s0008-6215(00)00128-2. [DOI] [PubMed] [Google Scholar]
- 17.Strenge K, Schauer R, Bovin N, Hasegawa A, Ishida H, Kiso M, Kelm S. Eur J Biochem. 1998;258:677–85. doi: 10.1046/j.1432-1327.1998.2580677.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General procedures for preparations of sialoside analog library, a complete list of printed analogs, procedures for printing and analysis of GBPs and analytical data for MAA and SNA plant lectins.


