Abstract
Diabetes (DM) contributes to the exacerbation of left ventricle (LV) dysfunction after myocardial infarction (MI). Activation of ERK5, an atypical mitogen activated protein kinase with transcriptional activity, inhibits apoptosis and LV dysfunction after doxorubicin treatment. SUMOylation has been proposed as a negative regulator of various transcription factors. In the current study, we investigated the role of ERK5-SUMOylation in ERK5 transcriptional activity as well as on DM-mediated exacerbation of LV dysfunction and apoptosis after MI. ERK5 wild type transcriptional activity was inhibited by Ubc9 (SUMO E2 conjugase) or PIAS1 (E3 ligase), but not in the ERK5-SUMOylation-site defective mutant (K6R/K22R). H2O2 and high glucose, two well-known mediators of diabetes, induced ERK5-SUMOylation, and the K6R/K22R mutant, dominant negative form of Ubc9, and siRNA-PIAS1 reversed H2O2-mediated reduction of ERK5 transcriptional activity in cardiomyocytes, indicating the presence of SUMOylation-dependent ERK5 transcriptional repression. Constitutively active form of MEK5α (CA-MEK5α) inhibited ERK5-SUMOylation independent of kinase activity, but dependent on MEK5-ERK5 association. To investigate the pathological role of ERK5-SUMOylation in DM mice after MI, we utilized cardiac specific CA-MEK5α transgenic mice (CA-MEK5α-Tg). MI was induced in streptozotocin (STZ)-injected (DM + MI group) or vehicle-injected mice (MI group) by ligating the left coronary artery. The ERK5-SUMOylation was increased in the DM + MI, but not in the MI group. ERK5-SUMOylation, the exacerbation of LV dysfunction, and the number of TUNEL positive cells in DM + MI was significantly inhibited in CA-MEK5α-Tg mice. Of note, we could not detect any difference of cardiac function after MI in non-diabetic CA-MEK5α-Tg and non-transgenic littermate control mice. These results demonstrated that ERK5 transcriptional activity is subject to down regulation by diabetes-dependent SUMOylation, which resulted in a pro-apoptotic condition contributing to poor post-MI LV function.
Keywords: ERK5, SUMOylation, diabetes, myocardial infarction, apoptosis
INTRODUCTION
Diabetes is an independent risk factor for both mortality and morbidity after myocardial infarction (MI) 1, 2. A number of clinical studies have shown that the post-MI left ventricular (LV) function is significantly worse in diabetic compared with non-diabetic patients 3, 4. However, what is lacking is a plausible relationship between diabetes and any of the known regulators of myocyte apoptosis known to play a significant role in the post-MI cardiac dysfunction. Previously, we have demonstrated that down-regulation of phosphodiesterase 3A (PDE3A) is associated with apoptosis and induction of inducible cAMP early repressor (ICER), a proapoptotic transcriptional repressor, providing a mechanistic framework for how angiotensin II (Ang II) regulates myocyte apoptosis 5, 6. Sustained elevation of ICER favors apoptosis through inhibition of cAMP response element binding protein (CREB)-mediated transcription and down-regulation of Bcl-2. Interactions between PDE3A and ICER constitute an auto regulatory positive feedback loop (PDE3A-ICER feedback loop) likely to determine the fate of injured myocytes. ERK5, an atypical mitogen activated protein kinase with transcriptional activity, negatively regulates the executor PDE3A-ICER feedback loop and subsequent apoptosis 7.
Our recent data indicated that ERK5 transcriptional activity itself is subjected to down regulation by reactive oxygen species (ROS) and advanced glycogen endproducts (AGE)-dependent small ubiquitin-related modification (SUMO), and inhibits KLF2 and eNOS expression in endothelial cells 8. ERK5-SUMOylation at the NH2-terminal region (K6 and K22) significantly inhibits COOH-terminal ERK5 transcriptional activity 8. Posttranslational modification by SUMO commonly regulates the assembly and disassembly of protein complexes, protein localization, stability, and function 9. SUMOylation is known to be highly substoichiometric because it often generates intermediates that result in new protein interactions or conformational states that persist even after SUMO removal 9.
In the current study, we showed that ERK5 is one of the major targets of SUMOylation in diabetic hearts. We found that SUMOylation-dependent ERK5 transcriptional repression induced by ROS and high glucose. Constitutively active form of MEK5α (CA-MEK5α) inhibited ERK5-SUMOylation independent of its kinase activity, but dependent on MEK5-ERK5 association. Diabetic hearts demonstrating exacerbation of LV dysfunction after myocardial infarction (MI) were accompanied by enhanced ERK5-SUMOylation. The inhibition of ERK5-SUMOylation by CA-MEK5α significantly improved the cardiac function after MI in diabetic mice, but not in non-diabetic mice. These data suggested the importance of ERK5-SUMOylation on ROS-mediated ERK5 transcriptional repression, which contributes to poor cardiac function after MI in diabetes.
MATERIALS and METHODS
An expanded Materials and Methods is available in the online-only Data Supplement
Details on reagents, antibodies, plasmid and adenovirus vectors construction, cell culture, mammalian one-hybrid analysis and transfection of cells, immunoprecipitation (SUMOylation assay) and Western blot analysis, transfection of the PIAS1 siRNA, analysis of apoptosis, animal models, streptozotocin (STZ) injections, coronary ligation surgery, echocardiographic analysis and in vivo hemodynamic measurements, and statistical analysis are also provided in the online Data Supplement.
RESULTS
Streptozotocin (STZ)-induced hyperglycemia exacerbates LV dysfunction and failure after an experimental MI
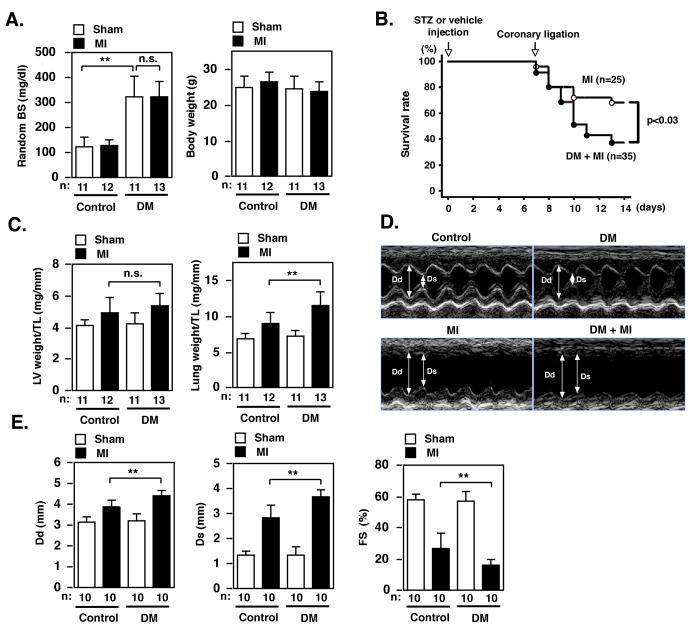
Diabetes adversely affects LV dysfunction after MI and leads to a higher incidence of heart failure but the exact mechanism of diabetes-mediated exacerbation of LV dysfunction remains largely unknown. First, we established a diabetic mouse model in our lab and demonstrated the development of heart failure after MI as previously described10. Four groups of mice were studied. Diabetes mellitus (DM) was induced in male FVB mice (5 to 8 weeks old and 25 to 35 g body weight) by intraperitoneal injection of STZ (200 mg/kg body weight)10, 11. Tail vein blood glucose samples were measured 6 days after injection to ensure induction of hyperglycemia. As a control, vehicle (0.1 mol/l citrate buffer, pH 4.5) was injected in another group of mice. At 7 days after injection, MI was induced in STZ-injected (DM+MI group) or vehicle-injected mice (MI group) by ligating the left coronary artery10, 12. We have carefully characterized the fasting glucose level after different doses of intraperitoneal injection of STZ in FVB mice and found that one injection of 200 mg/kg body weight STZ resulted in fasting blood glucose (FBG) of around 200 mg/dl at one week after injection. Since mice with extremely high glucose level (> 400 mg/dl) did not tolerate the coronary ligation surgery well, we chose a STZ dose that resulted in this moderately high FBG as the diabetic mice model for our study. A sham operation without ligating the coronary artery was also performed in additional groups of STZ-injected (DM group) and saline-injected mice (control group). All four groups of mice (control, DM, MI, and DM+MI) were followed up for another one week (one-week post-MI study). STZ treatment significantly increased random blood sugar (BS) levels 2 weeks after injection (Fig.1A, left panel). Of note, we did not observe any significant body weight difference between the MI and DM + MI groups at one week after injection and at the time of surgery (Fig.1A, right panel), and at one week after surgery (Table S1). Survival at one week after MI was significantly lower in the DM + MI versus MI group (Fig.1B).
Figure 1. STZ-induced hyperglycemia exacerbates LV dysfunction and mortality after MI.
(A) Random blood sugar (BS) and body weight one week after injection of STZ (DM) or vehicle (control) in sham or coronary ligation (MI) groups. (B) Kaplan-Meier survival analysis. Survival rate in diabetes mellitus (DM) + myocardial infarction (MI) (n = 35) and MI (n = 25) mice were plotted. Overall survival was significantly lower in DM + MI compared with MI mice. (C) LV weight/tibial length (TL) and lung weight/TL one week after sham or coronary ligation (MI) in STZ (DM) or vehicle (control) treated groups. (D, E) Echocardiogram obtained from control (sham and no STZ), DM (sham and STZ), MI (coronary ligation and no STZ), and DM + MI (coronary ligation and STZ) mouse one week after surgery. Dd = end-diastolic diameter, Ds = end-systolic diameter. FS = fractional shortening. n = number of the animals.
We could not detect any difference in LV weight/ tibial length (TL) between the MI and DM + MI groups. But lung weight/ TL was increased in the MI group, which was significantly exacerbated in the DM + MI group (Fig.1C), suggesting a more severe congestive heart failure in the DM + MI group than MI group. Echocardiography one week after ligation showed LV dilatation and dysfunction in the MI group but with both measures exaggerated in the DM + MI group (Fig.1D, E, and Table S1).
ERK5-SUMOylation in DM + MI model
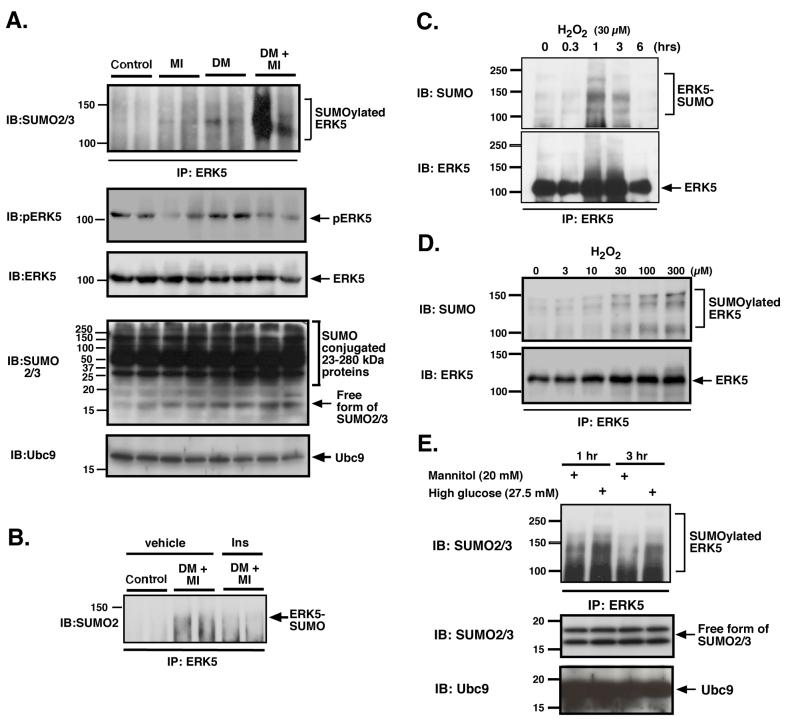
Recently, we have reported the importance of ERK5-SUMOylation on regulating its transcriptional activity 8. H2O2 and advanced glycation end products (AGE), two-well-known mediators of diabetes, negatively regulated ERK5 transcriptional activity via ERK5-SUMOylation in endothelial cells. Since ERK5 demonstrates a cardio-protective effect 7, we investigated the effect of STZ-mediated hyperglycemia and MI on the ERK5-SUMOylation. As shown in Fig.2, we found a slight increase of ERK5-SUMOylation in both the DM (with sham operation) and MI (without STZ treatment) groups. In contrast, the ERK5-SUMOylation was significantly exaggerated in the DM + MI group. These data suggested the possible role of ERK5-SUMOylation on the exaggerated LV dysfunction and development of heart failure after MI in DM mice. In contrast, a reduction of ERK5 phosphorylation was observed in both MI and DM + MI groups compared with non-MI control and DM groups. No significant difference in ERK5 phosphorylation was observed in MI vs. DM + MI groups suggesting that ERK5 phosphorylation and its subsequent kinase activation may not be involved in the exaggerated LV dysfunction after MI in DM mice.
Figure 2. ERK5-SUMOylation was increased in DM + MI mice and induced by H2O2 and high glucose in cardiomyocytes.
Heart samples from 4 groups of mice (control, MI, DM, and DM + MI) were immunoprecipitated with anti-ERK5 antibody and Western blot analysis was performed with anti-SUMO2/3 antibody (top). A significant decrease in ERK5 phosphorylation was observed in both MI and DM + MI groups (2nd from top) but with no difference in the amount of total ERK5 (3nd from top). Immunoblot with SUMO2/3 antibody (4th from top) and Ubc9 antibody (bottom) confirmed no significant differences in the general SUMOylation of proteins and the Ubc9 expression between samples. (B) Heart samples from control and DM+MI mice without or with pre-operative insulin treatment subjected to IP with ERK5 antibody and IB with anti-SUMO2/3 antibody. The pre-operative insulin treatment decreased ERK5-SUMOylation (arrow) in the DM+MI group. Cardiomyocytes were treated with H2O2 (C, D) or high glucose and mannitol (E) and probed for SUMOylated ERK5. (C) Cells treated for the indicated time were lysed and immunoprecipitated using anti-ERK5 antibody, then blotted with anti-SUMO2/3 (top) and ERK5 (bottom) antibodies. (D) H2O2-mediated ERK5-SUMOylation was measured at one hr at the indicated concentration of H2O2. (top). No significant difference of ERK5 expression among the samples was observed (bottom). (E) After treatment of cardiomyocytes with high glucose or mannitol, cells were lysed and immunoprecipitated with anti-ERK5 antibody and Western blot analysis was performed with anti-SUMO2/3 antibody (top). No significant difference of free forms of SUMO2/3 (middle) and Ubc9 (bottom) expression among the samples was observed. Data is representative of results from experiments performed on at least three separate occasions.
We investigated the possible cardio-toxic effect of STZ, as opposed to hyperglycemia, by administering insulin (60 units/kg subcutaneous b.i.d. NPH human insulin (HumulinN; Eli Lilly, Indianapolis, IN)) 1 - 2 days prior to the coronary ligation surgery to normalize the FBG and determined infarct size, cardiac function and the biochemical markers such as ERK5-SUMOylation after MI as previously described13. We demonstrated that pre-operative insulin treatment of STZ-injected mice decreased the FBG to 157.6 ± 49.5 mg/dl. Such pre-operative insulin-treatment did not result in significant differences in basal cardiac function from nondiabetic MI control (data not shown). In addition, we could not detect any increase in ERK5-SUMOylation after insulin-treatment, which was observed in the hyperglycemic DM + MI mice (Fig.2B). Therefore, we concluded that the exacerbation of cardiac damage and ERK5-SUMOylation in DM + MI model was due to hyperglycemia and not to STZ toxicity.
One of the major concerns of this study is whether the observed differences between the groups can be accounted for by metabolic perturbations (particularly malnutrition and weight loss in the diabetic animals), or by the direct effects of STZ on the myocardium. We carefully examined this issue and found that both MI alone and DM + MI animals had equally low body weights, but DM + MI mice showed greater cardiac damage (Fig.1A, Table S1).
H2O2 inhibited ERK5 transcriptional activity and induce apoptosis via ERK5-SUMOylation
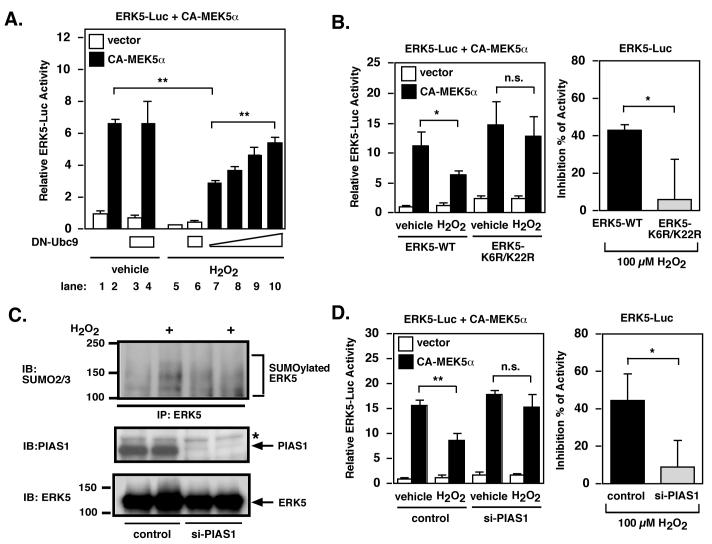
Since we found ERK5-SUMOylation in the DM + MI group, and ERK5-SUMOylation could decrease ERK5 transcriptional activity and possibly its cardio-protective effect, we investigated whether H2O2 and high glucose can induce endogenous ERK5-SUMOylation. As shown in Fig.2C and D, H2O2 significantly increased ERK5-SUMOylation in a time and dose dependent manner. High glucose, but not mannitol (20 mM) as a control, also increased ERK5-SUMOylation (Fig.2E). Previously, we reported that H2O2 and advanced glycation end products (AGE) inhibited ERK5 transcriptional activity via ERK5-SUMOylation in endothelial cells 8. Since we found that H2O2 and high glucose could induce ERK5-SUMOylation in cardiomyocytes (Fig.2C-E), we investigated whether ERK5-SUMOylation was important in the H2O2-mediated reduction of ERK5 transcriptional activity in cardiomyocytes. If SUMOylation of ERK5 underlies the H2O2-mediated reduction of its transcriptional activity, inhibition of SUMOylation by co-expression of the dominant negative form of Ubc9 (DN-Ubc9) should interfere with this reduction in transcriptional activity. As shown in Fig. 3A, H2O2 (30 μM) decreased ERK5 transcriptional activity by approximately 56 %, but this reduction in the transcriptional activity was less in DN-Ubc9 transfected cardiomyocytes in a DN-Ubc9 dose-dependent manner.
Figure 3. H2O2 inhibits ERK5 transcriptional activity through SUMOylation in cardiomyocytes.
(A) CA-MEK5α-induced ERK5 transcriptional activity was measured with DN-Ubc9 in a dose-dependent manner (lane 3-4 and 10, 1 μg/3 wells, lane 7, 0 μg/3 wells, lane 8, 0.1 μg/3 wells, lane 9, 0.5 μg/3 wells) with vehicle (lane 1-4) or H2O2 (lane 5-10) as indicated. For all figures without indication data are representative of triplicates using 2 or more different preparation of cells. Results are mean ± SD. *P < 0.05, **P < 0.01. (B) CA-MEK5α-induced ERK5 transcriptional activity was measured for wild-type ERK5 or ERK5 (K6/22R)-mutant in the presence of H2O2 (B) in cardiomyocytes (left panel). The right panel shows as % inhibition of reporter activity and calculated from the activity of the reporter under each condition in the presence and absence (taken as 100 %) of H2O2 as indicated. (C) Cardiomyocytes were transfected with siRNA targeting PIAS1 or control RNA. After 24 hrs, cells were treated with H2O2 for 1 hr. Cells were lysed and immunoprecipitated using anti-ERK5 antibodies, followed by immunoblotting with anti-SUMO2/3 and anti-ERK5 antibodies. Total cell lysates (50 μg/lane) were separated by 8% SDS-PAGE and immunoblotted with anti-PIAS1 and ERK5 antibodies. (D) CA-MEK5α-induced ERK5 transcriptional activity with or without H2O2 was measured, as described for panel B, but in myocytes treated with PIAS1 si-RNA.
Next, we compared the H2O2 (30 μM)-induced reduction of transcriptional activity in the wild type and SUMOylation sites-mutant (K6R/K22R) ERK5. As shown in Fig. 3B, H2O2-induced reduction of ERK5 transcriptional activity was significantly less in the ERK5-K6R/K22R mutant. To further confirm the involvement of ERK5-SUMOylation in transcriptional regulation, we tested whether deletion of PIAS1 using PIAS1 siRNA could inhibit the H2O2-mediated reduction of ERK5 transcriptional activity. PIAS1 siRNA inhibited PIAS1 expression and H2O2-mediated ERK5-SUMOylation in cardiomyocytes (Fig.3C). Furthermore, PIAS1 siRNA significantly prevented H2O2-mediated reduction of ERK5 transcriptional activity (Fig 3D). Taken together, these data strongly suggested the critical role of ERK5-SUMOylation in H2O2-mediated reduction of ERK5 transcriptional activity.
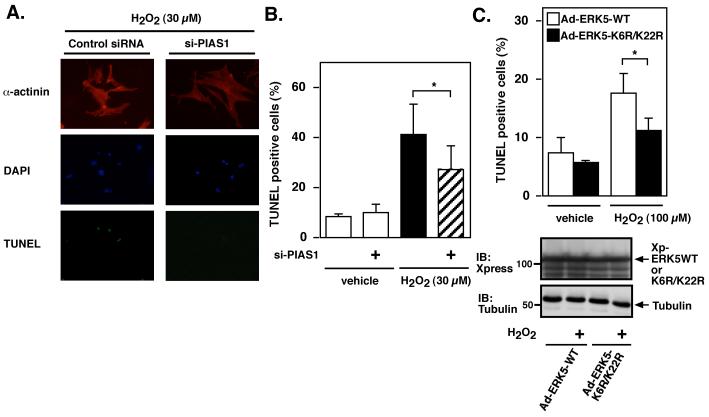
We have reported previously the importance of ERK5 in the regulation of cardiomyocyte apoptosis 7. Therefore, we investigated whether ERK5-SUMOylation was involved in the H2O2-mediated apoptosis. As shown in Fig.4, in this model the deletion of PIAS1 significantly inhibited H2O2-mediated apoptosis. To further support the critical role of ERK5-SUMOylation in regulating cardiomyocyte apoptosis, we generated two adenovirus vectors expressing the ERK5 wild type and ERK5 K6R/K22R mutant. Sixteen hrs after transduction with adenovirus vector containing Xpress tagged-ERK5 wild type (Ad-ERK5-WT) or ERK5 K6R/K22R mutant (Ad-ERK5 K6R/K22R), cardiomyocytes were treated with H2O2 (100 μM) for 24 hrs, and apoptosis measured by TUNEL staining. We found that expression of ERK5-SUMOylation site-mutant significantly inhibited H2O2-induced apoptosis (Fig.4C). No difference in the amount of ERK5 wild type and K6R/K22R mutant expression was confirmed in samples by immunoblotting with anti-Xpress antibody (Fig.4C). Of note, we needed to increase the concentration of H2O2 from 30 μM to 100 μM in this set of experiments because we could not detect significant increase of TUNEL positive cells in Ad-ERK5 WT tranduced cells with 30 μM H2O2 treatment probably due to the protective effect of ERK5 wild type overexpression.
Figure 4. Role of PIAS1 in H2O2-mediated cardiomyocytes apoptosis.
Cardiomyocytes were transfected with (A-B) PIAS1 or control siRNA as described in methods and (C) transduced with adenovirus vector containing Xpress-tagged ERK5 wild type (Ad-ERK5-WT) or ERK5 K6R/K22R mutant (Ad-ERK5 K6R/K22R) as we described previously 6. After 24 hrs of serum deprivation, cells were treated with H2O2 (A-B, 30 μM or C, 100 μM) for 24 hrs. Cardiomyocytes apoptosis was measured by TUNEL staining. (A) Representative pictures showed TUNEL, anti-α-actinin and 4′,6-diamidino-2-phenylindole (DAPI) staining. (B and C) Data represent mean ± SD of ≥ 3 culture preparation. *P < 0.05. (C) No difference in the amount of ERK5 wild type, K6R/K22R mutant and tubulin expression was observed in samples by Western blot analysis with anti-Xpress or tubulin antibody.
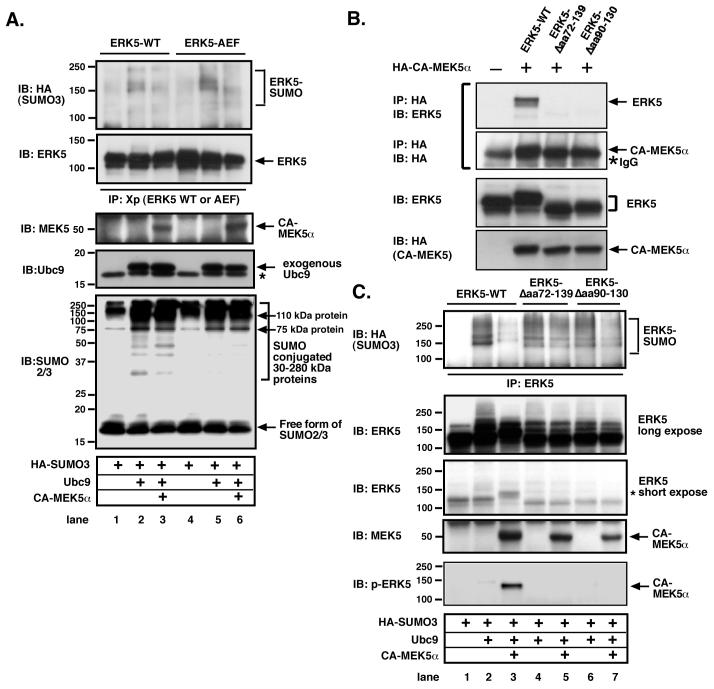
Interaction of MEK5α with ERK5 inhibits ERK5-SUMOylation but not MEK5α-mediated ERK5 kinase activation
It has been reported that ERK5 activation inhibits MEF2-SUMOylation via MEF2-Ser179 phosphorylation, and increases MEF2 transcriptional activity14. Therefore, we investigated whether MEK5 activation induced by CA-MEK5α can similarly inhibit Ubc9 and SUMO3-mediated ERK5-SUMOylation. As shown in Fig.5A, CA-MEK5α significantly inhibited Ubc9 and SUMO3-mediated ERK5-SUMOylation (lane1-3). These data suggested that CA-MEK5α could potentially transcriptionally activate ERK5 via inhibiting SUMOylation, and provides a novel mechanism of MEK5α activation of ERK5 via inhibition of ERK5-SUMOylation.
Figure 5. Association of MEK5 with ERK5, but not ERK5 kinase activation and phosphorylation, inhibits ERK5-SUMOylation.
(A) CA-MEK5α-mediated reduction of ERK5-SUMOylation was observed in both ERK5 wild type and kinase dead TEY motif mutant (ERK5-AEF). CHO cells were co-transfected with HA-SUMO3, Ubc9, DN-Ubc9 and Xp-tagged ERK5 (WT or ERK5-AEF kinase dead mutant) with CA-MEK5α or control cDNA as indicated and ERK5-SUMOylation assay performed as described above. No difference in the amount of ERK5 wild type and mutants (2nd from the top), CA-MEK5α (3rd from top), or Ubc9 (4th from top) was observed in samples by Western blot analysis. The expression of free forms of SUMO2/3 and SUMO conjugated 30-280 kDa proteins were detected by immunoblotting with anti-SUMO2/3 antibody (bottom). (B) Both MEK5 binding site truncated mutants of ERK5 (ERK5-Δaa72-139, and ERK5-Δaa90-130) did not associate with MEK5α. Cells were co-transfected with Xp-tagged ERK5 (WT or ERK5-Δaa72-139, and ERK5-Δaa90-130) with HA tagged CA-MEK5α or control cDNA as indicated. After 24 hrs of transfection, whole cell extract was immunoprecipitated with anti-HA antibody and Western blot analysis was performed with anti-HA (upper) or ERK5 (lower) antibody. No difference in the amount of ERK5 wild type and mutants or HA-CA-MEK5α was observed in samples by Western blot analysis. (C) Both MEK5 binding site truncated mutants of ERK5 (ERK5-Δaa72-139, and ERK5-Δaa90-130) inhibited CA-MEK5α-mediated reduction of ERK5-SUMOylation. Cells were co-transfected with HA-SUMO3, Ubc9, DN-Ubc9 and Xp-tagged ERK5 (WT or ERK5-Δaa72-139, and ERK5-Δaa90-130) with CA-MEK5α or control cDNA as indicated and performed ERK5-SUMOylation assay as described above. No difference in the amount of ERK5 wild type and mutants (2nd and 3rd from the top), or CA-MEK5α (4th from the top) was observed in samples by Western blot analysis. * activated (phosphorylated) form of ERK5 showed band shift as previously described 19 (3rd from the top and bottom). Data is representative of results from experiments performed on at least three separate occasions.
To determine the effect of MEK5α-mediated ERK5 phosphorylation on ERK5-SUMOylation, we mutated the TEY phosphorylation motif of ERK5, which is critical for ERK5 kinase activation15, and investigated whether these mutations can affect the CA-MEK5α-mediated inhibition of ERK5-SUMOylation. As shown in Fig. 5A (lane 4-6), although it is well known that the ERK5 kinase cannot be activated without the TEY motif phosphorylation 15, mutation of this motif had no effect on CA-MEK5α-mediated reduction of ERK5-SUMOylation. This suggested that both ERK5 kinase activation and TEY motif phosphorylation were not involved in the CA-MEK5α-induced inhibition of ERK5-SUMOylation. Of note, despite the presence of CA-MEK5α-induced inhibition of ERK5-SUMOylation, no significant difference in the SUMO conjugated 75 and 110 kDa proteins and other SUMO conjugated proteins between 30 to 280 kDa with or without CA-MEK5α transfection was noted. These data suggested that CA-MEK5α may have a unique regulatory role in ERK5-SUMOylation distinct from the general SUMOylation process induced by Ubc9/SUMO3.
Residues 78-139 of ERK5 constitutes the MEK5α binding domain 16. To investigate the contribution of MEK5-ERK5 association on CA-MEK5α-induced inhibition of ERK5-SUMOylation, we generated two deletion mutants of the MEK5α-ERK5 association site, Δ72-139 and Δ90-130, with respective deletions of the numbered residues. Co-immunoprecipitation studies confirmed the lack of association between these Δ-mutants and MEK5α (Fig.5B). Cotransfection of HA-SUMO3 and Ubc9 induced ERK5-SUMOylation, which was inhibited by CA-MEK5α induction (Fig. 5C, lane 1-3). As shown in Fig.5C (lane 4-7), both MEK5 binding site Δ-mutants were SUMOylated, but CA-MEK5α no longer substantially inhibited ERK5-SUMOylation compared with WT. These data indicate the critical role of ERK5-MEK5 association, but not ERK5 kinase activation and TEY motif phosphorylation, on CA-MEK5α-mediated inhibition of ERK5-SUMOylation.
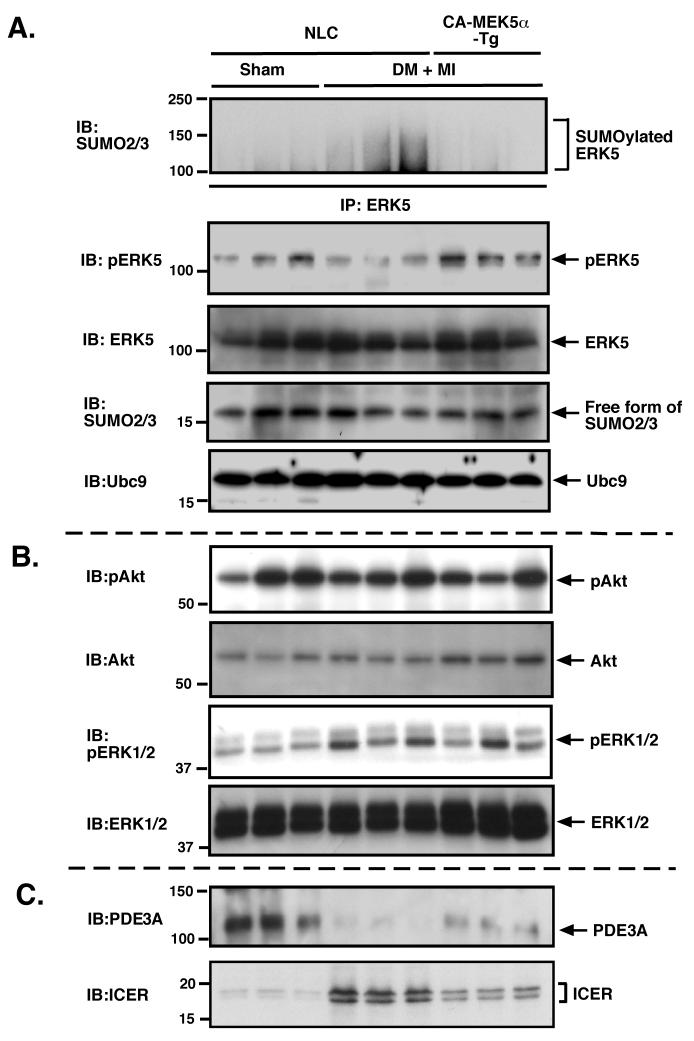
ERK5-SUMOylation, PDE3A-ICER feedback loop, and apoptosis in DM + MI mice was significantly inhibited in CA-MEK5α-Tg mice
As shown in Fig.2, we found that ERK5-SUMOylation was significantly increased in DM + MI mice heart. However, the involvement of ERK5-SUMOylation on diabetes-mediated exacerbation of LV dysfunction after MI remains unclear. Since CA-MEK5α-mediated MEK5-ERK5 association inhibited ERK5-SUMOylation, in vitro, we investigated whether DM-mediated ERK5-SUMOylation might be inhibited in cardiac specific CA-MEK5α-Tg mice. As shown in Fig.6A (top panel), ERK5-SUMOylation was significantly increased at one week after MI in diabetic non-transgenic littermate control (NLC) mice. In contrast, we did not find any significant MI-induced increase in ERK5-SUMOylation in diabetic CA-MEK5α-Tg mice. Previously, we have reported on the critical role of ERK5 activation in regulating the PDE3A/ICER feedback loop in a heart failure model 7. Since we found that ERK5-SUMOylation inhibited ERK5 transcriptional activity, we determined whether the induction of PDE3A/ICER feedback loop in DM + MI mice can also be inhibited in the CA-MEK5α-Tg mice. As shown in Fig.6C, we found a reduction of PDE3A and ICER induction at one week after MI in diabetic NLC mice but significantly inhibited in CA-MEK5α-Tg mice. ERK5 phosphorylation was decreased in diabetic NLC mice after MI as we have shown in Fig. 2A, but was recovered in CA-MEK5α-Tg mice (Fig.6A, 2nd panel from the top). No significant differences in Akt and activation between NLC and CA-MEK5α-Tg mice were observed in this model (Fig.6B, top and 2nd from the top). In contrast to ERK5, we found that ERK1/2 phosphorylation was increased in both diabetic NLC and CA-MEK5α-Tg mice after MI, which suggested the different regulatory mechanism of ERK1/2 compared with ERK5 after MI in DM mice (Fig.6B, 3rd from the top and bottom). Thus ERK5 activation as well as subsequent inhibition of ERK5-SUMOylation regulates the PDE3A/ICER feedback loop in DM + MI model.
Figure 6. ERK5-SUMOylation and PDE3A-ICER feedback loop after MI in diabetic-CA-MEK5α-Tg mice.
(A) Heart samples from control and DM + MI groups in NLC mice and DM + MI group in CA-MEK5α-Tg mice were collected and ERK5-SUMOylation assay performed as described in methods (top). ERK5 phosphorylation was detected by anti-phospho specific ERK5 antibody (2nd from top). No difference in the amount of ERK5 (3rd from top), free form of SUMO2/3 (4th from top), and Ubc9 (bottom) were observed in samples by Western blot analysis. Western blots showing Akt and ERK1/2 activity (B), and PDE3A and ICER expression (C) in the same samples as we previously described 7.
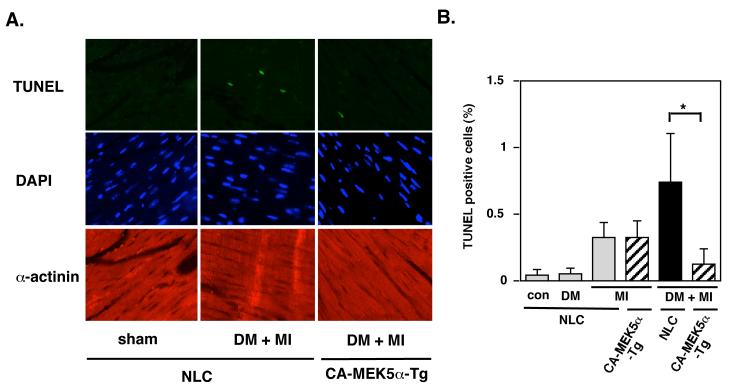
Apoptosis is believed to be important for the development of heart failure 17. In NLC mice hearts one week after MI, the number of in situ TUNEL positive cells increased to 0.32 ± 0.12 %, with a greater increase to 0.91 ± 0.39 % in the remote areas from the DM + MI model (Fig.7B). We found significantly decreased TUNEL positive cells in diabetic CA-MEK5α-Tg mice compared with diabetic NLC mice at the same time point after MI (Fig.7B). These data support the antagonistic role of ERK5 activation in DM-mediated exacerbation of cardiac apoptosis after MI.
Figure 7. Apoptosis in the remodeling area was enhanced in DM + MI group compared with MI alone group, which was significantly attenuated in CA-MEK5α-Tg mice.
TUNEL-positive cardiomyocytes were counted in the remodeling area of each animal. The animals were subjected to either MI or sham and allowed to survive for one week. TUNEL-positive myocytes were counted among more than 1000-4000 nuclei in the remodeling area of each animal. (A) Representative pictures of TUNEL (top), 4’,6-diamidino-2-phenylindole (DAPI) (middle), and α-actinin (bottom) staining of the remote areas from NLC and CA-MEK5α-Tg subjected to MI with or without diabetes as indicated. Note that there were significantly fewer TUNEL-positive myocytes in the remote area after MI in diabetic CA-MEK5α-Tg than in NLC mice. (B) A bar graph summary of TUNEL positive cells (%). Data represent mean ± SD (n = 5). *P < 0.05.
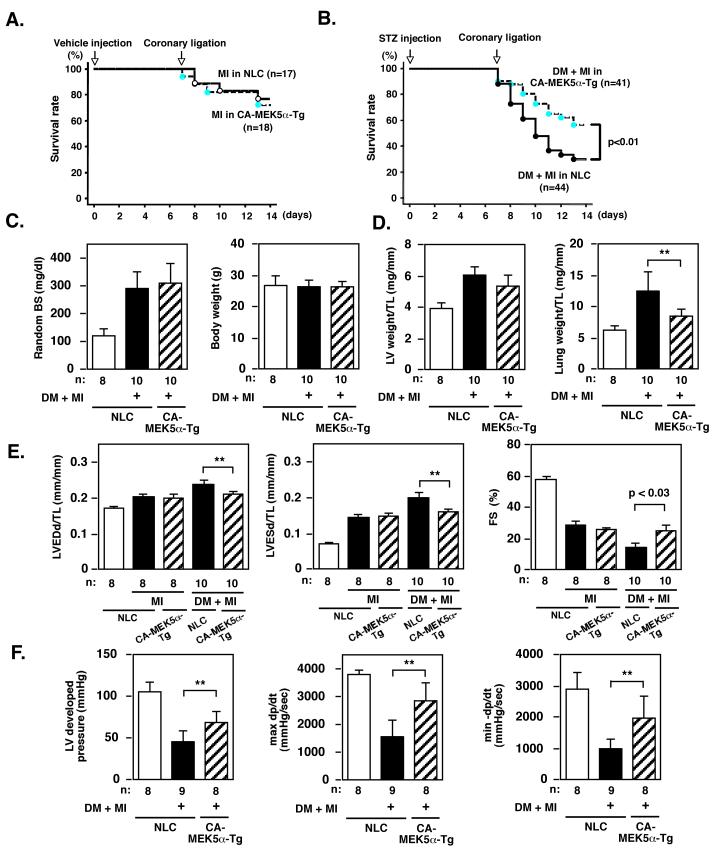
Cardiac dysfunction one week after MI in DM, but not in non-DM mice, was rescued in CA-MEK5α-Tg mice
Since we found an inhibitory role for CA-MEK5α on ERK5-SUMOylation and subsequent PDE3A and the ICER feedback loop and apoptosis, we next investigated whether the inhibition of ERK5-SUMOylation by CA-MEK5α can prevent the exacerbation of LV dysfunction and heart failure after MI in DM mice in vivo. No survival difference was noted between NLC and CA-MEK5α-Tg mice at the one week time point after MI in non-diabetic mice (Fig.8A). In contrast, in STZ-treated DM mice, the survival rate for CA-MEK5α was significantly higher than for NLC mice (Fig.8B). Random BS levels were elevated in both NLC and CA-MEK5α-Tg mice one week after STZ injection and at the time of surgery, but we did not find any difference in the body weight among these groups (Fig.8C).
Figure 8. MEK5 activation inhibited the exacerbation of LV dysfunction after MI in diabetic, but not in non-diabetic mice.
(A) Kaplan-Meier survival analysis in non-diabetic NLC and CA-MEK5α-Tg after MI. Survival rate in NLC (n = 17) and CA-MEK5α-Tg (n = 18) mice after MI were plotted. There was no significant difference between these two groups in the non-diabetic condition. (B) Kaplan-Meier survival analysis in diabetic NLC and CA-MEK5α-Tg after MI. Survival rate in NLC (n = 44) and CA-MEK5α-Tg (n = 41) mice after MI were plotted. Overall survival was significantly higher in CA-MEK5α-Tg compared with NLC mice. *p < 0.05 compared with the NLC group. (C) Random blood sugar (BS) and body weight one week after injection of STZ with coronary ligation (DM + MI) or vehicle injection with sham operation groups in NLC or CA-MEK5α-Tg mice as indicated. (D) LV weight/tibial length (TL) and lung weight/TL in STZ-treated coronary ligation (DM + MI) or vehicle-treated sham operation groups in NLC or CA-MEK5α-Tg mice one week after surgery as indicated. (E) Echocardiographic data obtained from STZ-treated coronary ligation (DM + MI), vehicle-treated coronary ligation (MI), or vehicle-treated sham operation groups in NLC or CA-MEK5α-Tg mice one week after surgery as indicated. LVEDd; left ventricle end-diastolic dimension, LVESd; left ventricle end-systolic dimension, TL; Tibial length. (F) Hemodynamic measurements in STZ-treated coronary ligation (DM + MI) or vehicle-treated sham operation groups in NLC or CA-MEK5α-Tg mice one week after surgery as indicated. All data are expressed as mean ± SD. **p < 0.01, *p < 0.05. n = number of the animals.
One week after coronary ligation, both LV weight/TL and lung weight/TL were increased in diabetic NLC mice. In CA-MEK5α-Tg mice lung weight/TL was significantly decreased, consistent with the idea that MEK5α activation reduces cardiac dysfunction and heart failure after MI in diabetic mice (Fig.8D, Table S2). In NLC mice, echocardiographic analysis showed that both LVEDD and LVESD after MI in DM mice were greater than in sham, and FS% also significantly decreased in DM + MI group (Fig.8E, Table S2). Increased in LVEDD and LVESD and the reduction of FS% after MI in diabetic NLC mice were significantly prevented in CA-MEK5α-Tg mice (Fig.8E). Of note, there was no significant difference in cardiac function (FS%) between NLC and CA-MEK5α-Tg mice at the same time point after MI in non-diabetic mice (Fig.8F), confirming the unique role of MEK5α activation on DM-mediated exacerbation of LV dysfunction after MI. In agreement with echocardiographic data, we observed a significant decrease in developed pressure (DP) and dP/dt max in diabetic NLC mice after MI. In contrast, diabetic CA-MEK5α-Tg mice showed significantly higher DP and dP/dt max (Fig.8G, Table S2). Thus physiological data also confirmed the critical role of MEK5 activation in ameliorating the exacerbation of cardiac dysfunction after MI by DM.
DISCUSSION
In this study we found that ERK5-SUMOylation was significantly increased after MI in diabetic mice. Diabetic CA-MEK5α-Tg mice demonstrated reduced ERK5-SUMOylation and improved LV function, and lung weight/TL after MI in comparison to the NLC mice. These data strongly suggested that the activation of MEK5, which inhibited diabetes-mediated ERK5-SUMOylation after MI, was cardio-protective against STZ-induced exacerbation of LV dysfunction after MI. Since both H2O2 and high glucose increased ERK5-SUMOylation, it is most likely that ROS production may be involved in this process, but the exact mechanism remains unclear. DN-Ubc9 and PIAS1 siRNA impaired ERK5-SUMOylation suggesting the involvement of Ubc9 and PIAS1 in ERK5-SUMOylation. Recently, the involvement of PIAS1 phosphorylation on regulating downstream events has been reported. PIAS1 can act by selectively inhibiting the recruitment of NF-κB/STAT1 to the endogenous gene products. IKKα is associated with PIAS1 and mediates the Ser 90 phosphorylation of PIAS1, which in turn is required for PIAS1 to block the promoter binding of p65. In this report, the importance of both Ser 90 phosphorylation and SUMO ligase activity of PIAS1 to repress transcriptional activity was proposed. However, the mutant of S90A PIAS1 did not decrease its ability to induce protein SUMOylation compared with wild type, suggesting additional regulatory mechanism of PIAS1 E3 ligase activity in addition to SUMOylation 18. The possible link between phosphorylation and SUMOylation is intriguing provided that ROS or high glucose-mediated kinase activation could be subject to PIAS1 phosphorylation coupled to ERK5-SUMOylation. Future research should focus on identification of the molecular mechanism of ROS and high glucose-mediated ERK5-SUMOylation since this process appears to be involved in the exacerbation of diabetes-mediated LV dysfunction after MI.
Supplementary Material
Acknowledgments
Source of Funding
This work is supported by grants from the Mochida Memorial Foundation for Medical and Pharmaceutical Research to Dr. Tetsuro Shishido, the America Heart Association to Dr. Woo (Postdoctoral fellowship 0625957T), and from the National Institute of Health to Dr. Abe (GM-071485, HL-077789 and HL-088637), Dr. Yan (HL-077789 and HL-088400), and Dr. Yang (GM-071485 and HL-088637). Drs. Abe and Yan are recipients of Established Investigator Awards of the American Heart Association (0740013N and 0740021N).
Footnotes
Disclosures
None.
Contributor Information
Tetsuro Shishido, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
Chang-Hoon Woo, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
Bo Ding, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
Carolyn McClain, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
Chen Yan, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
Jun-ichi Abe, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY 14586.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr., Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003;42(5):914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 4.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001;38(2):421–428. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- 5.Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111(19):2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding B, Abe J, Wei H, Xu H, Che W, Aizawa T, Liu W, Molina CA, Sadoshima J, Blaxall BC, Berk BC, Yan C. A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci U S A. 2005;102(41):14771–14776. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, Liu W, Xu H, McClain C, Molina CA, Blaxall BC, Abe J. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100(4):510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe JI. Extracellular Signal-Regulated Kinase 5 SUMOylation Antagonizes Shear Stress-Induced Antiinflammatory Response and Endothelial Nitric Oxide Synthase Expression in Endothelial Cells. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 9.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 10.Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, Wen J, Kubota T, Takeshita A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol. 2003;42(1):165–172. doi: 10.1016/s0735-1097(03)00509-6. [DOI] [PubMed] [Google Scholar]
- 11.Itoh S, Ding B, Shishido T, Lerner-Marmarosh N, Wang N, Maekawa N, Berk BC, Takeishi Y, Yan C, Blaxall BC, Abe J. Role of p90 ribosomal S6 kinase-mediated prorenin-converting enzyme in ischemic and diabetic myocardium. Circulation. 2006;113(14):1787–1798. doi: 10.1161/CIRCULATIONAHA.105.578278. [DOI] [PubMed] [Google Scholar]
- 12.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108(23):2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 13.Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51(4):1166–1171. doi: 10.2337/diabetes.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 14.Gregoire S, Yang XJ. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol. 2005;25(6):2273–2287. doi: 10.1128/MCB.25.6.2273-2287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. Embo J. 1997;16(23):7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan C, Luo H, Lee JD, Abe J, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001;276(14):10870–10878. doi: 10.1074/jbc.M009286200. [DOI] [PubMed] [Google Scholar]
- 17.Wollert KC, Drexler H. Regulation of cardiac remodeling by nitric oxide: focus on cardiac myocyte hypertrophy and apoptosis. Heart Fail Rev. 2002;7(4):317–325. doi: 10.1023/a:1020706316429. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, Loo JA, Shuai K. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129(5):903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272(33):20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.