Abstract
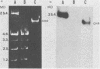
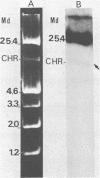
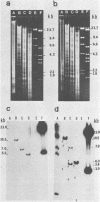
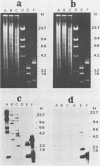
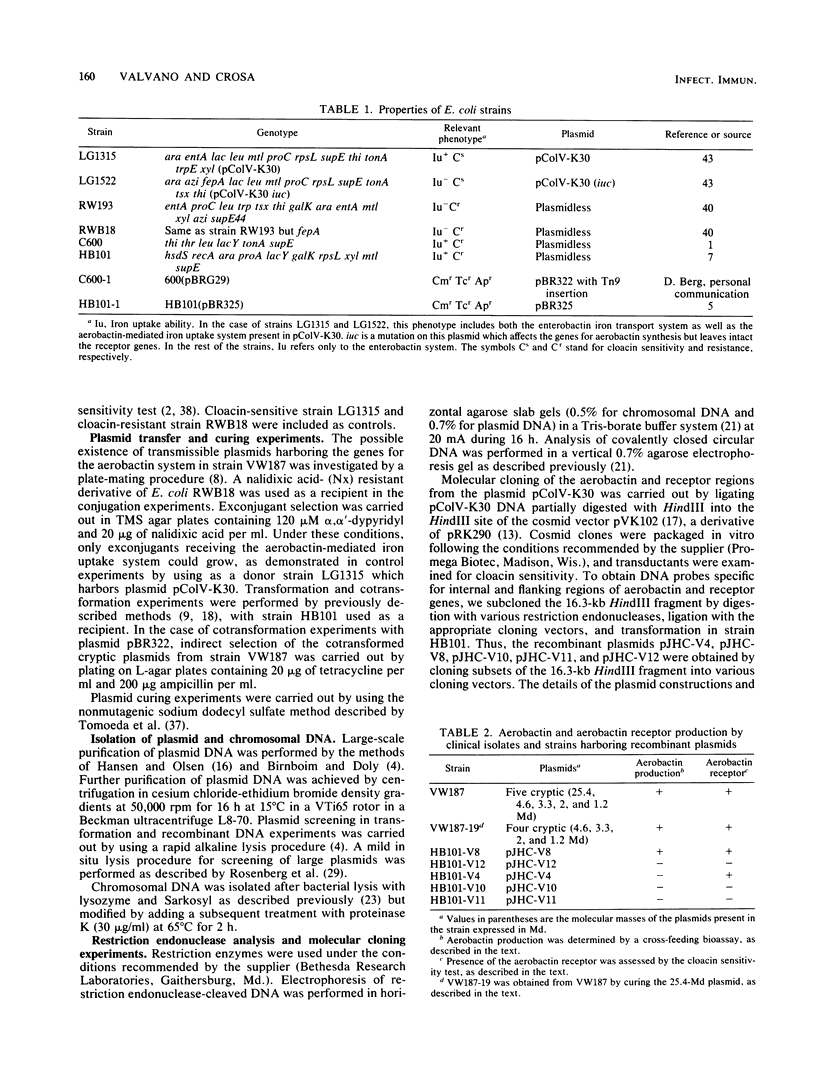
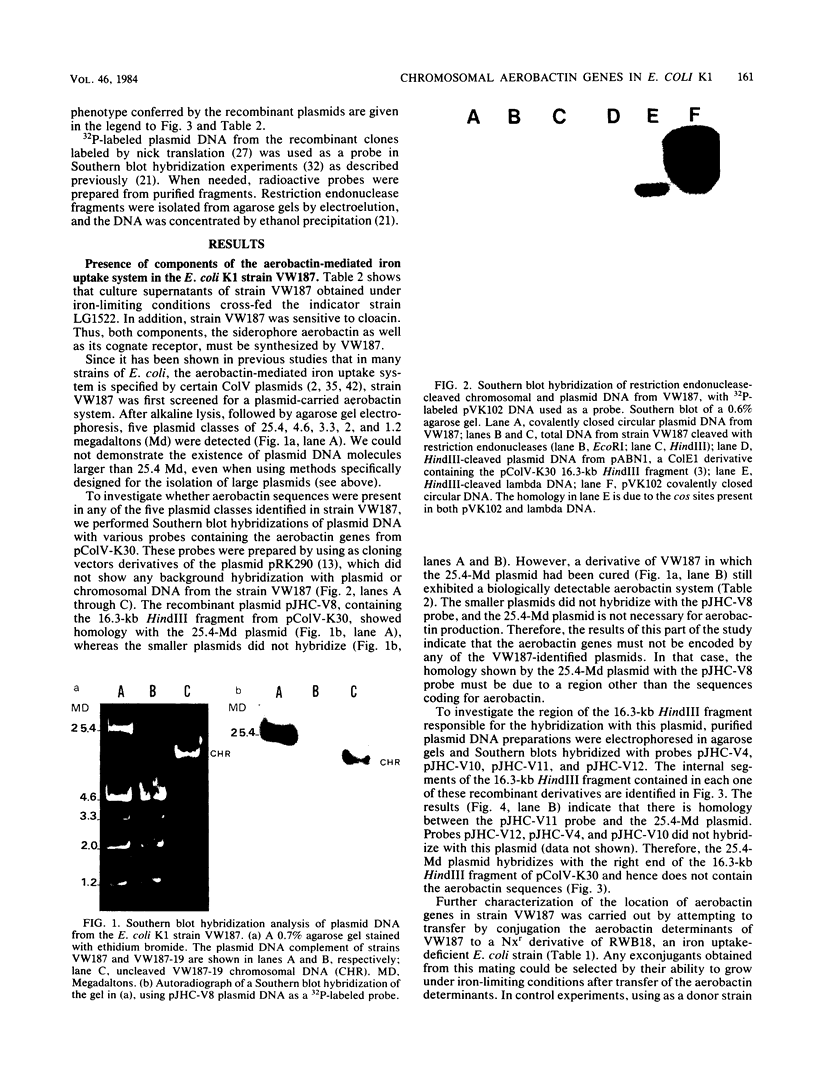
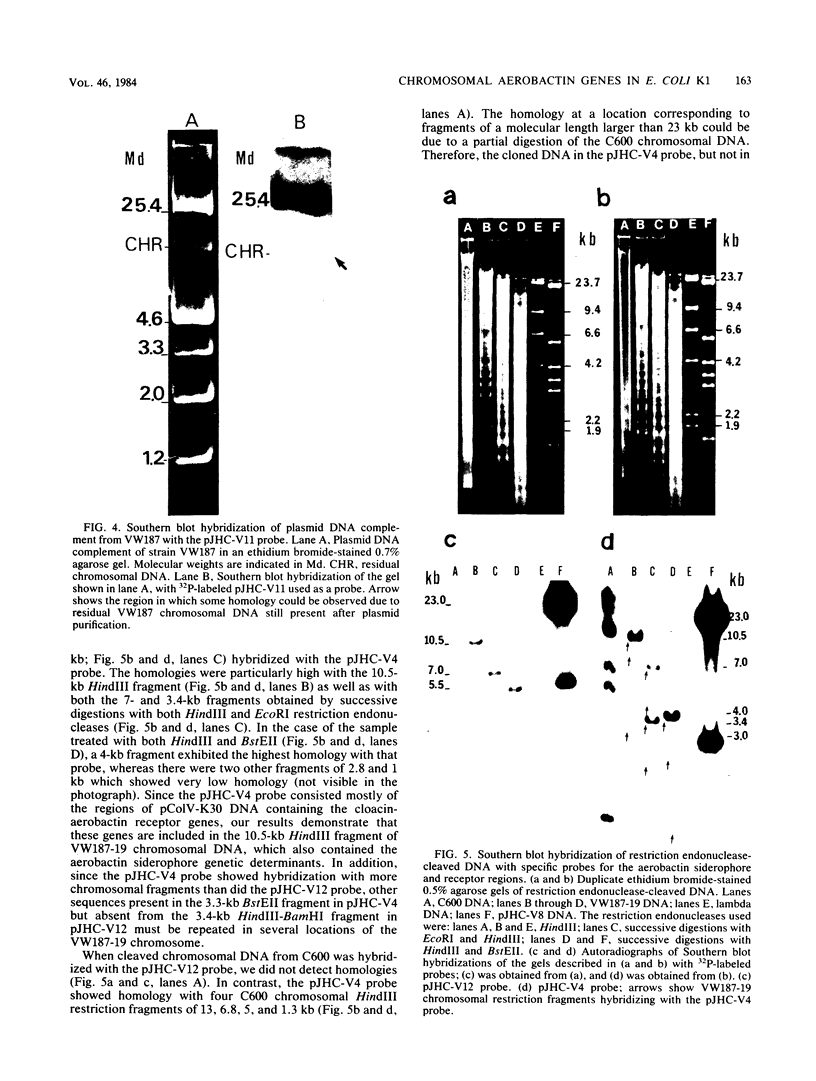
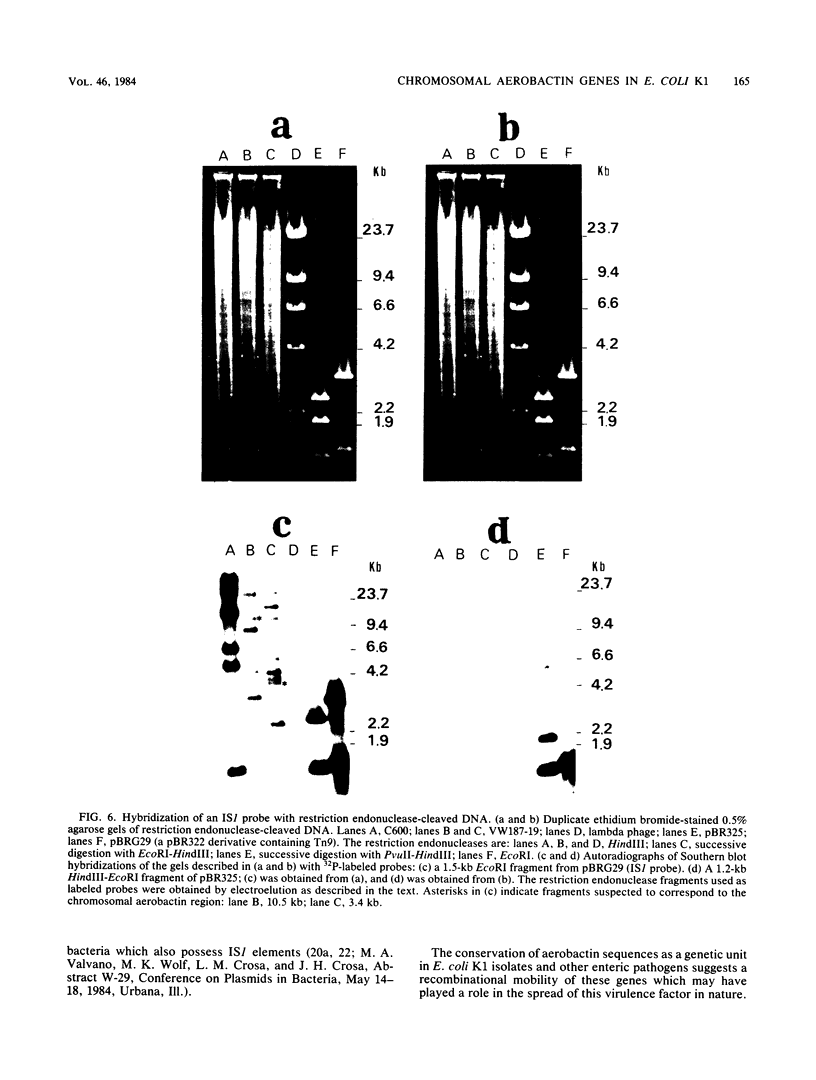
The aerobactin-mediated iron uptake system encoded by pColV-K30 and other ColV plasmids has been associated with the ability of Escherichia coli strains to cause disease. We investigated whether the pColV-K30 aerobactin system is present in E. coli K1 VW187 isolated from a human neonate with meningitis. This strain exhibited a functional aerobactin-mediated iron uptake system, as assessed by a cross-feeding bioassay and by its sensitivity to cloacin, a bacteriocin that recognizes the outer membrane receptor for iron-aerobactin complexes. By using a variety of techniques, we could not find any plasmid harboring the aerobactin genes. Hybridization of restriction endonuclease-cleaved chromosomal DNA from strain VW187 with various clones containing subsets of the pColV-K30 aerobactin region showed that the aerobactin genes were located on a 10.5-kilobase-pair chromosomal HindIII restriction fragment which also contained IS1-like insertion sequences. The chromosomal aerobactin region showed a high degree of conservation when compared with the homologous region in plasmid pColV-K30, although it was located on a different restriction endonuclease site environment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Cloning of the aerobactin-mediated iron assimilation system of plasmid ColV. J Bacteriol. 1983 Feb;153(2):1111–1113. doi: 10.1128/jb.153.2.1111-1113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Taylor D. E., Cohen D. R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Dietzman D. E., Fischer G. W., Schoenknecht F. D. Neonatal Escherichia coli septicemia--bacterial counts in blood. J Pediatr. 1974 Jul;85(1):128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Luirink J., Koningstein G., Oudega B., de Graaf F. K. Subcloning of the cloacin DF13/aerobactin receptor protein and identification of a pColV-K30-determined polypeptide involved in ferric-aerobactin uptake. J Bacteriol. 1983 Nov;156(2):945–948. doi: 10.1128/jb.156.2.945-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone W. J., Oudega B., Stegehuis F., de Graaf F. K. Cloning and expression of the cloacin DF13/aerobactin receptor of Escherichia coli (ColV-K30). J Bacteriol. 1983 Feb;153(2):716–721. doi: 10.1128/jb.153.2.716-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. M., Payne S. M. Aerobactin genes in Shigella spp. J Bacteriol. 1984 Oct;160(1):266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S., Neilands J. B. Plasmid- and chromosome-coded aerobactin synthesis in enteric bacteria: insertion sequences flank operon in plasmid-mediated systems. J Bacteriol. 1984 Jul;159(1):300–305. doi: 10.1128/jb.159.1.300-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Opal S., Cross A., Gemski P. K antigen and serum sensitivity of rough Escherichia coli. Infect Immun. 1982 Sep;37(3):956–960. doi: 10.1128/iai.37.3.956-960.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J. F., Crosa J. H. Aerobactin iron uptake sequences in plasmid ColV-K30 are flanked by inverted IS1-like elements and replication regions. J Bacteriol. 1984 Oct;160(1):256–265. doi: 10.1128/jb.160.1.256-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infect Immun. 1984 Feb;43(2):684–692. doi: 10.1128/iai.43.2.684-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens P., Huang S. N., Welch W. D., Young L. S. Restricted complement activation by Escherichia coli with the K-1 capsular serotype: a possible role in pathogenicity. J Immunol. 1978 Dec;121(6):2174–2180. [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]