Abstract
Purpose
To investigate the action of epidermal growth factor (EGF) on corneal keratocyte differentiation and its effects in conjunction with transforming growth factor (TGF)-β1.
Methods
Rabbit corneal keratocytes (RCK) were treated with EGF, TGF-β1 or EGF plus TGF-β1 in the presence or absence of inhibitors of EGF-receptor (EGF-R), neutralizing concentrations of EGF antibody and of signaling kinases for 2 days to 1 week. RCK differentiation to myofibroblasts was identified with anti-aldehyde dehydrogenase-1 (ALDH1) and α-smooth muscle actin (α-SMA) antibodies. Cell proliferation was evaluated with anti-Ki-67 antibody. Extracellular matrix (ECM) components were assayed by immunochemistry and Western blot. Cell migration images were captured with a camera attached to the microscope, and the wound area was calculated using MetaVue imaging software.
Results
RCK cultured in serum-free DMEM/F12 without frequent changes of medium maintained the phenotype for more than one month. EGF stimulated differentiation into a proto-myofibroblasts phenotype with loss of dendritic shape and expression of α-SMA. Treatment with TGF-β1 stimulated 12% of the cells to differentiate to defined myofibroblasts, but in the presence of EGF, TGF-β1 induced 90% of RCK to transform into myofibroblasts. Inhibition of EGF-R activation and of the phosphatidylinositol-3 kinase (PI-3K)/Akt-1 pathway prevented the action of EGF on TGF-β1 cell differentiation. TGF-β1 in the presence of EGF also increased cell migration, which is inhibited by blocking EGF-R activation.
Conclusions
Our data show that EGF contributes to differentiation and migration of myofibroblasts induced by TGF-β1 through EGF-R activation and is an important modulator of wound healing and scar tissue formation.
Introduction
The corneal stroma constitutes 90% of the corneal volume and consists of a highly organized and uniquely transparent extracellular matrix (ECM) of collagen fibrils and proteoglycans that provide both the refractive shape and the tensile strength of the tissue. Keratocytes are the principal cells of the stroma, which are responsible for the synthesis and maintenance of the ECM components. In normal adult cornea, keratocytes appear as a population of flat and dendritic cells, residing between the collagen lamellae and connecting to each other through a network of extensive processes.1–4 These cells are mitotically quiescent cells containing few mitochondria or endoplasmic reticulum and no nucleoli. Turnover of keratocytes is low (two to three years), and remodeling of the stromal ECM is undetectable over time.5–7 These homeostatic characteristics of stroma contribute to corneal transparency.
After injury, the quiescent keratocytes at the wound periphery become metabolically activated and transform to their repair phenotypes, corneal fibroblasts and/or myofibroblasts, which migrate into the damaged area, proliferate, and deposit a disorganized and fibrotic ECM to repair the wound.8–13 These cells differ from keratocytes in many aspects, including loss of dendritic shape, high proliferation rate, down-regulation of the expression of keratin sulfate (KS) proteoglycans and aldehyde dehydrogenase (ALDH), and up-regulation of chondroitin sulfate (CS) proteoglycans and fibronectin (FN).5–7,12,14 Both corneal fibroblasts and myofibroblasts contribute to normal wound healing, but myofibroblasts play a crucial role in tissue fibrosis because they produce ECM components at a high rate, and regulate contractile elements that generate the strength necessary for wound closure.6,7,15 If the remodeling of the ECM is not controlled, as in corneal disorders such as dystrophies, pterygia, pronounced wounds, or infections, it can lead to corneal opacity and loss of vision.
One of the first responses to corneal injury is inflammation, with the synthesis of several lipid mediators such as prostaglandins, lipoxygenase metabolites and platelet–activating factor10, 16 and several growth factors and cytokines7, 17; this is followed by apoptosis of keratocytes close to the wound.18, 19 The next step is a repair response with cell differentiation, proliferation of keratocytes to fibroblasts and myofibroblasts and changes in ECM components. Keratocyte differentiation is controlled by a variety of growth factors, including TGF-β, platelet-derived growth factor (PDGF), basic fibroblast growth factor (FGF2), and insulin-like growth factor 1 (IGF-1).20–23 TGF-β causes keratocyte differentiation to myofibroblasts with expression of α-SMA, along with increased CS proteoglycans and decreased KS proteoglycans.20,24 On the other hand, FGF2 and PDGF induce keratocyte differentiation to fibroblasts.22 In serum-cultured corneal fibroblasts, addition of FGF2 results in down-regulation of α-SMA and upregulation of KS proteoglycans.21
EGF, through binding to EGF-R, stimulates its tyrosine kinase activity leading to DNA synthesis, production of ECM molecules, and cell proliferation.25, 26 Receptor phosphorylation also leads to actin cytoskeletal rearrangement, which promotes cell motility. All three corneal cell types express EGF and its receptor, suggesting that EGF affects corneal cells in an autocrine, paracrine, or possible juxtacrine manner.17,27,28 EGF stimulates proliferation of corneal epithelial and endothelial cells, and accelerates epithelial wound healing.29–32 Topical application of EGF significantly increases the tensile strength of sutured or unsutured full thickness corneal incisions even under conditions where stromal healing was impaired by corticosteroids33–39; however, little is known about the cellular and molecular mechanisms by which EGF exerts this action. Here, we have investigated the effects of EGF on proliferation, differentiation and expression of ECM components in isolated keratocytes, which, in turn, could maintain its phenotype for extensive periods in culture, mimicking the in vivo situation. We have also investigated the involvement of the signaling pathways activated by the EGF-R. We have demonstrated that synergisms exist between EGF and TGF-β1 which increase differentiation and migration of myofibroblasts.
Methods
Materials
Recombinant human EGF, TGF-β and goat anti-EGF antibody (AB-236-NA) were purchased from R&D Systems, Inc. (Minneapolis, MN); 4-(3-chloroanilino)-6, 7-dimethoxyquinazoline (AG1478) from Biosource International, Inc. (Camarillo, CA). 4, 6-diamidino-2-phenylindole (DAPI), mouse monoclonal α-SMA, anti-vimentin (clone Vim-13.2), anti-chondroitin sulfate (clone CS-56), anti-collagen type IV (clone Col-94), anti-cellular fibronectin (clone FN-3E2), anti-human fibronectin (clone IST-3) and antilaminin (clone LAM-89), anti-proliferating cell protein Ki-67(clone pp-67) were purchased from Sigma (St. Louis, MO). Mouse anti-collagen type III (FH-7A) antibody (abcam7) and monoclonal anti-keratin sulfate antibody (clone 5-D-4) were purchased from Seikagaku Corporation (Chuo-ku, Tokyo, Japan). Mouse anti-TSP-1 was purchased from Abcam Inc. (Cambridge, MA). Mouse anti-Akt1 (2H10) and anti-phospho Akt (Ser473) monoclonal antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Goat anti-EGF receptor (sc-03-G) and p-EGF receptor (sc-12351) were from Santa Cruz Biotechnology (Santa Cruz, CA), and the MAPK-K inhibitor, PD98059, and Goat anti-aldehyde dehydrogenase 1(ALDH1) were from Calbiochem (San Diego, CA). The p38 inhibitor SB203580 was from Upstate Biotechnology (Lake Placid, NY). LY294002 was from Calbiochem (La Jolla, CA). All SDS reagents were from Bio-Rad (Hercules, CA); polyvinylidene difluoride (PVDF) membranes were from Amersham Pharmacia Biotech, Inc. (Piscataway, NJ).
Isolation and culture of rabbit corneal keratocytes
Rabbit eyes (Pel-Freez Biologicals, Rogers, AR) were shipped to the laboratory on ice in Hanks solution containing antibiotics and antimycotic and used within 24 hrs after enucleation. After marking with a 10-mm diameter trephine, the corneal epithelia together with a thin layer of stroma were removed by a superficial keratectomy under a dissecting microscope. The central corneal button was cut off and the endothelium together with the Descemet’s membrane was removed with a tooth-forceps under the microscope. The stromal buttons were cut into small pieces of 1 × 2 mm2 and digested with 3 mg/ml of collagenase in DMEM/F12 (1:1) for 4 hrs at 37°C. Isolated rabbit corneal keratocytes (RCK) were collected by centrifugation, resuspended in DMEM/F12 with 100 µg/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin, and seeded at the concentration of 1.5 × 105 cells/ml in the same medium in 12-well plates (2.25 × 105 cells per well) or 100-mm dishes (1.5 × 106 cells per dish). When the cells were attached, the media was changed to remove the dead cells and debris (1 ml or 10 ml, respectively). To maintain the cell phenotype, 1/10 of the original volume of new medium was added carefully every week (e.g. 100 µl per well for a 12-well plate, and 1 ml for a 100-mm dish). To stimulate the cells, RCK were incubated in the presence of EGF, TGF-β1, or a combination of TGF-β1 and EGF, added in a total volume of 100 µl DMEM/F12. Fibroblasts were obtained by incubating RCK in DMEM/F12 containing 10% fetal bovine serum (FBS). Differentiated myofibroblasts were obtained by subculture of fibroblasts at low density and incubated with 5% FBS for 3 days as previously described.14, 40
Immunofluorescence staining
Cells were washed in PBS and fixed with 2% paraformaldehyde in 0.1 M phosphate buffer for 30 min at 4°C and permeabilized with 0.3% Triton X-100 solution for 5 min on ice. The remaining procedures were performed at room temperature. Following three washes with PBS, the cells were incubated with 10% normal goat serum in PBS containing 0.1% bovine serum albumin (PBS-BSA) for 30 min to block non-specific binding. Afterwards, the cells were incubated for 1 hr with the corresponding primary antibodies at optimal dilutions in PBS containing 1.5% normal goat serum. After rising with PBS-BSA (3 × 5 min), cells were incubated with the corresponding secondary antibodies for 45 min. DAPI was used to counterstain the nuclei. In all assays, negative controls were prepared using normal mouse Ig G (Santa Cruz, sc-2025) or 0.1 M PBS instead of the primary antibody to exclude non-specific staining.
Western blot analysis
After the different treatments, cell cultures on 100-mm dishes were rinsed twice with PBS and harvested in modified RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 µg/ml each of aprotinin, pepstatin and leupeptin, 1 mM Na3VO4, 1 mM NaF) and analyzed by Western blot using different antibodies as described previously by our laboratory.14 Bound antibodies were visualized following chemiluminescence detection (ECL plus kit) on autoradiographic film.
Cell proliferation and migration
Seven-day cultures of RCK in 12-well plates were treated with EGF, TGF-β1 or a combination of both in the presence or absence of AG1478 for 48 hrs. Cultures were also treated with TGF-β1 and anti-EGF antibody. Cell proliferation was assessed by immunostaining with anti-Ki-67 antibody. DAPI was used to counterstain the nuclei. The number of Ki-67-positive nuclei versus all the nuclei as shown by DAPI staining were counted in a blind fashion in 10 different fields, clockwise at positions 3, 6, 9, 12 o’clock, and center at low magnification (10X) in two wells and averaged. Ki-67-positive cells were expressed as a percentage of total cells counted. To assay cell migration, the RCK cultures were wounded by a linear scratch with a sterile plastic tip in the center of the well and incubated for 24 hrs in DMEM/F12 with or without growth factors. In the experiments in which AG1478 or anti-EGF antibody were employed, they were added 30 min prior to the wound. Cell migration was determined by phase contrast images collected by a camera attached to the microscope, and the wounded area was calculated using MetaVue imaging software. Each point represents the analysis of images collected from 10 different wounded areas in two different wells.
Statistical analysis
Data are presented as means ±SD. Comparisons between groups at each time point of treatments were conducted by one-way ANOVA followed by Tukey’s test. P < 0.05 was considered as statistically significant.
Results
Keratocyte culture and phenotype maintenance
RCK cultured in serum-free DMEM/F12 without frequent changes of medium retained their phenotype for more than one month (Figure 1A). These cells showed typical dendritic morphology and were connected to each other with long processes to form a network as seen in vivo. They were strongly stained for vimentin, a general marker of mesenchyma-derived cells (Figure 1B), and showed an eccentric bean-shaped nucleus. RCK were also positively stained for ALDH1, a marker for keratocyte phenotype, but negatively stained for α-SMA (Figure 1C). In contrast, when the cells transformed to myofibroblasts by plating fibroblasts at low density14, 40, they showed the characteristic positive staining for α-SMA but negative staining for ALDH1. In addition, both keratocytes and myofibroblasts expressed the EGF-R. These results were further confirmed by immunoblotting (Figure 1D), in which ALDH1 appeared as a single band of about 50 kDa in the samples of RCK but not in the myofibroblasts, while α-SMA was present in the transformed cells with stronger EGF-R expression.
Figure 1. Keratocyte culture and phenotype identification.

(A) Phase contrast images of RCK at different times in culture (bar = 50 µm). (B) Immunofluorescence with anti- vimentin antibody, a general marker of cells originating in the mesenchyme, showed the morphology and cell-to-cell contacts of one week cultures of keratocytes. The nuclei, stained with DAPI in red, can be well distinguished at higher magnification (bar = 25 µm). (C) Immunofluorescence staining of α-SMA, ALDH1, and EGF-R in keratocytes and myofibroblasts obtained as described. (D) Duplicate samples analyzed by Western blot for α-SMA, ALDH1, and EGF-R in keratocytes and myofibroblasts. Anti-ALDH1 antibody recognized a single band at the expected size of 51 kDa in the sample of corneal keratocytes, while anti-α-SMA antibody detected a band of 46 kDa in myofibroblasts. Anti-EGF-R antibody detected a band at the expected size of 170 kDa in both keratocytes and myofibroblasts. Vimentin was used as a loading control. The experiment was repeated once with similar results.
EGF stimulates keratocyte differentiation to proto-myofibroblasts and promotes ECM expression and cell proliferation
One week cultures of RCK were treated with different concentrations of EGF for 2 days. Western blot showed that EGF induced α-SMA expression in a dose-dependent fashion, with optimal concentration at 50 ng/ml (Figure 2A). Immunostaining with α-SMA (Figure 2B) showed that the cells differentiated to a proto-myofibroblast phenotype in which the stain was prominent in the cytoplasm but was not organized into stress fibers, which are typical of a well-differentiated myofibroblasts.41 There was loss of dendritic morphology and process connection and up-regulation of CS expression and down-regulation of KS expression, characteristics of altered phenotype.6,7 EGF also stimulated the expression of several ECM components (Figure 2C), including FN, thrombospondin-1 (TSP-1), laminin (LM) and collagen (Coll) types III and IV. In addition, EGF treatment induced the expression of pro-inflammatory cytokines such as interleukin-1α, interferon-γ and metalloproteinase-2 (data not shown). Similar results were obtained when keratocytes were incubated with EGF for 1 week. In addition, EGF increased cell proliferation as detected by Ki-67 staining (Figure 2B). Cell proliferation was stimulated after 2 and 4 days and decreased by day 7 (Figure 2D).
Figure 2. Effects of EGF on keratocyte transformation, proliferation, and ECM expression.

(A) Seven-day RCK cultures were treated with different concentrations of EGF for 2 days and analyzed by Western blot. (B) Immunofluorescence was performed in RCK treated with 50 ng/ml of EGF to detect expression of α-SMA, CS, KS, and Ki-67; (B) Expression of FN, TSP-1, LM, Coll III and Coll IV in EGF- treated cells. DAPI was used to stain the nuclei. For better contrast of images, DAPI blue was converted to red. The images of controls in which those ECM components were not found have been omitted. Images shown are representative of three independent experiments, and; (C) Time course of cell proliferation induced by EGF. Data are expressed as average ± SE of percentages of Ki-67 positive cells versus total cells counted in a blind fashion in 10 different fields of two wells. The experiment was repeated twice with similar results.
EGF stimulation induces activation of Akt-1 and ERK1/2 MAP kinase in corneal keratocytes
To determine which signaling pathways are activated, RCK were stimulated with EGF (50 ng/ml) for different times and phosphorylation of Akt-1 and the mitogen-activated protein kinases (MAPK), ERK1/2, and p38 were determined by Western blot (Figure 3A). EGF rapidly phosphorylates Akt-1 at Ser473 with a peak at 15 min and a decrease by 60 min, although it was still higher than controls at 2 hrs. The growth factor also increased the phosphorylation of ERK1/2 with a peak at 15 min but did not have any effect on p38 activation. There were no changes in the expression of the total proteins. Immunofluorescence showed that Akt-1 in unstimulated cells was expressed throughout the cytoplasm, and treatment with EGF for 30 min induced the nuclear localization of p-Akt (Figure 3B).
Figure 3. EGF induced Akt-1 and ERK phosphorylation in corneal keratocytes.
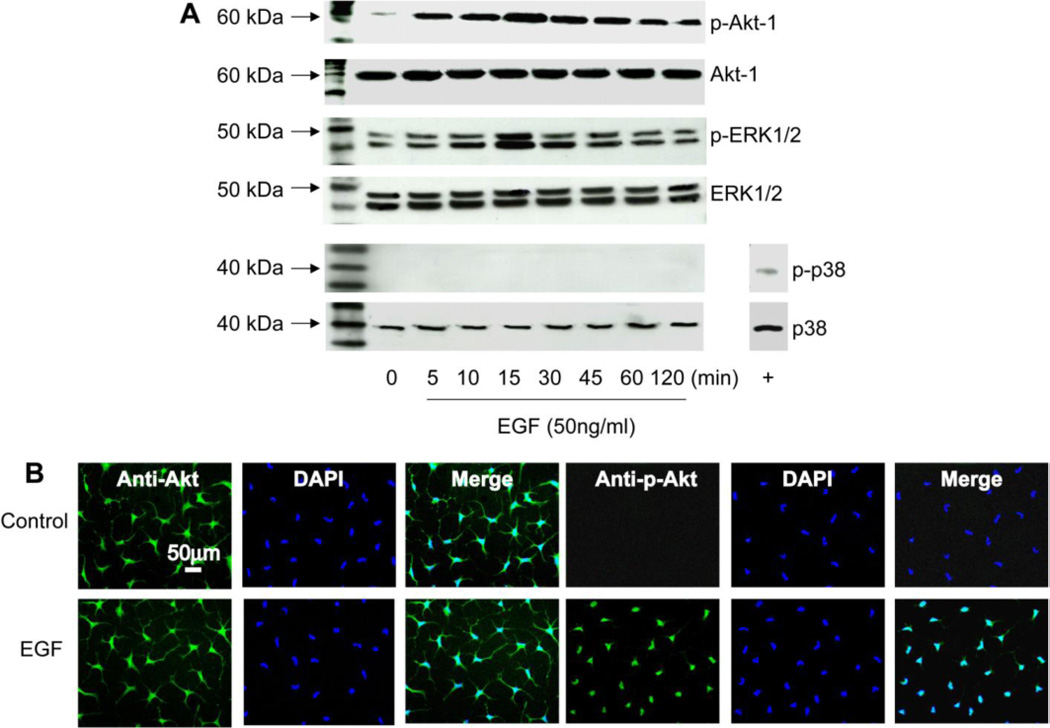
(A) Seven-day RCK cultures were stimulated with EGF (50 ng/ml) for 5, 10, 15, 30, 45, 60, and 120 min and analyzed by Western blot with antibodies for the phosphorylated form of Akt-1, ERK1/2, and p38. As positive control (+) for p-p38, a protein lysate of rabbit corneal epithelial cells stimulated with EGF was used. Antibodies for total Akt, ERK1/2 and p38 demonstrate similar gel loading. (B) Immunofluorescence shows the nuclear localization of p-Akt in RCK after stimulation with EGF (50 ng/ml) for 30 min. DAPI stain the nuclei (blue). Data shown are representative of three separate experiments.
EGF synergizes TGF-β1-induced transformation of keratocytes to myofibroblasts
To test what effect EGF has on TGF-β1-induced differentiation, RCK cultures were treated with EGF (50 ng/ml), TGF-β1 (10 ng/ml) or a combination of both growth factors for 7 days, and the expression of α-SMA and FN were analyzed by immunofluorescence and Western blot. Previous work had shown that this concentration of TGF-β induces keratocyte differentiation.24 As already shown, treatment with EGF induced transformation of 100% of the cells into a proto-myofibroblast phenotype that synthesized FN (Figure 4 A, B); treatment with TGF-β1 induced 88±4 % of RCK to differentiate into proto-myofibroblasts and 12±4 % into myofibroblasts, characterized by its large, spread morphology. However, treatment with TGF-β1 plus EGF induced 91±4 % of the cells to differentiate into definitive myofibroblasts, with significant increases in the expression of α-SMA and FN (Figure 4 A, C).
Figure 4. EGF and TGF-β1 promote keratocyte differentiation and fibronectin synthesis.

Seven-day RCK cultures were treated with EGF (50 ng/ml), TGF-β1 (10 ng/ml), or EGF plus TGF-β1 for one week. (A) Immunostaining with α-SMA and FN antibodies. Nuclei were counterstained with DAPI. The color of DAPI stain in the merged images with FN was changed from blue to red for better contrast. (B) Average percentage of proto-myofibroblasts versus differentiated myofibroblasts counted in a blind fashion in 10 different fields of two-well plates. The results are representative of two separate experiments. (C) Cell total homogenates were analyzed by Western blot with antibodies for α-SMA and FN. Vimentin was used as a loading control. The data are representative of three separate experiments. The relative signal strengths of α-SMA or FN to vimentin are presented as means ±SD.
Inhibition of EGF receptor or PI-3K signaling abolishes the synergistic effect on TGF-β1 keratocyte differentiation and FN expression
To investigate the interaction of EGF with TGF-β, RCK were treated for 7 days with EGF (50 ng/ml), TGF-β1 (10 ng/ml), or EGF plus TGF-β with or without AG1478 (10 µM), a specific inhibitor of EGF-R activation; LY294002 (15 µM), an inhibitor of PI-3K; PD98059 (50 µM), an inhibitor of MEK-K; and, SB203580 (20 µM), a p38 inhibitor. None of these inhibitors exhibited visible cell toxicity, as assayed by the LIVE/DEAD® Baclight™ Viability Kit (Molecular Probes®, Eugene, Oregon). Immunofluorescence (Figure 5A) showed that AG1478 completely inhibited EGF-induced keratocyte differentiation as well as FN expression. Similar inhibitory effects were observed in the presence of LY294002, but not in the presence of PD98059 or SB203580. When keratocytes were stimulated with both EGF and TGF-β1, inhibition of the EGF receptor or the PI-3K/Akt-1 signal decreased differentiation and FN secretion, while inhibition of the ERK1/2 or p38 pathways had no effect (Figure 5A). Western blot analysis revealed that blocking the EGF-R resulted in a significant inhibition (*p<0.05) in the TGF-β1 stimulated expression of α-SMA as well as in the FN secretion (Figure 5B). These results suggest that TGF-β1 acts through the EGF-R to induce keratocytes to differentiate and secrete FN.
Figure 5. Effect of EGF receptor and kinase inhibition on keratocyte differentiation and fibronectin expression.

Seven-day RCK cultures were treated with EGF (50 ng/ml) and EGF plus TGF-β1 (10 ng/ml) with or without AG1478 (10 µM), or LY294002 (15 µM), PD98059 (50 µM), or SB203580 (20 µM) for one week. (A) Representative images of immunofluorescence with α-SMA and FN. DAPI stain the nuclei (blue). (B) Western blot shows that inhibition of EGF receptor not only blocked the effect of EGF but also of TGF-β1 on expression of α-SMA and FN (*p<0.05). The relative signal strengths of α-SMA or FN to vimentin are presented as means ±SD.
Neutralization of EGF and inhibition of EGF-R on TGF-β1-induced a-SMA and FN expression
To determine whether TGF-β1 acts through EGF-R activation or by inducing EGF synthesis, RCK were treated with TGF-β1 in the presence of anti-EGF antibody at different concentrations (100 ng to 100 µg/ml) or AG1478 (10 mM) for 2 days, and expression of α-SMA and FN were assayed by immunofluorescence and Western blot (Figures 6A and B). Neutralization of EGF with anti-EGF antibody (10 µg/ml) did not prevent the TGF-β1-induced α-SMA and FN expression. Increasing the concentration of antibody up to 100 µg/ml showed similar results (data not shown). On the other hand, inhibition of EGF-R by AG1478 significantly decreased α-SMA and FN expression. These results suggest that TGF-β1 may act through the transactivation of EGF-R. To further test this hypothesis, RCK were stimulated with TGF-β1 (10 ng/ml) at different times and phosphorylation of EGF-R was determined by Western blot. TGF-β1 rapidly induced EGF-R activation with a peak at 10 min and a decrease by 60 min (Figure 6C). TGF-β1-induced EGF-R phosphorylation was completely inhibited by AG1478 (Figure 6D). These studies strongly suggest that TGF-β1 acts through transactivation of EGF-R to promote RCK differentiation and ECM expression.
Figure 6. Effect of neutralization of EGF or inhibition of EGF-R on TGF-β1-stimulated α-SMA and FN expression.
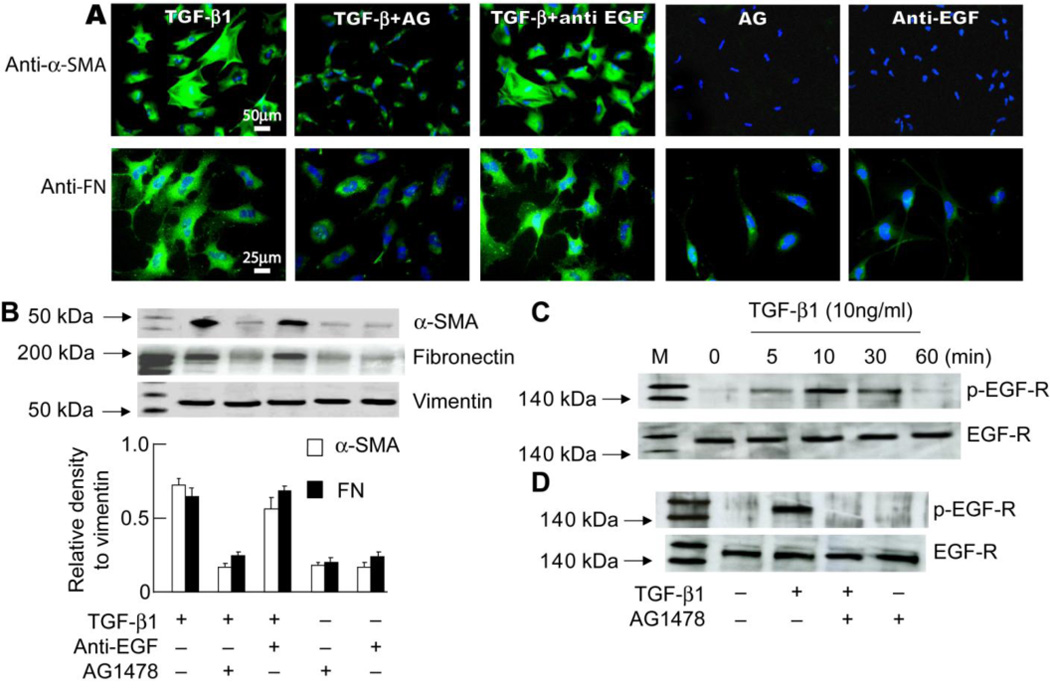
Seven-day RCK cultures were treated with TGF-β1 (10 ng/ml) with or without anti-EGF antibody (10 µg/ml) or AG1478 (10 µM) for 2 days. (A) Representative images of immunofluorescence with α-SMA and FN. DAPI stain the nuclei (blue). (B) Western blot shows that inhibition of EGF receptor, but not neutralization of EGF, decreased the effect of TGF-β1 on expression of α-SMA and FN. The relative signal strengths of α-SMA or FN to vimentin are presented as means ±SD. (Data are representative of three separate experiments). (C) Seven-day RCK cultures were stimulated with TGF-β1 (10 ng/ml) for 5, 10, 30, and 60 min and analyzed by Western blot with antibodies for the phosphorylated form of EGF-R. Antibodies for total EGF-R demonstrate similar gel loading. (D) Seven-day RCK cultures were pretreated with AG1478 (10 µM) for 30 min and then stimulated with TGF-β1 (10 ng/ml) for 10 min. Cell lysates were prepared for Western blot with antibodies for EGF-R and the phosphorylated form of EGF-R.
Inhibition of EGF-R activation prevents EGF-stimulated cell proliferation and migration
Proliferation and migration are two important mechanisms by which corneal stromal cells respond to injury. To determine the relationship between EGF and TGF-β in these two cellular responses, one week cultures of RCK were treated with EGF (50 ng/ml), TGF-β1 (10 ng/ml), or EGF plus TGF-β with or without AG1478(10 µM) for 1 day (migration) and 3 days (proliferation). To determine whether TGF-β1 acts directly through the EGF receptor or by inducing EGF, cells were incubated with AG1478 or anti-EGF antibody and stimulated with TGF. Both EGF and TGF-β1 promoted keratocyte proliferation (Figure 7A), but proliferation in the presence of EGF was significantly higher than with TGF-β1 alone, and there was no synergistic effect when both growth factors were combined. Inhibition of the EGF-R decreased proliferation induced by EGF and TGF-β1 to levels lower than TGF-β1 alone (*p<0.05) and completely blocked EGF-stimulated proliferation. However, neutralization of EGF by treatment with anti-EGF did not significantly decrease the action of TGF-β1, demonstrating that the small proliferative action of TGF-β1 involves the transactivation of the EGF-R. Both EGF and TGF-β1 stimulated keratocyte migration (Figure 7B). Migration was more rapid with TGF-β1, and further increase took place when EGF and TGF-β1 were combined. Inhibition of EGF-R by AG1478 completely prevented EGF-induced migration and significantly decreased TGF-β1-induced cell migration (*p<0.05), while treatment with anti-EGF antibody in the presence of TGF-β1 had no effect on migration.
Figure 7. Effect of EGF on cell proliferation and migration.

(A) To assay cell proliferation, seven-day RCK cultures were incubated in the presence of EGF (50 ng/ml), TGF-β1 (10 ng/ml) or both with or without AG1478 (10 µM) and in the presence of TGF-β1 and anti-EGF antibody. Photographs are representative of images taken at 72 hrs. Cell proliferation was expressed as average ± SD of percentages of Ki-67 positive cells versus total cells counted in a blind fashion in ten different fields of two wells (*p<0.05, with respect to TGF-β1). (B) To measure cell migration, confluent RCK were scraped in the center of the well with a sterile plastic tip to create a linear wound and incubated for 24 hrs. Photographs are representative of wounds taken at 0 and 24 hrs (bar = 100 µm). Cell migration was quantified by measuring the width of wounded area. Data represents means ± SD (n=10). The experiment was repeated twice with similar results. E=EGF; T=TGF-β1; AG=AG1478; Ab=anti-EGF antibody.
Discussion
The cornea contains no blood vessels, and unlike the epithelial or endothelial layers, which can derive nutrients directly from tear fluid and aqueous humor, the keratocytes obtain their supply exclusively by diffusion. This relatively insufficient supply of nutrients may contribute to keratocyte quiescence, stromal homeostasis, and corneal transparency. In the present study, by culturing RCK in serum-free DMEM/F12 without frequent changes of the medium, we were able to maintain the keratocyte phenotype for long times in vitro. The cells exhibited dendritic morphology, eccentric bean-shaped nuclei, and long intercellular processes which connected extensively to form a network like that seen in situ.1–4
Immunofluorescence showed that the cells were strongly stained for KS and ALDH1, but weakly stained for CS, and negatively stained for α-SMA and FN, typical of keratocytes seen in situ.6, 7 Expression of EGF-R was detected in both keratocytes and myofibroblasts. Also, treatment with EGF induced 100% transformation of keratocytes into a proto-myofibroblast phenotype expressing α-SMA in the cytoplasm, but without the well-organized stress fibers found in myofibroblasts. Proto-myofibroblasts proliferate and also express FN, TSP-1, LM and Coll III and IV. These ECM components were not found in the unstimulated keratocytes, but had been reported in the fibroblasts of corneal repair tissue42–45, indicating that EGF-stimulated cells have the characteristics of repair fibroblasts. EGF showed similar effects at longer times (1 week) of incubation, suggesting that the growth factor-induced differentiation is not altered after long exposure to EGF. Our findings suggest that EGF is an important mediator in the initial activation of stromal keratocytes through stimulation of the EGF-R and that the PI-3K/Akt-1 signaling pathway plays a key role in the EGF-induced keratocyte differentiation to proto-myofibroblasts, proliferation, and ECM production. Akt-1 regulation requires activation of PI-3K to recruit Akt-1 to the plasma membrane where it is first phosphorylated (activated) and then detached from the membrane in order to phosphorylate substrates from the cytosol and nuclei. Under our conditions, EGF induced a strong and prolonged (more than 2 hrs) Akt activation, and stimulating RCK for 30 min induced the translocation of phosphorylated Akt-1 to the nuclei, suggesting that regulation of transcription factors by Akt-1 could be an important mechanism for the differentiation of keratocytes stimulated with EGF. Studies on breast epithelial cells stimulated with IGF-1 have shown an active role of PI-3K/Akt in phosphorylation as well as in an epithelial to mesenchymal transition.46 Since no organized α-SMA stress fibers were found in the EGF-treated cells, we conclude that EGF is not sufficient to induce keratocyte differentiation to myofibroblasts, requiring other mediators to cooperate in completion of cell differentiation. A diversity of growth factors and cytokines are present after injury in the cornea, and it is possible that more than one component is required for myofibroblast differentiation.
TGF-β1 has been shown to be a potent inducer of myofibroblast transformation in a variety of cells of different tissues, including cornea.24, 47–51 Under our culture conditions, TGF-β1 induced 12% of the cells to differentiate into myofibroblasts. However, EGF plus TGF stimulated 90% of cells to differentiate into myofibroblasts, with a significant up-regulation of FN expression. Our results using chemical inhibition show that the synergism between TGF-β1 and EGF involves the activation of the EGF-R and PI-3K/Akt-1 signaling but not ERK1/2 or p38 activation. By blocking EGF-R activation or the PI-3K/Akt-1 pathway, there was a significant decrease in α-SMA and FN expression when RCK were stimulated with TGF-β1 (Figure 5), suggesting that this specific cytokine could act through the EGF-R. In addition, our experiments with neutralizing anti-EGF antibody demonstrate that the action of TGF-β1 is not mediated by promoting the synthesis of EGF but rather by inhibiting the activation of the EGF-R (Figure 6). There are earlier studies supporting this possibility, e.g., it has been shown that TGF-β induces the expression of high affinity EGF-R in stroma fibroblasts30, and in the A431 epidermal cell line, TGF-β1 caused an increased tyrosine phosphorylation of the EGF-R that was not dependent of protein synthesis.52 Other studies have shown that TGF-β amplifies the content of EGF-R in granulose cells from rat ovaries and increases EGF-R transcription in kidney fibroblasts.53, 54 Our studies have shown that activation of EGF-R occurs rapidly when stimulated with TGF-β1. Addition of EGF to TGF-β1 does not affect proliferation stimulated by EGF, but in the presence of AG1478, there was a small but significant decrease in proliferation stimulated by TGF-β1 (Figure 7B), suggesting that TGF-β1 is able to induce proliferation of keratocytes by pathways that involve EGF-R activation but not synthesis of EGF. However, addition of EGF to TGF-β1 promoted cell migration that was significantly impaired when the EGF-R was blocked, suggesting that EGF-R signaling is important in cell migration. This data differs from earlier publications describing that EGF increases migration, chemotaxis, and proliferation of stroma fibroblasts differentiated by serum, and that TGF-β1 decreases proliferation and migration of the cells.55–57 However, none of these studies investigate the combined action of EGF and TGF-β1 in non-differentiated keratocytes, as in the ones found “in situ” in the cornea.
In corneal epithelial cells stimulated with hepatocyte growth factor, p38 activation has been shown to be important for cell migration58; however, we could not demonstrate activation of p38 by EGF in RCK in our experiments. It is possible that other signals, such as PI-3K/Akt-1 are important in migration. The role of PI-3K/Akt-1 in migration seems to be cell- and tissue-specific and dependent on the potential targets of Akt. Phosphorylation of Akt has been linked to organization of the actin cytoskeleton, as well as expression of genes involved in migration and phosphorylation of proteins involved in adhesion such as focal adhesion kinase and paxillin.59–61 The increased expression of FN contributes to the combined effect of TGF-β1 and EGF on cell migration. This glycoprotein mediates a wide variety of cellular interactions with the matrix, and plays important roles in cell adhesion, migration, growth and differentiation.62 The up-regulated expression of FN has been found at sites of stromal wounds and is supposed to provide a substrate for the attachment and facilitate the migration of corneal fibroblasts.42,63,64 In vitro models of wound healing have also shown that fibronectin promotes corneal fibroblast-mediated collagen gel contraction and contributes to the maintenance of corneal shape by corneal fibroblasts during stromal wound healing.65,66 In the present study, we have shown that EGF and TGF-β1 separately induced FN expression in corneal keratocytes. However, there was a significant increase when both growth factors were added, suggesting that during stromal injury, these two growth factors may synergistically function to promote wound healing by up-regulation of fibronectin expression and to promote migration.
Future studies to investigate the actions of the growth factors on message levels could preclude variables such as protein secretion to the media and protein degradation, further insight into the mechanisms of gene induction of FN and other ECM components.
In conclusion, our studies demonstrate that EGF synergizes TGF-β1 induction of keratocytes to myofibroblast differentiation, increases secretion of FN, and produces change in ECM components through activation of EGF-R and PI-3K/Akt-1 signaling. These results provide insight as to why the use of EGF in penetrating wounds, although effective in closing the wounds, promotes scar tissue formation and compromises corneal transparency.
Acknowledgements
The project described was supported by grants R01 EY004928 and R01 EY006635 from the National Eye Institute (NEI). The content is solely the responsibility of the authors and does not necessarily represent the official views of NEI or the National Institutes of Health.
Supported by NIH grants R01 EY004928 and R01 EY006635 from the National Eye Institute (NEI).
References
- 1.Nishida T, Yasumoto K, Otori T, Desaki J. The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Invest Ophthalmol Vis Sci. 1988;29:1887–1890. [PubMed] [Google Scholar]
- 2.Poole CA, Brookes NH, Clover GM. Keratocyte networks visualized in the living cornea using vital dyes. J Cell Sci. 1993;106:685–692. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- 3.Jester JV, Barry PA, Lind GL, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–735. [PubMed] [Google Scholar]
- 4.Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225–236. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davison PF, Galbavy EJ. Connective tissue remodeling in corneal and scleral wounds. Invest Ophthalmol Vis Sci. 1986;27:1478–1484. [PubMed] [Google Scholar]
- 6.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 7.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 8.Cintron C, Hassinger LC, Kublin CL, Cannon DJ. Biochemical and ultrastructural changes in collagen during corneal wound healing. J Ultrastruct Res. 1978;65:13–22. doi: 10.1016/s0022-5320(78)90017-5. [DOI] [PubMed] [Google Scholar]
- 9.Garana RMR, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992;33:3271–3282. [PubMed] [Google Scholar]
- 10.Bazan HEP. Cellular and molecular events in corneal wound healing: Significance of lipid signaling. Exp Eye Res. 2005;4:453–463. doi: 10.1016/j.exer.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Ottino P, He J, Axelrad TW, Bazan HEP. PAF-induced furin and MT1-MMP expression is independent of MMP-2 activation in corneal myofibroblasts. Invest Ophthalmol Vis Sci. 2005;46:487–496. doi: 10.1167/iovs.04-0852. [DOI] [PubMed] [Google Scholar]
- 12.Esquenazi S, He J, Bazan NG, Bazan HEP. Comparison of corneal wound healing response in photorefractive keratectomy and laser-assisted subepithelial keratectomy. J Caract Refract Surg. 2005;31:1632–1639. doi: 10.1016/j.jcrs.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.He J, Bazan NG, Bazan HEP. Alkali-induced corneal stromal melting prevention by a novel platelet-activating factor (PAF) receptor antagonist. Arch Ophthalmol. 2006;124:70–78. doi: 10.1001/archopht.124.1.70. [DOI] [PubMed] [Google Scholar]
- 14.He J, Bazan HEP. Synergistic Effect of Platelet-activating Factor and Tumor Necrosis Factor-α on Corneal Myofibroblast Apoptosis. Invest Ophthalmol Vis Sci. 2006;47:883–891. doi: 10.1167/iovs.05-0581. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 16.Bazan HEP, Ottino P. The role of platelet-activating factor in the corneal response to injury. Prog Ret Eye Res. 2002;21:449–464. doi: 10.1016/s1350-9462(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 17.Imanishi J, Kamiyama K, Iguichi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 18.Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- 19.Esquenazi S, He J, Bazan HE, Bazan NG. Prevention of experimental diffuse lamellar keratitis using a novel platelet-activating factor receptor antagonist. J Cataract Refract Surg. 2004;30:884–891. doi: 10.1016/j.jcrs.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Funderburg JL, Funderburg ML, Mann M, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 22.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 23.Musselmann K, Alexandrou B, Kane B, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280:32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- 24.Jester JV, Barry-lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 25.Jorisson RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signaling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 26.Nishida T, Tanaka H, Nakagawa S, Sasabe T, Awata T, Manabe R. Fibronectin synthesis by the rabbit cornea: effect of mouse epidermal growth factor and cyclic AMP analogs. Jpn J Ophthalmol. 1984;28:196–202. [PubMed] [Google Scholar]
- 27.Wilson SE, He Y-G, Lioyd SA. EGF, EGF receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts. Invest Ophthalmol Vis Sci. 1992;32:1756–1765. [PubMed] [Google Scholar]
- 28.Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptors (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–517. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa T, Kinoshita S, Fujita K, Araki K, Watanabe H, Ohashi Y, Manabe R. The mechanism of accelerated corneal epithelial healing by human epidermal growth factor. Invest Ophthalmol Vis Sci. 1990;31:1773–1778. [PubMed] [Google Scholar]
- 30.Hongo M, Itoi M, Yamaguchi N, Imanishi J. Distribution of epidermal growth factor (EGF) receptors in rabbit corneal epithelial cells, keratocytes, and endothelial cells, and the changes induced by transforming growth factor-β1. Exp Eye Res. 1992;54:9–16. doi: 10.1016/0014-4835(92)90063-x. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado BA, Furcht LT. Epidermal growth factor stimulates integrin-mediated cell migration of cultured human corneal epithelial cells on fibronectin and arginine-glycine-aspartic acid peptide. Invest Ophthalmol Vis Sci. 1995;36:2120–2126. [PubMed] [Google Scholar]
- 32.Zieske JD, Takahashi H, Hutcheon AEK, Dabbone AG. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 33.Brightwell JR, Riddle SL, Eiferman RA, Valenzuela P, Barr PJ, Merryweather JP, et al. Biosynthetic human EGF accelerates healing on neodecadron-treated primate corneas. Invest Ophthalmol Vis Sci. 1985;26:105–110. [PubMed] [Google Scholar]
- 34.Woost PG, Brightwell J, Eiferman RA, Schultz GS. Effect of growth factors with dexamethasone on healing of rabbit corneal stromal incisions. Exp Eye Res. 1985;40:47–60. doi: 10.1016/0014-4835(85)90107-1. [DOI] [PubMed] [Google Scholar]
- 35.Petroutsos G, Sebag J, Courtois Y. Epidermal growth factor increases tensile strength during wound healing. Ophthalmic Res. 1986;18:299–300. doi: 10.1159/000265452. [DOI] [PubMed] [Google Scholar]
- 36.Mathers WD, Sherman M, Fryczkowski A, Jester JV. Dose-dependent effects of epidermal growth factor on corneal wound healing. Invest Ophthalmol Vis Sci. 1989;30:2403–2406. [PubMed] [Google Scholar]
- 37.Leibowitz HM, Morrello S, Jr, Stern M, Kupferman A. Effect of topically administered epidermal growth factor on corneal wound strength. Arch Ophthalmol. 1990;108:734–737. doi: 10.1001/archopht.1990.01070070120048. [DOI] [PubMed] [Google Scholar]
- 38.Brazzell RK, Stern ME, Aquavella JV, Beuerman RW, Baird L. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci. 1991;32:336–340. [PubMed] [Google Scholar]
- 39.Beaubien J, Boisjoly HM, Gagnon P, Guidoin R. Mechanical properties of the rabbit cornea during wound healing after treatment with epidermal growth factor. Can J Ophthalmol. 1994;29:61–65. [PubMed] [Google Scholar]
- 40.Masur SK, Dewall HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 42.Suda T, Nishida T, Ohashi Y, Nakagawa S, Manabe R. Fibronectin appears at the site of corneal stromal wound in rabbits. Curr Eye Res. 1981;1:553–556. doi: 10.3109/02713688109069181. [DOI] [PubMed] [Google Scholar]
- 43.Anderson JA, Binder PS, Rock ME, et al. Human excimer laser keratectomy. Immunohistochemical analysis of healing. Arch Ophthalmol. 1994;114:54–60. doi: 10.1001/archopht.1996.01100130050008. [DOI] [PubMed] [Google Scholar]
- 44.Latvala T, Tervo K, Mustonen R, et al. Expression of cellular fibronectin and tenascin in the rabbit cornea after excimer laser photorefractive keratectomy: a 12 month study. Br J Ophthalmol. 1995;79:65–69. doi: 10.1136/bjo.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armstrong DJ, Hiscott P, Batterbury M, Kaye S. Corneal stromal cells (keratocytes) express thrombospondins 2 and 3 in wound repair phenotype. Int J Biochem Cell Biol. 2002;34:588–593. doi: 10.1016/s1357-2725(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 46.Yu JT, Foster RG, Dean DC. Transcriptional repression by Rb-E2F and regulation of anchorage-independent survival. Mol Cell Biol. 2001;21:3325–3335. doi: 10.1128/MCB.21.10.3325-3335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arciniegas E, Sutton AB, Allen TD, Shor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103:521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 48.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta1 in quiescent human breast gland fibroblasts. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 49.Verbeek MM, Otte-Holler I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- 50.Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994;13:885–890. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- 51.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure. A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldkorn T, Mendelsohn J. Transforming growth factor beta modulates phosphorylation of the epidermal growth factor receptor and proliferation of A431 cells. Cell Growth Differ. 1992;3:101–109. [PubMed] [Google Scholar]
- 53.Bendell JJ, Dorrington JH. Epidermal growth factor influences growth and differentiation of rat granulosa cells. Endocrinology. 1990;127:533–540. doi: 10.1210/endo-127-2-533. [DOI] [PubMed] [Google Scholar]
- 54.Thompson KL, Assoian R, Rosner MR. Transforming growth factor-beta increases transcription of the genes encoding the epidermal growth factor receptor and fibronectin in normal rat kidney fibroblasts. J Biol Chem. 1988;263:19519–19524. [PubMed] [Google Scholar]
- 55.Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17:79–87. doi: 10.1076/ceyr.17.1.79.5261. [DOI] [PubMed] [Google Scholar]
- 56.Andresen JL, Ledet T, Ehlers N. Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Curr Eye Res. 1997;16:605–613. doi: 10.1076/ceyr.16.6.605.5081. [DOI] [PubMed] [Google Scholar]
- 57.Pancholi S, Tullo A, Khaliq A, Foreman D, Boulton M. The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefes Arch Clin Exp Ophthalmol. 1998;236:1–8. doi: 10.1007/s004170050034. [DOI] [PubMed] [Google Scholar]
- 58.Sharma GD, He J, Bazan HEP. P38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 59.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, et al. Akt/PKB regulates actin organization and cell motility via girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Galaria II, Nicholl SM, Roztocil E, Davies MG. Urokinase-induced smooth muscle cell migration requires PI3-K and Akt activation. J Surg Res. 2005;127:46–52. doi: 10.1016/j.jss.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Leventhal PS, Shelden EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 62.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 63.Tervo K, van Setten GB, Beuerman RW, Virtanen I, Tarkkanen A, Tervo T. Expression of tenascin and cellular fibronectin in the rabbit cornea after anterior keratectomy. Immunohistochemical study of wound healing dynamics. Invest Ophthalmol Vis Sci. 1991;32:2912–2918. [PubMed] [Google Scholar]
- 64.Andresen JL, Ledet T, Hager H, Josephen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of corneal fibroblasts. Exp Eye Res. 2000;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- 65.Taliana L, Evans MD, Dimitrijevich SD, Steele JG. Vitronectin or fibronectin is required for corneal fibroblast-seeded collagen gel contraction. Invest Ophthalmol Vis Sci. 2000;41:103–109. [PubMed] [Google Scholar]
- 66.Liu Y, Yanai R, Lu Y, Kimura K, Nishida T. Promotion by fibronectin of collagen gel contraction mediated by human corneal fibroblasts. Exp Eye Res. 2006;83:1196–1204. doi: 10.1016/j.exer.2006.06.008. [DOI] [PubMed] [Google Scholar]


