Abstract
NG2 cells express the chondroitin sulfate proteoglycan NG2 and are a fourth type of glia distinct from astrocytes, oligodendrocytes, and microglia. NG2 cells generate oligodendrocytes but have also been reported to represent neuronal progenitor cells in the postnatal mouse subventricular zone (SVZ). We performed a detailed immunohistochemical analysis of NG2 cells in the mouse SVZ, rostral migratory stream (RMS), and olfactory bulb granule cell layer (OB GCL), which constitute a neurogenic niche in the postnatal forebrain. NG2 cells in the SVZ and RMS expressed the oligodendrocyte precursor cell antigen platelet-derived growth factor receptor-α but did not express antigens known to be expressed by neuronogenic cells in the SVZ, such as doublecortin, PSA-NCAM, beta-tubulin, Dlx2, or GFAP. More than 99.5% of the proliferating cells in the SVZ were NG2 negative. In the olfactory bulb, NG2 cells were found to generate primarily oligodendrocytes and a small number of astrocytes but not neurons. In the SVZ and RMS, NG2 cells were sparse and made up a much smaller fraction of the cells compared with the surrounding nonneurogenic parenchyma. Parenchymal NG2 cells were often located along the border of the SVZ and RMS. The abundance of NG2 cells increased in the distal parts of the RMS and especially in the OB GCL, where NG2 cell processes were seen in close proximity to many maturing interneurons. Our findings indicate that NG2 cells do not represent neuronal progenitor cells in the postnatal SVZ but are likely to be oligodendrocyte precursor cells.
Keywords: neural progenitor cells, neural stem cell, neurogenesis, olfactory bulb, oligodendrocyte precursor cells, rostral migratory stream
In the adult brain, NG2 glia are a fourth major type of glia that is distinct from astrocytes, oligodendrocytes, and microglia (Nishiyama, 2007). These cells express the chondroitin sulfate proteoglycan NG2 and possess typical morphology, with multiple branched processes (Stallcup et al., 1983; Nishiyama et al., 1999). NG2 cells are also known as oligodendrocyte progenitor cells based on their ability to differentiate into oligodendrocytes in vitro (Levine and Stallcup, 1987; Stallcup and Beasley, 1987) and in vivo in developing and adult CNS (Horner et al., 2000; Bu et al., 2004; Zhu et al., 2008) and after a demyelinating injury (Reynolds et al., 2002; Watanabe et al., 2002; Polito and Reynolds, 2005). NG2 cells are ubiquitous in gray and white matter and constitute 2-9% of total cells in the adult rodent brain (Dawson et al., 2003). The physiological functions of NG2 cells in the adult brain are currently the subject of intense study. What has attracted a lot of interest lately is the neurogenic potential of NG2 cells. Specialized in vitro conditions have been shown to reprogram oligodendrocyte progenitor cells into multipotent neural stem cells that can generate neurons, astrocytes, and oligodendrocytes (Kondo and Raff, 2000). Other reports have highlighted the role of NG2 cells as neuronal precursors in vitro as well as in vivo in the postnatal brain (Belachew et al., 2003; Aguirre and Gallo, 2004; Aguirre et al., 2004). However, more recent studies have reported a lack of neurogenesis from NG2 cells (Buffo et al., 2008; Zhu et al., 2008).
As reviewed elsewhere, it is well-established that the adult brain contains two distinct regions where neural stem and progenitor cells reside and generate new neurons and glia throughout life (Emsley et al., 2005). These areas are the subgranular zone/granule cell layer of the dentate gyrus in the hippocampus (Altman and Das, 1965) and the forebrain subventricular zone (SVZ; Hinds, 1968a,b). Large numbers of new neurons are generated continuously in the adult SVZ, and newly generated neuroblasts migrate via the rostral migratory stream (RMS) to the olfactory bulbs (OB), where most become granule cells (Luskin, 1993; Lois and Alvarez-Buylla, 1994; Betarbet et al., 1996; Jankovski and Sotelo, 1996; Lois et al., 1996; Winner et al., 2002; Merkle et al., 2007; Young et al., 2007). It has so far been shown that GFAP-expressing cells (type B cells) in the SVZ represent multipotent self-renewing neural stem cells that generate neuroblasts (type A cells) via a transiently amplifying precursor cell type (type C cells; Doetsch et al., 1997, 1999). To examine more closely the relation between NG2 cells and previously identified cell populations in the rostral forebrain neurogenic niche, we performed a detailed phenotypical analysis of the SVZ, RMS, and OB granule cell layer.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut. Mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred and maintained in the University of Connecticut animal research facility. Postnatal day 3, 30, 42, and 120 C57BL/6J mice were used. To label proliferating cells in S-phase of the cell cycle, mice received a single injection of the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) intraperitoneally (50 mg/kg body weight; Roche), and were killed 2 hours postinjection. For fate mapping of NG2 cells in the OB, we used transgenic mice expressing the bacteriophage Cre recombinase specifically in NG2 cells (NG2creBAC) and crossed them to either Z/EG (Novak et al., 2000) or ROSA26R (Soriano, 1999) Cre reporter mice, thereby generating NG2CreBAC:ZEG or NG2CreBAC:ROSA26R double transgenic (tg) mice as previously reported (Zhu et al., 2008).
Tissue processing
Mice were anesthetized with isoflurane and killed by transcardiac perfusion with 2% paraformaldehyde solution in phosphate buffer containing 0.1 M lysine and 0.01 M sodium metaperiodate (paraformaldehyde-lysine-periodate fix; McLean and Nakane, 1974). The brains were postfixed in the same fixative for 2 hours, followed by washes in 0.2 M sodium phosphate buffer (pH 7.4). Coronal and sagittal sections (50 μm) were cut with a vibratome (VT1000S; Leica, Deerfield, IL).
Antibody characterization
The mouse monoclonal antibody clone CC1-adenomatosis polyposis coli (APC; clone CC1) antibody was raised against amino acids 1-226 of recombinant human APC (Calbiochem, Gibbstown, NJ; catalog No. OP80, lot No. 1279501-2). This antibody is reported to reveal a single band of 300 kDa by immunoblotting (Bhat et al., 1996) and stained cells with the classic morphology and distribution of oligodendrocytes in this study and in our previous study (Zhu et al., 2008).
The mouse monoclonal anti-BrdU was raised against a 5-iodo-uridine/ovalbumin conjugate (Amersham, Piscataway, NJ; catalog No. RPN 202, lot No. 307024). The specificity of the antibody has been tested by competitive ELISA. No cross-reactivity was observed with thymidine (manufacturer's technical information). In our study, no staining was observed when this anti-BrdU antibody was used to stain brains from mice that had not received BrdU injections.
The mouse monoclonal anticlass III β-tubulin was raised against a chemically synthesized peptide corresponding to amino acids 443-450 of human β-tubulin isotype III conjugated to bovine serum albumin (BSA; Sigma, St. Louis, MO; catalog No. T8660, lot No. 104K4875). This antibody is reported to stain a single band of 46 kDa on Western blot (manufacturer's technical information). It strongly stained cells with the morphology and distribution of immature neurons in our study.
The mouse monoclonal antiglial fibrillary acidic protein (GFAP) antibody was raised against purified GFAP from pig spinal cord (Sigma-Aldrich, St. Louis, MO; catalog No. G3893, clone G-A-5, lot No. 037K4759). The antibody specifically localizes GFAP in immunoblotting assays (manufacturer's technical information) and stained cells with the classic morphology and distribution of fibrillary astrocytes in our study.
The mouse monoclonal anti-Ki67 antigen antibody was raised against a recombinant protein encoded by nucleotides 1159-1522 of the human Ki67 gene (Vision Biosystems, Boston, MA; catalog no. NCL-Ki67-MM1). This antibody exhibited nuclear immunoreactivity in cell types with known high cell proliferation rates but did not react with cells known to be in a resting state, as was seen in our study and as previously reported (Gerdes et al., 1983).
The mouse monoclonal antimicrotubule-associated protein 2 (MAP2; 2a + 2b) was raised against purified bovine MAP2 (Sigma; catalog No. M1406, clone AP-20). The antibody localizes the high-molecular-weight forms of MAP2, namely, MAP2a and MAP2b (manufacturer's technical information) and showed selective dendritic labeling in neurons in our study.
The mouse monoclonal antineuron-specific nuclear protein (NeuN) antibody was raised against purified cell nuclei from mouse brain (Chemicon, Temecula, CA; catalog No. MAB377, clone A60). Immunostaining with this antibody is observed in most postmitotic neurons, and Western blotting with this antibody shows three bands in the 46-48-kDa range (Mullen et al., 1992).
The mouse monoclonal antipolysialylated neural cell adhesion molecule (PSA-NCAM) antibody was raised against a living cell suspension obtained from the forebrain of embryonic day 18 rats (MAb 12E3; a gift from Dr. Tatsunori Seki, Tohoku University, Sendai, Japan). The antibody recognizes the PSA portion of the high-molecular-weight neural cell adhesion molecule expressed in immature neurons (Seki and Arai, 1991).
The mouse monoclonal antibody anti-S100β was raised against purified bovine brain S100β (Sigma; catalog No. S2532, lot No. 096K4868). This antibody is reactive in dot blots with denatured-reduced preparations of S100β (manufacturer's technical information) and stained the same population of cells that was stained with a rabbit antibody, which had been used to isolate the S100β mRNA and clone the cDNA (Kuwano et al., 1984).
The rabbit polyclonal anti-NG2 antibody was raised against purified rat NG2 chondroitin sulfate proteoglycan (Chemicon; catalog No. AB5320). This antibody identifies both the intact proteoglycan and the 300-kDa rat NG2 core glycoprotein by Western blot and stained cells with the morphology and distribution of oligodendrocyte precursor cells characteristic of those in our previous study with other anti-NG2 antibodies.
The rabbit polyclonal antiphosphorylated histone H3 antibody was raised against the peptide corresponding to amino acids 7-20 of human histone H3 (Upstate, Charlottesville, VA; catalog No. 06-570, lot No. 32219). This antibody detected a single band at ~17 kDa (manufacturer's technical information) and stained cell nuclei characteristic of mitotic cells in areas with high cell proliferation in our study.
The polyclonal rabbit antiplatelet-derived growth factor receptor-α (PDGFRα) antibody was kindly provided by Dr. William Stallcup (Burnham Institute, La Jolla, CA). The antibody was raised against a recombinant protein that spanned amino acids 24-524 and corresponded to the entire extracellular domain of rat PDGFRα protein. The antibody was affinity purified on a column made from the same PDGFRα ectodomain.
The polyclonal guinea pig anti-Dlx2 antibody was raised against purified maltose-binding protein-mouse Dlx2 fusion protein (amino acids 1-154; Kuwajima et al., 2006; kindly provided by Dr. Kazuaki Yoshikawa, Osaka University, Osaka, Japan). On Western blot, this antibody recognizes a 45-kDa band in the lysates from E13.5 forebrains.
The polyclonal guinea pig anti-NG2 antibody was raised against a recombinant protein that spanned amino acids 30-2225 and corresponded to the entire extracellular domain of rat NG2 core protein. The antibody was affinity purified on a column made from the same ectodomain (Ozerdem et al., 2001).
The polyclonal goat antidoublecortin (DCX) antibody was raised against a synthetic peptide corresponding to amino acids 385-402 at the C-terminus of human doublecortin (Santa Cruz Biotechnology, Santa Cruz, CA; catalog No. sc-8066). Western blotting with this antibody detects a single band of 40 kDa molecular weight (Brown et al., 2003). Immunostaining with DCX stained the same population of cells stained by other immature neuronal markers such as PSA-NCAM and class III β-tubulin in our study.
The monoclonal rat anti-AN2 antibody was raised against rat Oli-Neu cells. It was kindly provided by Dr. Jacqueline Trotter, University of Mainz. The antibody detects a single band at 330 kDa on Western blot. Immunostaining with this antibody stained the same type of cells as those stained by rabbit and guinea pig anti-NG2 as seen in our study.
Immunohistochemistry
Sections were incubated in blocking/permeabilizing solution [5% normal goat serum or horse serum and 0.1% Triton X-100 in phosphate-buffered saline (PBS), pH 7.4] for 1 hour and then incubated with primary antibodies (mouse monoclonal antiadenomatosis polyposis coli (APC; clone CC1), 1:200; anti-BrdU, 1:2,000, with nuclease 1:20; anticlass III β-tubulin, 1:6,000; anti-GFAP, 1:400; anti-Ki67 IgG, 1:300; antimicrotubule-associated protein (MAP2ab), 1:100; anti-NeuN, 1:5,000; anti-PSA-NCAM, 1:800; anti-S100β, 1:2,000; rabbit polyclonal anti-NG2, 1:500; antiphosphorylated histone H3 IgG, 1:3,000; anti-PDGFRα, 1:1,000; guinea pig polyclonal anti-Dlx2, 1:3,000; anti-NG2, 1:500; goat polyclonal anti-DCX, 1:400; monoclonal rat anti-AN2, 1:3,000) in blocking solution (5% normal goat serum or horse serum) overnight at 4°C. To detect BrdU, sections were pre-treated for 10 minutes in 2 N HCl at room temperature, followed by neutralization in 0.1 M sodium borate buffer, pH 8.6. The primary anti-BrdU antibody solution contained nuclease for production of single-stranded DNA.
Subsequently, all sections were reacted with fluorochrome-conjugated species-specific secondary antibodies (Alexa 488-conjugated antibodies at 1:1,000 from Molecular Probes, Eugene, OR; FITC- and Cy5-conjugated antibodies at 1:150 or Cy3 antibodies at 1:500 from Jackson Immunoresearch, West Grove, PA) for 1 hour at room temperature in blocking solution. Sections were dried onto Superfrost glass slides (Fisher Scientific, Pittsburgh, PA). Stained sections were mounted in Vectashield mounting medium containing the nuclear stain DAPI (Vector Laboratories, Burlingame, CA).
Microscopy and photomicrograph production
Confocal images were obtained on a Zeiss Axiovert 200M microscope equipped with an Axiocam camera and Apotome 3D fluorescence imaging system (Zeiss, Göttingen, Germany). Serial z-stack images were taken, and maximum-projection images were produced with Zeiss AxioVision 4.6 software. Figures were prepared in AxioVision 4.6 software, Adobe Photoshop 9.0, and Adobe Illustrator CS3 (Adobe Systems Inc., San Jose, CA). Image manipulations were limited to linear gray-scale level adjustment. Adobe Photoshop was used for color assignment.
Preembedding immunoelectron microscopy
P30 mice were anesthetized and perfused transcardially with fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde (Electron Microscopy Science, Fort Washington, PA) in 0.1 M phosphate buffer, pH 7.2. The brains were then dissected out and postfixed for 1 hour at 4°C in the same fixative. After washing with phosphate buffer, 50-μm coronal sections were cut with a vibratome. Sections were first immersed in 3% normal goat serum in PBS for 1 hour at room temperature and then incubated at 4°C overnight in a rabbit antibody to the extracellular domain of rat NG2 (NG2EC; a gift from Dr. W. Stallcup) diluted 1:2,000 in 3% normal goat serum in PBS. The primary antibody was detected by the ABC/peroxidase method with diaminobenzidine (DAB) as a substrate (Vectastain Elite Kit; Vector Laboratories). Sections were postfixed with 1% OsO4 in 0.12 M cacodylate buffer, pH 7.2, for 45 minutes at room temperature; dehydrated through a series of ethanol; and infiltrated in Embed-812 resin (Electron Microscopy Science). Sections were flat embedded and polymerized at 60°C for 2 days. The area of interest was excised from the embedment and affixed to a blank Epon block. Ultrathin sections were cut on a Leica microtome and placed on 200 mesh copper/rhodium grids. The sections were either counterstained with 2% aqueous uranyl acetate and Sato's lead stain for 90 seconds or left without counterstain, then viewed under a FEI Technai electron microscope. Images were captured digitally.
Quantification of NG2 cell number
Regions quantified
The number of NG2-immunopositive nonvascular cells (NG2 cells) was quantified in P30 SVZ and nonneurogenic parenchyma that included the striatum, corpus callosum, and cortex and in P42 anterior SVZ (aSVZ), RMS, and OB, as indicated in Figure 1. Five animals of each age were used for the quantifications.
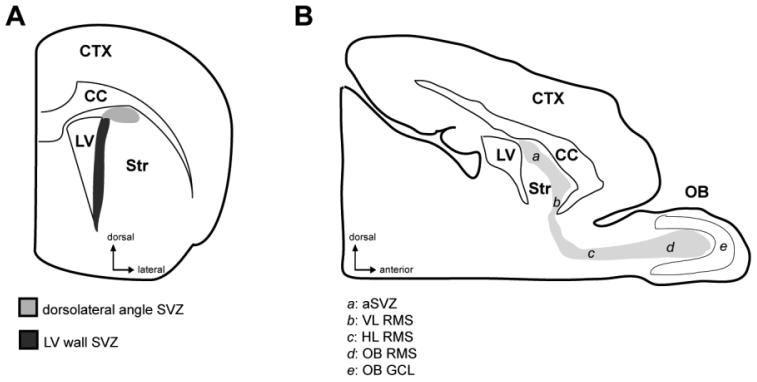
Figure 1.
Schematic drawings of a coronal forebrain section (A) and a sagittal brain section (B) showing the different subregions of the SVZ and RMS that were analyzed. aSVZ, anterior part of the subventricular zone; CC, corpus callosum; CTX, cortex; HL RMS, horizontal limb of the rostral migratory stream; LV, lateral ventricle; OB, olfactory bulb; OB GCL, granule cell layer of the olfactory bulb; OB RMS, olfactory bulb portion of the rostral migratory stream; Str, striatum; SVZ, subventricular zone; VL RMS, vertical limb of the rostral migratory stream.
The SVZ was identified in coronal forebrain sections, anterior to the crossing of the anterior commissure. For this quantification, we used three or four sections 200 μm apart. As indicated in Figure 1A, in the present study, the SVZ was subdivided into two regions using coronal sections: the dorsolateral angle SVZ, situated at the dorsolateral edge of the lateral ventricle and extending dorsolaterally between the corpus callosum and the striatum, and the lateral ventricle wall SVZ, extending from the dorsolateral edge of the lateral ventricle down to the ventral tip of the lateral ventricle along its lateral wall. The aSVZ/RMS/OB pathway, which, we collectively refer to as theRMS, was identified in sagittal sections. The subregions of the RMS that were analyzed were the aSVZ, the vertical and horizontal limbs of the RMS (VL RMS, HL RMS), the RMS within the OB (OB RMS), and the granule cell layer of the OB (OB GCL). The nuclear stain DAPI was used to distinguish the SVZ, the RMS, and the nonneurogenic parenchyma. The boundary between the cellular organization of the SVZ, RMS, and parenchyma was defined as the boundary between the highly cellular SVZ and RMS, and the less cellular nonneurogenic parenchyma. The OB GCL was identified by its characteristic layers of tightly packed granule cells.
Sampling
We performed systematic random sampling as described below. The sampling of the nonneurogenic parenchyma and OB GCL was performed as follows. For the striatum, the sampling was done 250 μm away from the lateral ventricle, and each grid box was 100 μm apart along the lateral ventricle wall starting from the dorsolateral angle. The entire width of the corpus callosum overlying the dorsal ventricle wall was sampled, and the grid boxes were placed immediately adjacent to one another. For the cortex, the grid boxes were placed in the cingulate cortex and sampled the entire thickness of the cortex. For the OB GCL, the sampling was performed 100 μm from the outer border of the OB RMS, and the grid boxes were placed 500 μm apart starting from the beginning of the ventral portion of the OB GCL.
NG2 cell counts
NG2 cells were quantified throughout the thickness of 50-μm sections by using a ×40 objective lens on a Leica DMR epifluorescence upright microscope (Leica Microsystems, Bannockburn, IL) equipped with a digital camera (ORCA, Hamamatsu, Japan). Only NG2 cells with clearly visible nuclei were counted. NG2 cells were categorized as within the SVZ or the RMS if they were one or more cell layers deep in the SVZ or RMS, counting from the border with the parenchyma. For the SVZ and RMS, the numbers of NG2 cells were counted in the entire SVZ in coronal sections or RMS in sagittal sections, and the areas of the SVZ and RMS were measured in IPLab imaging software (Scanalytics, Fairfax, VA). For the OB GCL and nonneurogenic parenchyma, a grid box corresponding to a sample area of 0.06 mm2 was used in five different regions, and the numbers of NG2 cells were counted in each grid.
Total cells
Because of the large number of total cells, DAPI+ cells were quantified using a ×100 objective. For quantification of the total number of DAPI-labeled cells, a grid box corresponding to a sample area of 0.0027 mm2 was used in three different regions for the SVZ and RMS and in nine different regions for the nonneurogenic parenchyma and OB GCL.
Cell density and cell numbers
The measured areas were multiplied by the section thickness (50 μm) to acquire a sample volume, and the data were expressed as NG2 cell density (number of NG2 cells/mm3) and the percentage of NG2 cells among total cells. Measurements of the volumes of the SVZ and RMS were performed as follows. Serial cresyl violet-stained sections were used to estimate the total volume of the SVZ and RMS. The areas of the SVZ and RMS in each section were multiplied by the distance between the sections, and the values of the series were added together according to the Cavalieri principle (Gundersen et al., 1988). For volume measurement of the SVZ, we used four coronal sections 200 μm apart, starting from the appearance of the anterior horn of the lateral ventricle to the crossing of the anterior commissure. For the volume measurement of the RMS, we used five serial sagittal sections 100 μm apart, starting from the appearance of the anterior horn of the lateral ventricle to the appearance of the intrabulbar part of the anterior commissure. To estimate the total number of NG2 cells in the SVZ and RMS, the cell density in the SVZ or RMS was multiplied by the total volume of the SVZ and RMS, respectively.
To avoid cell overcounting, all cell counts were corrected for nuclear size according to Abercrombie's formula (Abercrombie, 1946). The nuclear diameter along the axis perpendicular to the plane of section was measured with the ×100 objective in 15-25 NG2 cells and 25 DAPI-positive cells in each of the sampled anatomical regions. A ratio was calculated by dividing the 50-μm section thickness by a value consisting of the average nuclear diameter added to the section thickness. The previously quantified cell numbers were subsequently multiplied by the calculated ratio for each cell class.
Quantification of proliferating NG2 cells
The following markers were used to identify proliferating cells: 1) Ki67, which is expressed by all actively cycling cells (Gerdes et al., 1983); 2) the thymidine analogue bromodeoxyuridine (BrdU), which is incorporated into proliferating cells in the S-phase of the cell cycle (Gratzner et al., 1976; Miller and Nowakowski, 1988; del Rio and Soriano, 1989); and 3) phosphorylated histone H3, which is expressed by cells in the M-phase of the cell cycle (Hendzel et al., 1997). In sections doubly labeled for Ki67, BrdU, or phosphorylated histone H3 and NG2 from five, five, and one mouse, respectively, the total number of cells positive for Ki67, BrdU, or phosphorylated histone H3 and the number of cells doubly labeled for Ki67, BrdU, or phosphorylated histone H3 and NG2 was counted. The numbers of cells counted for Ki67, BrdU, and phosphorylated histone H3 were 236, 190, and 64 in the SVZ, respectively, and 36, 38, and 3 for each marker in the nonneurogenic parenchyma, respectively.
Fate mapping of NG2 cells in the OB
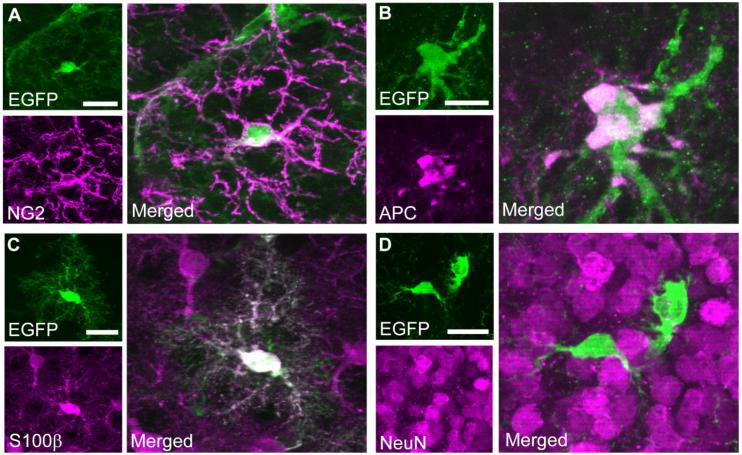
To examine the fate of NG2 cells in the OB, the total number of enhanced green fluorescent protein (EGFP)-positive cells and the number of cells that were doubly labeled with EGFP and NG2, APC, S100β, or NeuN were counted from the glomerular layer to the internal granule cell layer in sagittal OB sections from three P14 NG2creBAC:ZEG double tg mice with a ×40 objective. For each marker, between 171 and 215 EGFP cells were evaluated. Data are presented as the percentage of colabeled EGFP-expressing cells among total EGFP cells. Cells were counted with the Leica DMR epifluorescence microscope.
Quantification of contacts between neuroblasts and NG2 cells
Contacts between neuroblasts and NG2 cells were assessed in serial z-stack images taken with a ×40 objective lens. The RMS subregions indicated in Figure 1B were analyzed in NG2, DCX, and GFAP triple-labeled sagittal sections from five different P42 animals. Three consecutive images, 0.35 μm apart, were merged together into a maximum-projection image, and three different projected images per anatomical region were evaluated. The percentage of DCX-positive neuroblasts that were contacted by NG2 cells was expressed as a fraction of the total number of DCX-labeled neuroblasts in each region. Only neuroblasts with DCX-immunopositive cytoplasm along the entire perimeter of the cell were counted, and only direct apposition of doublecortin-positive cell body to NG2 cell body or process/processes was counted as contact. Subsequently, GFAP expression in the triple-stained sections was used to exclude the presence of fine GFAP-labeled processes interspersed between apposed NG2 cell process and DCX-positive neuroblast.
Triple labeling with NG2, the immature neuronal markers DCX or PSA-NCAM, and the mature neuronal markers NeuN or Map2ab was used to evaluate the correlation between presence of NG2 cell contacts and neuroblast maturity. Maturing neurons were identified as cells that coexpressed DCX and NeuN, DCX and MAP2ab, PSA-NCAM and NeuN, or PSA-NCAM and MAP2ab.
RESULTS
NG2 cells are less abundant in the SVZ than in the nonneurogenic parenchyma
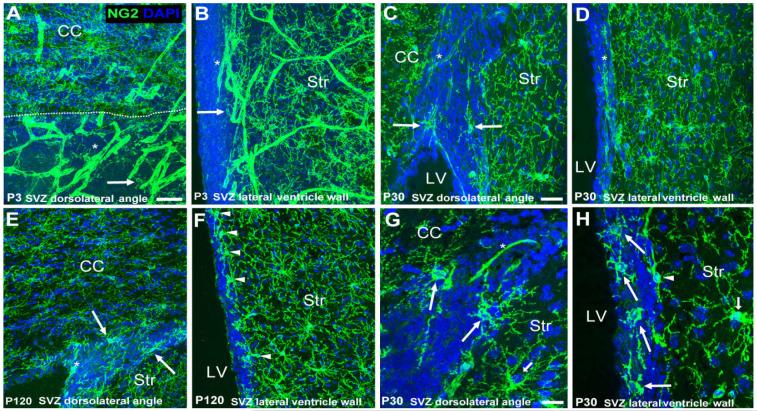
We examined the distribution of NG2 cells in the SVZ identified by DAPI nuclear stain in mice of different ages (P3, P30, P42, and P120). NG2 cells with typical multiprocessed morphology were ubiquitous in the parenchyma (Nishiyama, 2007; Fig. 2). NG2 is expressed not only on process-bearing glial cells but also on pericytes in the vasculature as previously described (Ozerdem et al., 2002; Zhu et al., 2008), and vascular expression of NG2 was more prominent in the early postnatal brains than in the adult. The termNG2 cellsin this paper refers to nonvascular cells in the CNS that express NG2. In the nonneurogenic parenchyma of P30 mice, the density of NG2 cells was 2,309 ± 634 cells/mm3, representing 2.81% ± 0.78% of total cells (mean values ± SD; Table 1). By contrast, NG2 cells were much more sparse in the SVZ at all ages examined (Fig. 2). In P30 mice, the density of NG2 cells in the SVZ was 1,255 ± 607 cells/mm3, representing 0.15% ± 0.07% of total cells, which was more than 18-fold lower than the proportion of NG2 cells among total cells in the parenchyma (Table 1). The number of NG2 cells in the entire SVZ was estimated to be 162 ± 78 cells. NG2 cells were found scattered both in the dorsolateral angle and in the lateral ventricle wall SVZ. There were no pronounced differences in the distribution of NG2 cells along the dorsoventral extent of the SVZ. Within the SVZ, NG2 cells were more prevalent in the outer regions, close to the border with the nonneurogenic parenchyma (Fig. 2B,C,E,G). Parenchymal NG2 cells were uniformly distributed and were often found aligned along the border with the SVZ.
Figure 2.
Distribution of NG2 cells in the mouse SVZ. A-H: Coronal forebrain sections showing the distribution of NG2 cells (green) in the mouse SVZ compared with the surrounding nonneurogenic parenchyma (striatum and corpus callosum) in P3 (A,B), P30 (C,D,G,H), and P120 (E,F) forebrain. The SVZ was identified with DAPI nuclear stain (blue). Long arrows indicate NG2 cells in the SVZ. Arrowheads show parenchymal NG2 cells along the border with the SVZ. Asterisks show NG2-immunopositive vasculature. G,H: Higher magnification images illustrating morphologies exhibited by NG2 cells in the SVZ. G shows an NG2 cell with multiple slender and branched processes (polydendrocyte) in the dorsolateral angle SVZ. H shows four cells with fewer, thicker, and less well branched processes located in the lateral ventricle wall SVZ. Short arrows indicate parenchymal polydendrocytes for comparison. NG2 cells are less frequent in the SVZ compared with the parenchyma. Scale bars = 50 μm in A,C; 20 μm in G.
TABLE 1.
Quantification of NG2 Cell Numbers in the SVZ and Nonneurogenic Regions Indicated in the Schematic Drawings in Figure 11
| Region | NG2 cells/ mm3 | Percentage NG2 cells of total cells |
|---|---|---|
| Total SVZ | 1,255 ± 607 | 0.15 ± 0.07 |
| Dorsolateral angle | 1,306 ± 762 | 0.15 ± 0.09 |
| LV wall | 1,205 ± 637 | 0.16 ± 0.08 |
| Total parenchyma | 2,309 ± 634 | 2.81 ± 0.78 |
| Striatum | 1,803 ± 361 | 2.03 ± 0.41 |
| Corpus callosum | 3,344 ± 1,116 | 3.98 ± 1.33 |
| Cortex | 1,781 ± 485 | 2.42 ± 0.66 |
Data are presented as numbers of NG2 cells/mm3 of specific area as well as the percentage of NG2 cells among total numbers of DAPI-positive cells. Numbers are from five different postnatal day 30 mice and represent mean values ± SD.
Next, to examine whether NG2 cells in the SVZ were similar to those found in the parenchyma, we studied the expression of another oligodendrocyte progenitor cell antigen, PDGFRα (Nishiyama et al., 1996) on NG2-expressing cells in the SVZ. In NG2- and PDGFRα-immunolabeled sections, NG2 cells in the SVZ coexpressed PDGFRα in early postnatal as well as in older mice (Fig. 3A-C). NG2 cells in the parenchyma were also colabeled with PDGFRα (Fig. 3A), which is consistent with our previous study (Nishiyama et al., 1996). We can therefore conclude that NG2 cells in the SVZ belonged to the oligodendrocyte lineage and were antigenically indistinguishable from NG2 cells in the surrounding parenchyma. The majority of the NG2 cells in the SVZ had long, slender, branched processes typical of NG2 cells (polydendrocytes) in the parenchyma (Fig. 2G). There were some NG2 cells in the SVZ that exhibited a more immature morphology, with fewer, shorter processes that were less well branched (Fig. 2H), which were often located close to the ventricular surface.
Figure 3.
A,B: Colabeling of NG2 (green) with the oligodendrocyte precursor cell antigen platelet-derived growth factor receptor (PDGFRα; magenta). NG2 cells in the SVZ (A) and RMS (B) of P30 mice coexpress NG2 and PDGFRα. The SVZ and RMS are displayed as nuclear stain (DAPI; blue) or are outlined by dashed lines accordingly. Arrows indicate NG2 cells in the SVZ. Asterisks show NG2-immunopositive vasculature. Arrowheads show parenchymal NG2 cells along the interface with the SVZ and RMS. NG2 cells in the SVZ and RMS coexpress PDGFRα (white when merged) and are therefore antigenically indistinguishable from NG2 cells in the parenchyma. NG2-immunopositive vasculature did not colabel with PDGFRα. C: High-power view of an NG2 and PDGFRα outlined in A. A single image from a z-stack is displayed. Scale bar = 50 μm.
We found that, in the RMS, similarly to the SVZ, NG2 cells were also rather infrequent, and those that were present in the RMS coexpressed PDGFRα (Fig. 3B). NG2 cells within the RMS exhibited the mature, multiprocessed morphology similar to that of those in the parenchyma. As we observed in our analysis of the SVZ, NG2 cells were often found outside the RMS, aligned close to the parenchymal border to the RMS.
NG2 cells in the SVZ and RMS are distinct from type A, B, or C cells
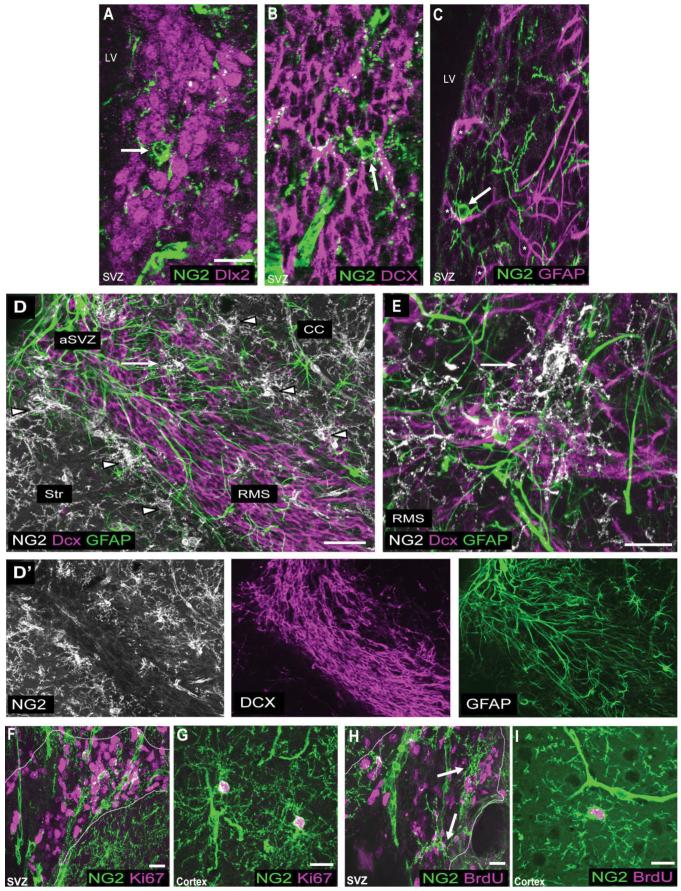
To determine the relation between NG2 cells in the SVZ and previously identified cellular constituents of the SVZ known as type A, B, or C cells (Alvarez-Buylla et al., 2002), we compared the expression of NG2 and antigens known to be expressed by each SVZ cell type. Type C cells, which are the transit-amplifying precursor cells of the SVZ, express the homeobox transcription factor Dlx2, which is also expressed by type A cells (Doetsch et al., 2002). Figure 4A shows that NG2 cells in the SVZ do not express Dlx2. Type A cells, which represent SVZ neuroblasts, express antigens such as DCX, PSA-NCAM, and class III β-tubulin (Doetsch et al., 1997; Nacher et al., 2001). When sections were doubly labeled for NG2 and DCX, we detected no overlap between NG2 cells in the SVZ and cells that expressed DCX (Fig. 4B). Moreover, double labeling with NG2 and PSA-NCAM or class III β-tubulin did not show any overlap between NG2 and neuroblast antigens in the SVZ. Type B cells, which are the neural stem cell of the SVZ that can generate neurons and glia, have been shown to express GFAP. When sections were doubly labeled for NG2 and GFAP, we detected no overlap between NG2 cells and cells that expressed GFAP (Fig. 4C). These data suggest that NG2 cells are distinct from type A, B, and C cells that have previously been identified in the SVZ. Furthermore, NG2 cells in the RMS did not express DCX or GFAP. NG2 cells in the RMS were therefore also found to be distinct from type A cells and type B cells that have previously been described for the RMS (Doetsch et al., 1997; Fig. 4D,E).
Figure 4.
A-E: Immunolabeling for NG2 and antigens expressed by SVZ type A, B, and C cells. P30 coronal sections costained with NG2 (green) and Dlx2 (magenta; A), NG2 (green) and DCX (magenta; B), and NG2 (green) and GFAP (magenta; C). Triple labeling for NG2 (white), DCX (magenta), and GFAP (green) in a P30 sagittal section (D) and a P120 sagittal section (E). D′ represents the separate color channels shown in D. F-I: Double labeling with NG2 and proliferative cell markers. P30 coronal sections colabeled for NG2 (green) and Ki67 (magenta; F,G) or NG2 (green) and BrdU (magenta; H,I). The SVZ is outlined by dashed lines based on DAPI nuclear stain. Arrows indicate NG2 cells in the SVZ. Arrowheads show parenchymal NG2 cells along the border with the SVZ and RMS. NG2 cells in the SVZ and RMS do not express antigens expressed by type A, B, or C cells. The vast majority of proliferating cells in the SVZ lack NG2 expression. NG2 cells are, however, the main dividing cell population in the nonneurogenic parenchyma. Scale bars = 20 μm in A,E-I; 50 μm in D.
The transit-amplifying cells or type C cells in the SVZ represent the most rapidly proliferating cell population in the SVZ (Doetsch et al., 1997). To determine further whether NG2 cells in the SVZ represent type C cells, we combined NG2 immunolabeling with three different methods of detecting proliferating cells: 1) Ki67 immunodetection, to identify cycling cells during all the active phases of the cell cycle; 2) immunodetection of the thymidine analogue BrdU injected 2 hours prior to sacrifice to identify cells in the S-phase of the cell cycle; and 3) immunolabeling for phosphorylated histone H3, which is detected in cells in the M-phase of the cell cycle. In the SVZ of P30 mice, only 0.2% ± 0.5% of Ki67-positive cells and 0.35% ± 0.67% of BrdU-positive cells coexpressed NG2 (mean values ± SD; Fig. 4F,H). By contrast, in the parenchyma, 94.8% ± 3.9% of Ki67-positive cells and 95.3% ± 4.6% of BrdU-positive cells were colabeled with NG2 (Fig. 4G,I). Similarly, there was little colocalization of NG2 and Ki67 in the RMS (not shown). Analysis of a third proliferative marker, the mitotic cell marker phosphorylated histone H3, showed that none of the detected mitotic cells in the SVZ coexpressed NG2, whereas all phosphorylated histone H3-immunopositive cells observed in the parenchyma were NG2 immunopositive. Taken together, these observations indicate that NG2 cells in the SVZ do not constitute SVZ type C cells.
NG2 cell processes are inserted between SVZ cells
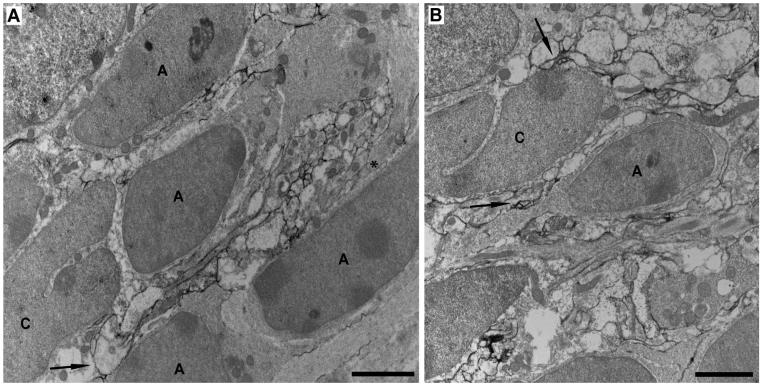
To visualize better the relationship between NG2 cells and SVZ cells, we performed preembedding immunoelectron microscopy with an antibody directed against the ectodomain region of the NG2 molecule. We found numerous thin and delicate NG2-immunolabeled profiles often distributed in clusters within the SVZ (Fig. 5A,B). The clustered arrangement of NG2-immunolabeled profiles is likely to be caused by the visualization of tortuous distal NG2 cell processes in the section. Many of the NG2-immunoreactive profiles were often found to surround and insert between SVZ cells, sometimes contacting their cell surface. SVZ cells that were adjacent to NG2-labeled profiles consisted of cells with elongated cell bodies and dark nuclei with one or several prominent nucleoli, which were likely to be type A cells, and cells with cleaved nuclei, which were likely to be type C cells according to the established nomenclature (Doetsch et al., 1997). SVZ type A or C cells themselves did not exhibit NG2 immunolabeling (Fig. 5A,B). In summary, the ultrastructural studies demonstrate that NG2 cell processes were found to be intimately associated with SVZ cells.
Figure 5.
A,B: Preembedding NG2 immunoelectron microscopy of the SVZ in a P30 mouse using a rabbit anti-NG2 antibody against the ectodomain of the NG2 core protein. NG2-immunolabeled section without lead or uranyl acetate counterstain (A) or with lead and uranyl acetate counterstain (B). Cells with elongated dark nuclei with one or several prominent nucleoli are likely SVZ type A cells. Cells exhibiting a deep cleft in their nuclei are likely SVZ type C cells. A cluster of NG2-immunolabeled profiles (black DAB product) of varying sizes and shapes is inserted between three SVZ type A cells and a type C cell (A). Asterisk indicates an unlabeled process of larger diameter with bundles of intermediary filaments, which likely belongs to an astrocyte. Arrow indicates an NG2 cell process contacting both a type A and a type C cell. B: Arrow indicates contact between NG2-immunolabeled profiles and an SVZ type A cell, possessing migratory-like morphology with an elongated cell body and a leading process. Another arrow indicates NG2-immunolabeled profiles that surround and contact an SVZ type C cell. Scale bars = 2 μm.
NG2 cells generate predominantly NG2 cells and oligodendrocytes in the OB
With NG2CreBAC tg mice generated in our laboratory, we previously reported that NG2 cells generate oligodendrocytes and some protoplasmic astrocytes (Zhu et al., 2008). Here, we examined the fate of NG2 cells in the OB by using NG2CreBAC:ZEG double-transgenic mice in which Cre expressed in NG2 cells permanently induces the expression of EGFP. The majority of NG2-positive cells in the OB expressed EGFP, as previously shown in the forebrain (Fig. 6A; Zhu et al., 2008). Among the EGFP-positive cells, 81.4% ± 2.6% expressed NG2, 28.6% ± 2.4% expressed APC (Fig. 6B), and 1.2% ± 0.2% expressed S100 β and displayed the morphology of protoplasmic astrocytes (Fig. 6C). None of the EGFP-positive cells was NeuN immunopositive (Fig. 6D). Similarly, we did not detect β-galactosidase and NeuN double-positive cells in P14 NG2CreBAC:ROSA26R double-tg mice. These findings suggest that the progeny of NG2 cells are predominantly oligodendrocytes. NG2 cells appear to generate a small number of astrocytes but not neurons in the postnatal OB.
Figure 6.
Fate mapping of NG2 cells in the OB from postnatal day 14 NG2CreBAC:ZEG double-transgenic mice. In these mice, Cre recombinase, expressed in NG2 cells, permanently induces the expression of the reporter EGFP (Zhu et al., 2008). Sections doubly labeled with EGFP (green) and NG2 (magenta; A), EGFP (green) and APC (magenta; B), EGFP (green) and S100β (magenta; C), and EGFP (green) and NeuN (magenta; D). EGFP reporter-positive cells expressed NG2, APC, and S100β but did not express NeuN. Scale bars = 20 μm.
Maturing OB neuroblasts exhibit increased contacts with NG2 cells
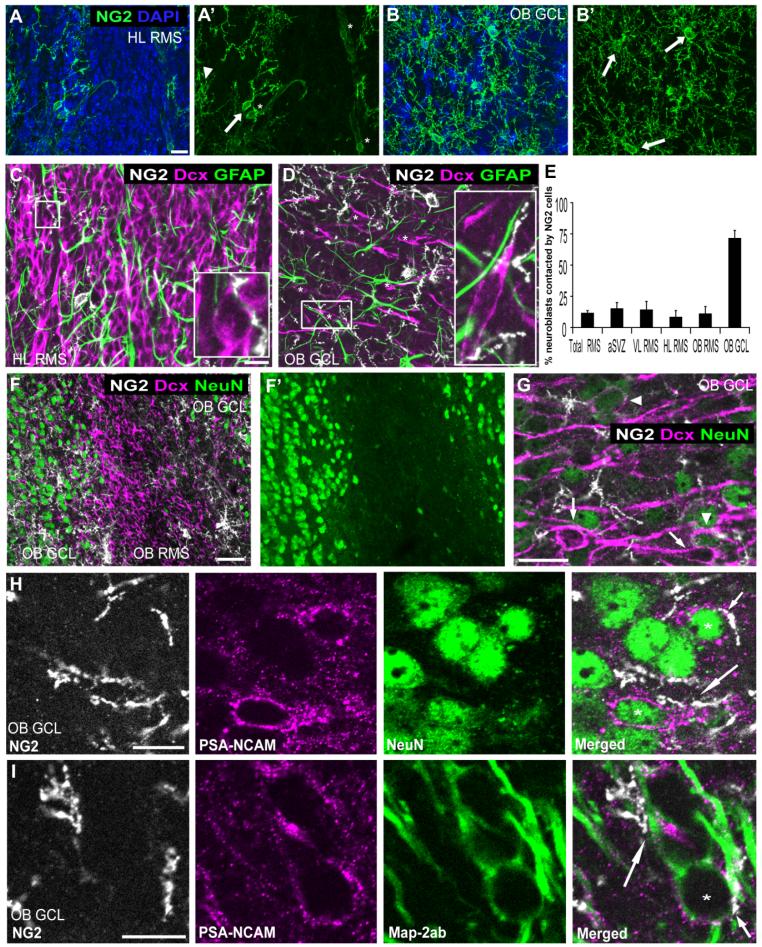
We had found that NG2 cells were less abundant among immature cells in the SVZ compared with the nonneurogenic parenchyma. Next, we quantified the distribution of NG2 cells in the different subregions of the RMS in sagittal sections from P42 mice, as specified in Figure 1B. Quantification of NG2 cells in the RMS starting at the rostral tip of the lateral ventricle showed that the aSVZ and VL RMS together contained 1,344 ± 376 cells per mm3, representing 0.17% ± 0.05% of total cells (mean values ± SD); the HL RMS contained 1,752 ± 595 cells per mm3, representing 0.25% ± 0.09% of total cells; the OB RMS contained 2,306 ± 853 cells per mm3, representing 0.34% ± 0.13% of total cells; and the OB GCL contained 2,012 ± 338 cells per mm3, representing 0.85% ± 0.14% of total cells. The number of NG2 cells in the entire RMS was estimated to be 413 ± 116 cells. The quantification showed that NG2 cell density increased distally in the RMS. Representative images of the differences in NG2 cell density are shown in Figure 7A,B. The images show that NG2 cells were more frequent in the OB GCL (Fig. 7B) than in regions located more proximally to the SVZ, such as the HL RMS (Fig. 7A). The greater abundance of NG2 cells in the distal part of the RMS pathway appeared to correlate with increased neuroblast maturity, insofar as the newly generated neurons are known to migrate rostrally.
Figure 7.
Relation between NG2 cell processes and RMS neuroblasts. A,B: Projected images of z-stacks through 12 μm of P42 mouse sagittal section immunolabeled for NG2 (green) and counterstained with DAPI (blue), showing the density of NG2 cells in the HL RMS (A) and in the OB GCL (B). Arrows indicate NG2 cells in the RMS or the OB GCL. Arrowhead shows a parenchymal NG2 cell at the interface with the RMS. Asterisks show NG2-immunopositive vasculature. The density of NG2 cells is higher in the OB GCL than in the HL RMS. C,D: Projected images of of z-stacks through 1 μm of tissue triple labeled for NG2 (white), DCX (magenta), and GFAP (green). The images were obtained from a portion of the 12-μm z-stack presented in A and B, respectively. Insets in C and D correspond to the boxed areas. Inset in D has been rotated 90° clockwise. Asterisks in D indicate individual DCX-positive neuroblasts. E: Percentages of DCX-positive neuroblasts that were contacted by NG2 cells in different subregions of RMS in P42 mice. F,G: Projected images from a P30 mouse coronal section through the OB triple labeled for NG2 (white), DCX (magenta), and NeuN (green) showing a low-magnification image of the OB RMS and OB GCL (F,F′) and a high-magnification image of the OB GCL (G). Most DCX-positive neuroblasts in the OB RMS lack NeuN expression, whereas, in OB GCL, both NeuN-negative (arrows) and NeuN-positive (arrowheads) neuroblasts are seen. H,I: Relation between maturing PSA-NCAM-immunopositive neurons and NG2 cells in P30 mouse OB GCL. Triple labeling for NG2 (white), PSA-NCAM (magenta), and NeuN (green; H), and for NG2 (white), PSA-NCAM (magenta), and MAP2ab (green; I). NG2 cell processes contact the cell bodies (asterisks and short arrow) as well as neurites (long arrows) of PSA-NCAM and NeuN or PSA-NCAM and MAP2ab double-positive maturing neurons. Scale bars = 20 μm in A,C,G; 50 μm in F; 10 μm in H,I.
We observed that NG2 cell processes contacted neuroblasts in the SVZ and RMS (Figs. 4B,D,E, 5, 7C). To determine whether there was a correlation between the extent of NG2 cell-neuroblast interaction and the degree of neuroblast maturity, we quantified the percentage of DCX-immunopositive neuroblasts contacted by NG2 cells along the RMS (Fig. 7E). Our analysis showed that, on average, 10.7% ± 3.4% (mean values ± SD) of DCX-positive neuroblasts in the SVZ and RMS were contacted by NG2 cell processes and that there were no noticeable differences in the extent of neuroblast-NG2 cell contact among the different regions of the RMS, excluding the OB GCL (aSVZ: 13.7% ± 6.6%, VL RMS: 11.7% ± 7%, HL RMS: 7.1% ± 6.9%, OB RMS: 12.8% ± 6.9%). By contrast, most DCX-labeled cells in the OB GCL (71% ± 8.9%) were contacted by NG2 cells. Representative images of these differences in neuroblast-NG2 cell contact are shown in Figure 7C,D. Figure 7D shows that, in the OB GCL, a larger fraction of neuroblasts exhibited contacts with NG2 cells compared with regions located more proximally, such as the HL RMS, shown in Figure 7C.
We noticed differences in the organization and morphology of neuroblasts in the RMS and OB GCL, which may reflect the differences in the degree of neuroblast maturation. The neuroblasts in the RMS were tightly apposed to each other, had smaller cell bodies, and did not possess extensive neurites. By contrast, neuroblasts in the OB GCL were spread farther apart from each other and had larger cell bodies and longer neurites.
To evaluate the correlation between presence of NG2 cell contacts and neuroblast maturity, we used triple labeling with NG2, DCX, and the mature neuronal markers MAP2ab or NeuN, known to label postmitotic neurons (Garner and Matus, 1988; Tucker et al., 1989; Mullen et al., 1992). Maturing neurons were identified as cells that coexpressed DCX and NeuN or MAP2ab. More than 99% of DCX-positive neuroblasts in the OB RMS lacked NeuN expression (Fig. 7F,F′), whereas 53% of the DCX-positive neuroblasts in the OB GCL expressed NeuN, indicating increased neuronal maturation in the OB. Both NeuN-negative and NeuN-positive neuroblasts were contacted by NG2 cells in the OB GCL (Fig. 7G). Similar results were observed with NG2, DCX, and MAP2ab triple labeling. To examine more closely the contact between differentiating neuroblasts and NG2 cells, sections were triple labeled for NeuN or MAP2ab, NG2, and PSA-NCAM, which is expressed on the cell surface of neuroblasts and thus would be spatially closer to sites of contact than the cytoplasmic antigen DCX. We found that almost 90% of PSA-NCAM-positive neuroblasts in the OB GCL colabeled with NeuN or MAP2ab. The higher percentage of PSA-NCAM-positive neuroblasts that colabeled with mature neuronal markers compared with DCX-positive neuroblasts in the OB GCL suggests that PSA-NCAM expression is maintained longer than DCX expression in maturing neurons. We found that nearly all PSA-NCAM-positive neuroblasts that coexpressed NeuN or MAP2ab were contacted by NG2 cells (Fig. 7H,I). NG2 cell contacts were seen at the neuronal cell body as well as at proximal and distal neurites in neuroblasts. Mature OB neurons lacking DCX or PSA-NCAM expression also exhibited extensive contacts with NG2 cells. Taken together, our findings indicate that differentiating neuroblasts and mature neurons in the OB GCL are more extensively contacted by NG2 cells than more immature neuroblasts in the SVZ and RMS.
DISCUSSION
Our findings show that NG2 cells in the SVZ and RMS are antigenically indistinguishable from parenchymal NG2 cells and are distinct form SVZ type A, B, or C cells. Fate-mapping studies showed that NG2 cells give rise to oligodendrocytelineage cells but not neurons in the OB. Furthermore, we found that NG2 cells are less abundant in the SVZ and RMS than in the nonneurogenic parenchyma but were more prevalent in the OB, where they appear to contact maturing neurons.
NG2 cells were proposed to represent type C-like cells in the postnatal SVZ (Aguirre et al., 2004). To determine whether NG2 cells in the SVZ overlap with neuro(no)genic or multipotent cells in the SVZ, we performed a detailed phenotypical analysis of NG2 cells in the postnatal SVZ with cell-specific markers. Arturo Alvarez-Buylla and colleagues (2002) have characterized the main cell types that constitute the SVZ and demonstrated their lineage relationship. According to the authors' nomenclature, self-renewing multipotent neural stem cells in the SVZ express GFAP and are called SVZ type B cells. With our double-labeled sections, we found that NG2 cells in the SVZ did not express GFAP. SVZ type B cells generate neuroblasts (SVZ type A cells) via a transiently amplifying intermediate precursor cell type (SVZ type C cells; Doetsch et al., 1997, 1999). The homeobox transcription factor Dlx2 has been shown to be expressed in transit-amplifying type C cells and neuroblasts (type A cells) but not in neural stem cells (type B cells) in the adult SVZ (Porteus et al., 1994; He et al., 2001; Marshall and Goldman, 2002). In colabeling experiments, we found that NG2 cells did not express Dlx2, nor did NG2 cells express immature neuronal antigens such as DCX, PSA-NCAM, and class III β-tubulin known to be expressed by SVZ neuroblasts but not by type B or C cells (Rousselot et al., 1995; Doetsch et al., 1997; Gleeson et al., 1999; Nacher et al., 2001).
To confirm further that NG2 cells were distinct from the previously identified populations in the SVZ, we next focused on the proliferative cells of the SVZ. Doetsch and colleagues (1997) had shown by injecting mice with tritiated thymidine that dividing cells in the SVZ consisted of 52% type C cells, 15% type A cells, and 12% type B cells, and the remaining 20% of the proliferating cells could not be identified ultra-structurally. When we combined NG2 immunolabeling with three different methods of detecting proliferating cells (immunodetection of Ki67, phosphorylated histone H3, and administered thymidine analogue BrdU), although over 94% of proliferating cells in the forebrain outside of the neurogenic SVZ were NG2 cells, as previously described (Dawson et al., 2003), we found that fewer than 1% of proliferating cells in the SVZ were NG2 cells. Our finding that proliferating cells in the SVZ are NG2 negative further supports the notion that NG2 cells in the SVZ are a separate entity from SVZ type A, B, and C cells. Finally, NG2 cells constituted a very small fraction of total cells in the SVZ in our study (0.15%), which is lower than previously reported proportions of SVZ type A, B, and C cells, which were found to represent 34%, 25%, and 10% of cells, respectively (Doetsch et al., 1997). Our quantitative data showing the paucity of NG2 cells in the SVZ relative to surrounding nonneurogenic brain regions are consistent with previous qualitative findings from developing and adult rodent brain (Nishiyama et al., 1996; Diers-Fenger et al., 2001; Parras et al., 2007; Zhu et al., 2008). Taken together, our data strongly suggest that NG2 cells in the SVZ are distinct from SVZ type A, B, and C cells that are responsible for neurogenesis in this niche. This is corroborated by the lack of neuronal differentiation from NG2 cells in the OB in P14 NG2creBAC:ZEG double-tg mice.
Multipotentiality, i.e., the ability to generate the three neural lineages, neurons, astrocytes, and oligodendrocytes, has been suggested as a characteristic of NG2 cells in earlier studies. It was shown that A2B5-positive oligodendrocyte precursor cells isolated from postnatal rat optic nerve could under special in vitro conditions be reprogrammed to transition through an intermediate astrocytic stage into multipotent cells that could generate neurons, astrocytes, and oligoden drocytes (Kondo and Raff, 2000). Furthermore, human subcortical glial cells isolated by 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) promotor activity formed multipotent neurospheres and generated neurons when they were maintained under highly purified low-density culture conditions (Roy et al., 1999). The CNP promotor is active in oligodendrocyte precursor cells and mature oligodendrocytes. Similarly, by using transgenic mice expressing EGFP under the murine CNP promoter, Gallo and colleagues have demonstrated that purified EGFP reporter-positive NG2 cells, cultured with epidermal growth factor and basic fibroblast growth factor, generated neurospheres that gave rise to neurons, astrocytes, and oligodendrocytes (Belachew et al., 2003). Furthermore, neuronal differentiation was reported when purified NG2 cells were grafted into neurogenic regions in the embryonic or early postnatal host brain (Belachew et al., 2003; Nunes et al., 2003; Aguirre and Gallo, 2004; Aguirre et al., 2004).
Gallo and colleagues had also previously performed a phenotypical analysis of the EGFP-positive NG2 cells in the SVZ of EGFP-CNP mice. The authors found that NG2 cells in the early postnatal and adult SVZ expressed markers previously shown to be expressed by SVZ type C cells, such as Dlx gene family proteins (dlx1/2 and dlx5/6), as well as typical type A cell markers, such as PSA-NCAM, class III β-tubulin, and DCX (Belachew et al., 2003; Aguirre and Gallo, 2004; Aguirre et al., 2004). Our observations differ from these findings, insofar as we never saw NG2 cells in the postnatal SVZ colocalizing with any of these markers for SVZ type A or C cells. Even though NG2 cells were infrequent in the SVZ, parenchymal NG2 cells, which were often located close to the SVZ, extended processes into this germinal zone. Our ultrastructural analysis of the SVZ in NG2-immunolabeled sections demonstrated that ample slender NG2-immunoreactive profiles were often clustered together and found to surround and insert between SVZ cells, sometimes contacting their cell surface. Although we observed contact between NG2-labeled cells and what we could ultrastructurally identify as SVZ type A cells and C cells, NG2 immunolabeling was never observed on the type A or C cells themselves. It is thus possible that, in the SVZ, labeling of NG2 cell processes that are intimately associated with SVZ cells could be misinterpreted as expression of NG2 antigen by SVZ cells.
Our findings that NG2 cells are distinct from the neurogenic cell types in the SVZ, type A, B, and C cells, could explain the findings of a predominantly glia-restricted fate and lack of neurogenesis reported in our recent NG2 cell fate-mapping study in the forebrain (Zhu et al., 2008). In that study, DsRed-positive NG2 cells were purified from NG2DsRedBAC transgenic mice by fluorescence-activated cell sorting to study the fate of NG2 cells in vitro. When DsRed-expressing NG2 cells were purified from dissected material that contained the SVZ and the cells were cultured under in vitro conditions previously reported to promote neuronal differentiation from NG2 cells (Belachew et al., 2003), no neurons were generated. This indicates that the SVZ did not contain NG2 cells that were neuronogenic. Moreover, Cre recombinase fate-mapping data in the same study also showed that NG2 cells give rise to oligodendrocytes and a subpopulation of astrocytes in vivo, but there is no evidence of neuronal differentiation of NG2 cells (Zhu et al., 2008). Our data are further supported by another recent study demonstrating that NG2 cells, isolated from intact or lesioned adult brain, fail to generate multipotent neurospheres that give rise to neurons, astrocytes, and oligodendrocytes (Buffo et al., 2008).
NG2 cells in the SVZ coexpressed the oligodendrocyte precursor marker PDGFRα, as previously reported for gray and white matter NG2 cells (Nishiyama et al., 1996, 1997) and thus likely represent oligodendrocyte precursor cells. NG2 cells in the SVZ exhibited heterogeneous morphologies. Although the majority of NG2 cells in the SVZ exhibited long, slender, and highly branched processes typical of NG2 cells (polydendrocytes) in the parenchyma, there were a few NG2 cells exhibiting fewer and shorter processes with less branching that may represent more immature cells in the oligodendrocyte lineage. Previous studies employing retroviral lineage tracing have demonstrated that proliferating cells in the early postnatal SVZ can generate oligodendrocytes in the cerebral cortex, corpus callosum, and striatum (Levison and Goldman, 1993; Levison et al., 1999; Parnavelas, 1999). In adult mice, a demyelinating injury of the corpus callosum can induce SVZ cells to proliferate, and PSA-NCAM-expressing cells that could be a source of remyelinating cells were found in the lesion area (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002). A recent study has used retroviral tracing to demonstrate that SVZ type B cells generate oligodendrocytes in intact brain and after demyelination (Menn et al., 2006). In our immunolabeling experiments, we did not detect GFAP expression in NG2 cells in the SVZ. Our observation that NG2 cells in the SVZ and RMS were more abundant in the outer regions of the SVZ and RMS, closer to the parenchyma, is consistent with the notion that oligodendrocyte precursor cells migrate away from the SVZ as they differentiate.
The apparent delineation of the neurogenic niche from the nonneurogenic parenchyma by NG2 cells, located at the border of the SVZ and RMS, also raises the question of whether NG2 cells might be necessary to confine the migration of neuroblasts to the RMS. NG2 is a chondroitin sulfate proteoglycan (CSPG), and the NG2 molecule has been shown to inhibit axonal growth and to induce growth cone collapse (Dou and Levine, 1994; Ughrin et al., 2003). Observations from Marla Luskin's laboratory have shown that cells from SVZ/RMS explants did not migrate or elaborate processes outside of their niche and that CSPGs surround the RMS but are not expressed inside of it (Bonsall and Luskin, 2002, 2003). In addition, cells from SVZ explants did not adhere to stripes coated with CSPGs and did not extend processes toward CSPG-rich areas. It is possible that NG2-expressing cells surrounding the SVZ/RMS might be preventing neuroblasts from dispersing into the parenchyma.
However, we have shown that NG2 cells themselves provide a favorable substrate for growing axons and that axonal growth cones form extensive contacts with NG2 cells in the developing corpus callosum (Yang et al., 2006). In the current study, we found that NG2 cells were infrequent in the SVZ and proximal RMS but that their density increased distally in the RMS. The greater abundance of NG2 cells in the OB GCL correlated with increased expression of mature neuronal markers in neuroblasts. OB neuroblasts appeared to be more closely associated with NG2 cells than neuroblasts in the SVZ and RMS, which are presumably less mature. Whereas astrocytes regulate neuronal synaptogenesis via soluble factors(Pfrieger and Barres, 1997; Ullian et al., 2001; Christopherson et al., 2005), NG2 cells might be regulating neuronal maturation in the OB by a contact-dependent mechanism.
ACKNOWLEDGMENTS
Dr. W. Stallcup, Burnham Institute, La Jolla, CA (guinea pig anti-NG2 and rabbit anti-PDGFRα IgG); Dr. T. Seki, Tohoku University, School of Medicine, Sendai, Japan (mouse anti-PSA-NCAM IgM); Dr. J. Trotter, University of Mainz, Mainz, Germany (rat anti-AN2 IgG); and Dr. K. Yoshikawa, Osaka University, Osaka, Japan (guinea pig anti-Dlx2 IgG) are gratefully acknowledged for the gifts of antibodies.
Grant sponsor: National Institutes of Health; Grant number: RO1 NS049267 (to A.N.); Grant sponsor: CT State Stem Cell Research Grant; Grant number: 06SCB03 (to A.N.); Grant sponsor: Gothenburg Medical Society (to M.K.); Grant sponsor: Swedish Medical Society (to M.K.); Grant sponsor: Swedish Royal Academy of Sciences (to M.K.); Grant sponsor: Swedish Society for Medical Research (to M.K.); Grant sponsor: Wenner-Gren Foundation (to M.K.).
LITERATURE CITED
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Zigova T, Bakay RA, Luskin MB. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int J Dev Neurosci. 1996;14:921–930. doi: 10.1016/s0736-5748(96)00066-4. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bonsall JM, Luskin MB. The distribution of neuronal progenitor cells originating from the neonatal anterior subventricular zone cultured on cryostat sections of the forebrain. Soc Neurosci Abstr. 2002;423.6 [Google Scholar]
- Bonsall JM, Luskin MB. Chondroitin sulfate proteoglycans (CSPGs) may restrict migrating SVZa-derived progenitor cells to the rostral migratory stream in the rat. Soc Neurosci Abstr. 2003;138.5 [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bu J, Banki A, Wu Q, Nishiyama A. Increased NG2+ glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004;48:51–63. doi: 10.1002/glia.20055. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- del Rio JA, Soriano E. Immunocytochemical detection of 5′-bromodeoxyuridine incorporation in the central nervous system of the mouse. Brain Res Dev Brain Res. 1989;49:311–317. doi: 10.1016/0165-3806(89)90033-3. [DOI] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neuro-genesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Garner CC, Matus A. Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol. 1988;106:779–783. doi: 10.1083/jcb.106.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gratzner HG, Pollack A, Ingram DJ, Leif RC. Deoxyribonucleic acid replication in single cells and chromosomes by immunologic techniques. J Histochem Cytochem. 1976;24:34–39. doi: 10.1177/24.1.815428. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968a;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. II. Cell proliferation and migration. J Comp Neurol. 1968b;134:305–322. doi: 10.1002/cne.901340305. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Nishimura I, Yoshikawa K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci. 2006;26:5383–5392. doi: 10.1523/JNEUROSCI.1262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano R, Usui H, Maeda T, Fukui T, Yamanari N, Ohtsuka E, Ikehara M, Takahashi Y. Molecular cloning and the complete nucleotide sequence of cDNA to mRNA for S-100 protein of rat brain. Nucleic Acids Res. 1984;12:7455–7465. doi: 10.1093/nar/12.19.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo J-M, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridineimmunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yu M, Drazba JA, Tuohy VK. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG. Glial cell lineages in the rat cerebral cortex. Exp Neurol. 1999;156:418–429. doi: 10.1006/exnr.1999.7044. [DOI] [PubMed] [Google Scholar]
- Parras CM, Hunt C, Sugimori M, Nakafuku M, Rowitch D, Guillemot F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 1994;14:6370–6383. doi: 10.1523/JNEUROSCI.14-11-06370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Lois C, Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol. 1991;184:395–401. doi: 10.1007/BF00957900. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Arner LS, Levine JM. An antiserum against the PC12 cell line defines cell surface antigens specific for neurons and Schwann cells. J Neurosci. 1983;3:53–68. doi: 10.1523/JNEUROSCI.03-01-00053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Garner CC, Matus A. In situ localization of microtubule-associated protein mRNA in the developing and adult rat brain. Neuron. 1989;2:1245–1256. doi: 10.1016/0896-6273(89)90309-7. [DOI] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]