Abstract
Purpose
The expression of CD56, a natural killer (NK) cell-associated molecule, on αβ T lymphocytes correlates with their increased anti-tumor effector function. CD56 is also expressed on a subset of γδ T cells. However, anti-tumor effector functions of CD56+ γδ T cells are poorly characterized.
Experimental design
To investigate the potential effector role of CD56+ γδ T cells in tumor killing, we employed isopentenyl pyrophosphate (IPP) and IL-2 expanded γδ T cells from PBMC of healthy donors.
Results
Thirty to 70% of IPP+IL-2 expanded γδ T cells express CD56 on their surface. Interestingly, while both CD56+ and CD56− γδ T cells express comparable levels of receptors involved in the regulation of γδ T cell cytotoxicity (e.g. NKG2D and CD94) only CD56+ γδ T lymphocytes are capable of killing squamous cell carcinoma (SCC) and other solid tumor cell lines. This effect is likely mediated by the enhanced release of cytolytic granules, since CD56+ γδ T lymphocytes expressed higher levels of CD107a compared to CD56− controls, following exposure to tumor cell lines. Lysis of tumor cell lines is blocked by concanomycin A and a combination of anti-γδTCR + anti-NKG2D mAb, suggesting that the lytic activity of CD56+ γδ T cells involves the perforin-granzyme pathway and is mainly γδTCR/NKGD2 dependent. Importantly, CD56 expressing γδ T lymphocytes are resistant to Fas ligand and chemically induced apoptosis.
Conclusions
Our data indicate that CD56+ γδ T cells are potent anti-tumor effectors capable of killing SCC and may play an important therapeutic role in patients with head and neck cancer and other malignancies.
Keywords: gamma delta T lymphocytes, Human, T cells, Cytotoxic, Cell Surface Molecules, Tumor immunity
Introduction
Squamous cell carcinoma (SCC) is the most common malignancy of the upper aero-digestive tract. Despite continually evolving therapeutic options, we have witnessed little improvement in survival for patients with squamous cell carcinoma of the head and neck (SCCHN) over the past three decades (1). While immunotherapy has the potential to improve outcomes, broad clinical application will require improved understanding of the immuno-regulatory and cytotoxic potential of specific effector cell populations.
The overwhelming majority of experimental and clinical immunotherapeutic modalities for patients with SCCHN are directed towards the activation of major histocompatibility complex (HLA)-restricted αβ T lymphocytes (2, 3). However, such vaccination approaches designed to stimulate anti-tumor T cell immunity for SCCHN have demonstrated only limited efficacy (4). The root causes for such poor therapeutic benefit include an impaired αβ T cell response in patients with advanced disease, and down-regulation of HLA class I on the tumor cell surface, limiting the cytotoxic potential of αβ T lymphocytes (5–7). One alternative effector T cell population which may have therapeutic relevance in SCCHN is γδ T lymphocytes.
γδ T lymphocytes represent 1–10% of human peripheral T cells (8), and based on both their increased in frequency in PBMC of patients with SCCHN, and known cytotoxic potential towards tumor cell lines (9–14), are postulated to play a role in tumor immune surveillance (15). Unlike their αβ counterparts, γδ T lymphocytes recognize and respond to non-peptide antigens (e.g. phosphoantigens) in an HLA-unrestricted fashion (16). For example, γδ T cells recognize the mevalonate pathway-derived isopentenyl pyrophosphate (IPP) (17). It was shown that the IPP concentration is increased in malignant cells, suggesting that certain tumor cells are recognized by human γδ T cells on the basis of their enhanced IPP production (18, 19). While effector function is controlled by both TCR-dependent and TCR-independent mechanisms (20), including activating (NKG2D) (21) and inhibitory (CD94) NK cell receptors,(22) the phenotype of γδ T cells responsible for killing tumor cells is unknown.
Several recent reports indicate that CD56 is an important effector marker of NK and T lymphocytes (23–25). For example, while it appears that CD56 is not essential for cell-mediated cytotoxicity (26), bright staining CD56 NK cells have increased potential for cytokine production whereas dim staining is associated with enhanced cytotoxicity (27). Similarly, high levels of CD56 are also expressed on NKT cells, which demonstrate both cytotoxic and regulatory properties (28). Finally, CD56 expression is associated with potent effector function in conventional αβ T cells in both the human intestine and peripheral blood (23–25). While CD56 is expressed on γδ T cells (11, 29), its functional significance remains unknown.
In this study we sought to define the cytotoxic potential of γδ T lymphocytes against SCCHN and characterize the phenotype of γδ T lymphocytes responsible for tumor cell death. Our findings indicate that CD56+, but not CD56− γδ T cells, mediate anti-tumor cytotoxic effects. The killing of tumor cells by CD56+ γδ T cells is associated with increased expression of CD107a, a degranulation marker. Our findings demonstrate that CD56+ γδ T cells are functionally capable of SCCHN killing and suggest that these cells may play an important role in the immunotherapy of head and neck cancer.
Materials and Methods
Tumor cell lines
Tu159, Tu167, and MDA1986 head and neck tumor cell lines were graciously provided by Dr. Gary Clayman (M.D. Anderson Cancer Center). WMMSCC was a kind gift from Dr. Suyu Shu (Cleveland Clinic). 012SCC was genially received from Dr. Bert O’Malley (University of Pennsylvania). Daudi Burkitt’s lymphoma and K562 chronic myelogenous leukemia cell lines were purchased from American Type Culture Collection (ATCC CCL-213). All tumor cell lines and peripheral blood mononuclear cells (PBMC) were cultured in complete RPMI 1640 media (Gibco, Grand Island, NY) supplemented with 10% FBS (Atlanta Biologicals, Atlanta, GA), 2 mM L-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml) and 10 mM HEPES (all purchased from Gibco). The complete media for Daudi cell culture was supplemented with 4.5g/L D-glucose, 1.5g/L Sodium Bicarbonate, and 1mM Sodium Pyruvate (Gibco). To ensure the purity of cultured tumor cell lines we routinely performed PCR based haplotyping (One Lambda Inc., Canoga Park, CA) and/or flow cytometry staining with antibodies against HLA class I (BD Biosciences, San Jose, CA).
γδ T cell expansion
Whole blood or buffy coats from healthy donors were purchased through Biologic Specialty Corp. (Colmar, PA) under University of Maryland IRB exemption. For expansion of γδ T cells, whole PBMC were separated on Ficoll (Amersham Biosciences, Piscataway, NJ) and 1×106 cells/ml cultured in complete media with 15 μM isopentyl pyrophosphate (IPP) (Sigma) and 100 U/ml human recombinant IL-2 (Tecin, Biological Resources Branch, National Institutes of Health, Bethesda, MD) (30). Fresh complete medium and IL-2 supplement at 100 U/ml was added every 3 days. After 10 to 14 days of culture, cells were harvested and used in experiments. The percentage of γδ T cell in the culture was analyzed by flow cytometry.
Transwell co-culture
γδ T cell-depleted PBMC at 1×106 cells/ml (3 ml/well) were placed in 6-well plates equipped with Transwell inserts (Costar). γδ T cells, purified by negative selection, were resuspended at 5×104 and 1 ml of cells were added into the upper wells of the Transwell. In the Transwell γδ T cell-depleted PBMC were separated from purified γδ T cell by 1 mm, but soluble factors can diffuse through a micro-porous (pore diameter 0.4 μM) polycarbonated membrane between upper and lower wells. Cells in Transwell were cultured with IPP and IL-2 for 10–14 days as described above. Fresh complete medium and IL-2 supplement at 100 U/ml was added every 3 days.
Magnetic bead sorting and depletion
PBMC expanded with IPP and IL-2 were sorted for CD56+ and CD56− populations using a CD56 MultiSort Kit (Miltenyi Biotec). If further sorting was required, the CD56 magnetic particles were enzymatically released from the CD56+ fraction. CD56+ and CD56− cells were then sorted using anti-γδTCR magnetic beads (Miltenyi Biotec) according to the manufacturer’s instructions. In some experiments unaltered NK and γδ T cells were isolated from fresh PBMC by negative selection using NK cell and γδ T cell isolation kits, respectively (Miltenyi Biotec). For Transwell experiments γδ T cells were depleted from PBMC using anti-γδ TCR beads (MiltenyiBiotec). The purity of resulting cell populations were routinely checked by flow cytometry. We achieved 90 to 99% purity after magnetic bead sorting even for rare cell populations (e.g. NK cells in IPP expanded PBMC).
Flow Cytometry
Cells were stained for cell surface markers with the following antibodies: mouse anti-human γδ TCR-FITC, mouse anti-human CD3-PerCP, and mouse anti-human CD56-APC. All antibodies were purchased from BD Biosciences. To determine granule release by γδ T cells, IPP expanded PBMCs were cultured in 14 ml polypropylene tubes (BD Biosciences) with complete medium alone, Daudi cells, SCCHN cell lines (effector:target ratio 1:2), or phorbol-12-myristate-13-acetate (PMA) (5 ng/ml) and ionomycin (0.5 μg/ml) (Sigma). CD107a-PE Ab (BD Biosciences) was added directly to all tubes at the beginning of the culture. The cells were incubated for 1 h at 37 °C in 5% CO2. At this time 1 μg/ml monensin (Golgi-Stop, BD Biosciences) was added to the culture and the incubation continued for an additional 4 h. The cells were washed with FACS buffer and stained for surface cell markers (γδTCR, CD3, CD56). To measure the number of cells expressing IFN-γ and TNF-α, IPP expanded PBMCs were cultured with complete medium alone or PMA and ionomycin for 4 h in the presence of monensin. Cells were then stained with Ab against cell surface molecules (γδTCR, CD3, CD56). After cell surface staining the cells were fixed and permeabilized using the BD Cytofix/Cytoperm Kit as described by the manufacturer (BD Biosciences). After permeabilization, the cells were stained with PE-conjugated cytokine specific mAb. To determine granzyme B expression, permeabilized cells were stained with PE-conjugated anti-human Granzyme B (Caltag, Burlingame, CA) or the appropriate isotype control.
In most flow cytometry samples at least 3×104 gated γδ T lymphocytes (defined as CD3+ and γδ TCR+) were acquired using a BD LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ). All samples were analyzed using FACS Diva software (Becton Dickinson).
Cytotoxicity assay
The cytotoxicity of IPP expanded γδ T cells, their fractions, and γδ T lymphocytes and NK cells isolated from fresh PBMC was measured using standard 51Cr-release assay, as described previously (31). Briefly, target cells (2×106 in 0.3 ml of complete media) were incubated for 90 min at 37°C in 5% CO2 with 150 μCi of sodium 51chromate in saline solution (GE Healthcare, Piscataway, NJ). The labeled cells were then washed twice with media and incubated for an additional 30 min to reduce background radioactivity. The cells were then washed two more times and adjusted to a concentration of 5 × 104 cells per ml in complete media. Serial dilutions of effector cells were added into each well of 96-well V-bottomed plates (Corning, Corning, NY). Aliquots of 51Cr-labeled target cells (100 μl) were dispensed into wells containing effector cells. The plates were centrifuged at 200 rpm for 2 min and incubated at 37°C in 5% CO2. After 4 h of incubation, the plates were centrifuged again at 13,000 rpm for 5 min and 100 μl aliquots of the supernatants from each well were transferred to a new plate containing 100 μl/well of Optiphase Supermix scintillation fluid (Perkin Elmer, Boston, MA). The radioactivity was measured using 1450 Microbeta counter (Wallac, Turku Finland). In some experiments anti-γδ TCR (Clone: Immu 510; Biodesign, International, Saco, ME ), anti-NKG2D (Clone: 149810; R&D Systems, Minneapolis, MN), their combination or control mouse Ig (IgG1) were added at 10 μg/ml at the onset of the cytolytic assay before exposure to labeled target cells. In other experiments, effector γδ T cells were preincubated with 100 nM concanamycin A (CMA; Sigma-Aldrich) at 37°C for 20 min to block the perforin-granzyme pathway and added to 51Cr-labeled cells. No significant effector γδ T cell death was observed after CMA treatment as measured by trypan blue exclusion assay. The percentage of specific cytotoxicity was calculated as (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Spontaneous release was determined by incubating the targets with 100 μl of complete media, and maximum release was determined by incubating the target cells with 100 μl of 0.5% Triton-X.
Apoptosis assay
IPP + IL-2 expanded PBMC were washed with PBS and resuspended at 1×106 cells/ml. Anti-CD95 mAb (CH11; Upstate, Lake Placid, NY) at 1 μg/ml were added to the cultures and incubated for 18 h at 37°C. For evaluation of apoptosis, cells were stained with anti-CD56-APC and anti-γδTCR-FITC, then washed and stained with Annexin V-PE according to the manufacturer’s instructions and analyzed by flow cytometry.
RESULTS
IPP expanded γδ T cells efficiently kill SCCHN in vitro
Because γδ T lymphocytes represent only a small percentage of PBMC’s in healthy donors, we first employed well characterized methods to expand these cells. Freshly isolated PBMC contained 1–5% γδ T cells, after 10–14 days of culture with IPP + IL-2 the percentage of CD3+, γδ TCR+ was between 50 to 80%, indicating significant expansion of γδ T lymphocytes (Fig 1A). PBMC cultured with IL-2 alone contained approximately 10% γδ T cells (Fig 1A).
Figure 1. IPP activated γδ T cells efficiently kill HNSCC cell lines.
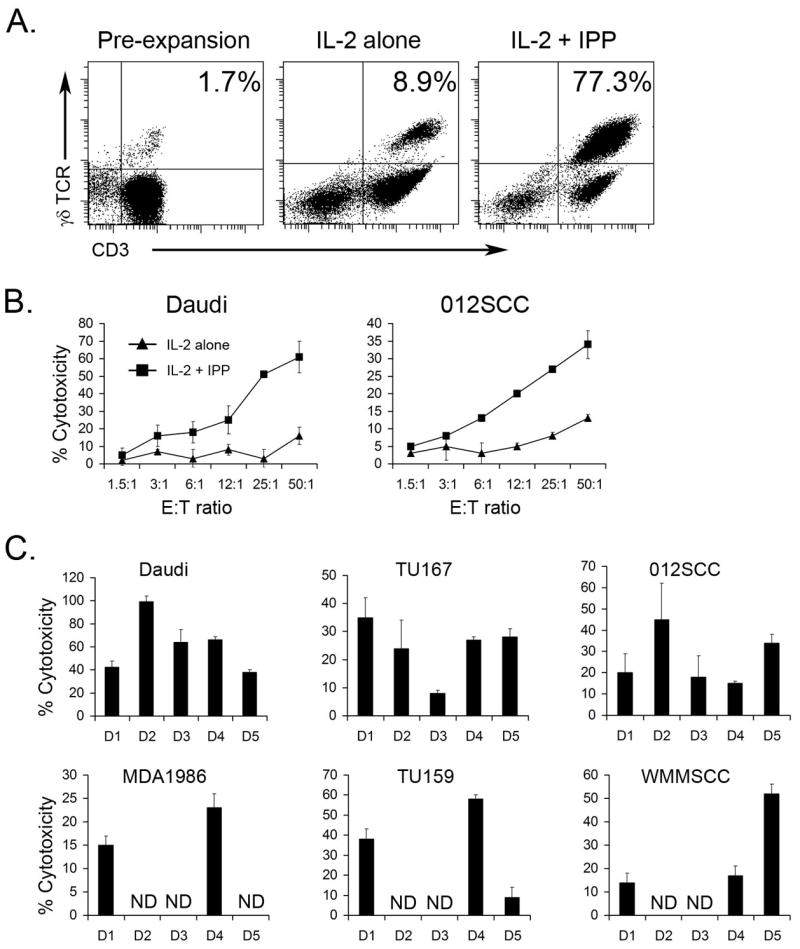
γδ T cells were generated by expanding PBMC from healthy donors with IPP and IL-2 (see Materials and Methods). A. Fresh PBMC, PBMC cultured with IL-2 alone or with IPP + IL-2 for 14 days were stained using anti-CD3 and anti-γδTCR mAb and analyzed by flow cytometry. The representative dot plots from 5 separate experiments are presented. B. Cytotoxic activity of PBMC cultured with IL-2 alone or with IPP + IL-2 was measured in standard 4 h 51Cr-release assay against Daudi cells and 012SCC HNSCC cell lines. One of three independent experiments is shown. C. The cytolysis of Daudi cells (used as a positive control) and five HNSCC cell lines (TU167, TU159, 012SCC, MDA1986 and WMMSCC) by γδ T cells expanded with IPP + IL-2 from PBMC of five separate healthy donors (D1, D2, D3, D4, D5) were measured in 51Cr-release assays. Cytotoxicity is shown for 12:1 Effector:Target ratio. Cytotoxicity data are shown as Mean±STD of triplicate wells. ND – not done.
To examine whether the expanded γδ T cells could kill γδ T lymphocyte-sensitive Daudi cells (positive control) and SCCHN cell lines, we performed standard 51Cr-release assays. γδ T cells expanded in the presence of IPP and IL-2 killed Daudi and 012SCC (SCCHN), while cells cultured with IL-2 alone were not cytotoxic (Fig. 1B). The cytotoxic effects were not donor specific, as IPP expanded cells from 5 different donors killed γδ T cell-sensitive Daudi cells and all five tested SCCHN lines (012SCC, MDA1986, Tu159, Tu167, WMSCC) when cultured at a 25:1 E:T ratio (Fig. 1C). Small variations in the anti-tumor activity of γδ T cells were observed among individual donors.
CD56 expressing, but not CD56 negative, IPP expanded PBMC kill SCCHN in a γδ TCR-dependent manner
It is known that CD56 expression on conventional T lymphocytes correlates with their effector activity (23–25). Using flow cytometry, we found that 30–70% of fresh or IPP + IL-2 expanded γδ T cells express CD56 on their surface (Fig 2A). To determine the contribution of IPP expanded CD56+ and CD56− cells in SCCHN killing we isolated CD56+ and CD56− fractions using magnetic beads and tested these cells in cytotoxicity assays. As can be seen in Fig. 2B, we were able to achieve about 90% enrichment of CD56+ cells, 77% of which were γδ TCR+. All IPP expanded populations including unseparated cells, CD56+, and CD56− lymphocytes were capable of killing Daudi cells. However, cytolytic activity of CD56− cells against Daudi lymphoma was significantly lower as compared to the bulk (unseparated) and CD56+ fractions. In contrast, only unseparated and CD56+ lymphocytes killed TU159 (Fig. 2C). At a 20:1 E:T ratio, CD56+ cells induced ~56% killing of TU159. At the same E:T ratio CD56− cells showed only 11% cytotoxicity against TU159. Similarly, we observed that CD56+ but not CD56− IPP expanded cells kill four additional SCCHN cell lines: MDA1986, 012SCC, TU167, WMMSCC (data not shown). In addition, the CD56+ fraction of IPP + IL-2 expanded PBMC were also highly cytotoxic towards CaCo2 colon carcinoma and transformed human kidney fibroblast HEK293 cell line, indicating CD56+ γδ T cell killing is not limited to SCCHN. The cytolytic activity of the CD56+ γδ T cell fraction was very reproducible, since we observed similar high levels of cytotoxicity by CD56+ γδ T cells expanded from PBMC of 15 independent donors. Importantly, only IPP expanded cells were cytolytic towards various tumors, as γδ T cells isolated from fresh PBMC did not lyse any tumors (Fig. 4).
Figure 2. CD56+ fraction of IPP expanded lymphocytes mediates killing of SCCHN.
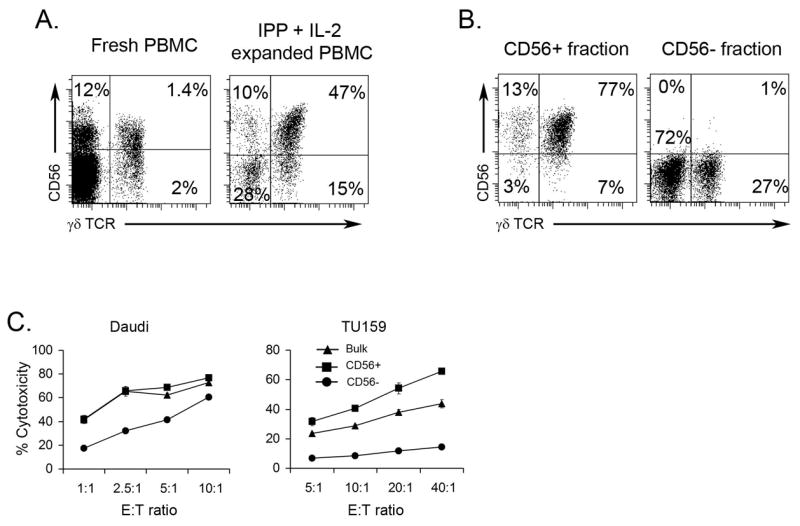
γδ T cells were generated by expanding PBMC (see Materials and Methods). A. Fresh PBMC or PBMC cultured with IPP + IL-2 for 14 days were stained using anti-γδTCR and CD56 mAb. Dotplots of gated total lymphocytes are presented. B. After expansion, CD56+ and CD56− populations were separated using magnetic beads. Sorted cells were stained anti-γδTCR mAb and anti-CD56 and analyzed by flow cytometry. The representative dot blots from multiple experiments are shown. C. Cytotoxic activity of PBMC cultured with IL-2 and IPP and sorted for CD56+ and CD56− populations was measured in a standard 4 h 51Cr-release assay against Daudi cells (positive control) and TU159 SCCHN cell lines. One of fifteen independent experiments is shown.
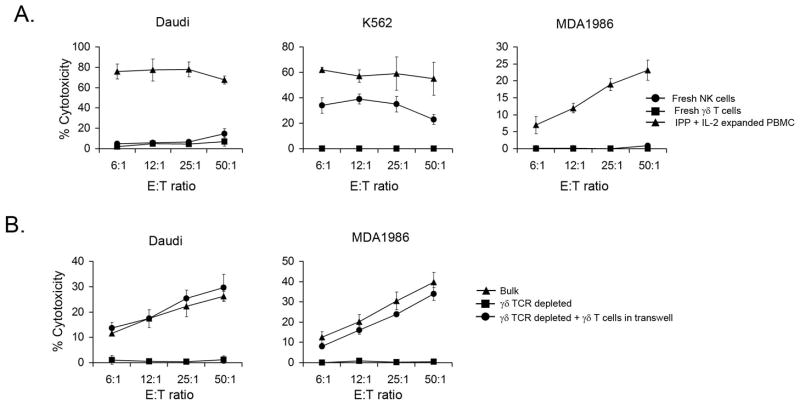
Figure 4. γδ T cells are required for IPP+IL-2-induced cytotoxicty against SCCHN.
A. IPP + IL-2 activated PBMC, NK cells and γδ T cells isolated from fresh PBMC were used in a standard 4 h 51Cr-release assay against Daudi, K562 and MDA1986 cell lines. One of three independent experiments is shown. B. Whole PBMC or PBMC depleted of γδ T cells were plated in the wells of 6-well plate were cultured with or without γδ T cells plated in 0.4-μm Transwell insert in the presence of IPP + IL-12 for 14 days. A standard 4 h 51Cr-release assay against Daudi and MDA1986 cells were performed with various effector cell populations. One of three independent experiments is shown.
CD56+ γδTCR+ and CD56+ γδTCR- cells are highly cytotoxic against SCCHN
Our initial studies indicated that CD56+ cells expanded from PBMC of individual donors can kill SCCHN cell lines in a dose dependent fashion. Because our cultures contained other cell types, the relative import of γδ T lymphocytes versus other CD56+ populations in tumor cell killing remained uncertain. For example, IPP expanded PBMC of a single donor depicted in Fig 3A (central dot plot) consisted of 4% CD56+CD3− NK cells, 36% CD56+CD3+ γδ T cells, 26% CD56− CD3+ γδ T cells and 34% CD56−CD3+ conventional αβ T cells. To determine the contribution of various IPP expanded PBMC in killing of SCCHN we used magnetic beads to isolate CD56+ and CD56− populations. From these populations we isolated γδTCR+ (Fig 3A two right dot plots) and γδTCR− (Fig 3A two left dot plots) cells. It is important to note that the CD56− γδTCR− population was predominately CD3+ (93%), indicating that these cells belong to a conventional αβ T lymphocyte lineage. All purified populations were tested for cytotoxic activity against SCCHN.
Figure 3. Purified CD56+ γδTCR+ and CD56+γδTCR- cells are highly cytotoxic against SCCHN.

γδ T cells were generated by expanding PBMC (see Materials and Methods). After expansion, cells were separated for CD56+ γδTCR+, CD56− γδTCR+, CD56+ γδTCR−, CD56− γδTCR− using magnetic beads. A. Purified cells were stained with anti-γδTCR mAb and anti-CD56 and analyzed by flow cytometry. The dot plots of CD56 and γδTCR stained gated lymphocytes are shown. The representative dot blots from three experiments are shown. B. Cytotoxic activity of purified IPP+IL-2 expanded PBMC fractions was measured in a standard 4 h 51Cr-release assay against MDA1986 and TU167. One of three independent experiments is shown.
As indicated in Fig. 3B, the populations depleted of CD56+ cells did not demonstrate cytotoxicity towards SCCHN. In contrast, unseparated IPP expanded PBMC, and IPP expanded populations containing CD56+γδTCR− (NK cells) and CD56+γδTCR+ lymphocytes killed MDA1986 and TU167. CD56+γδTCR− (NK cells) also showed significant killing of TU167 and MDA1986 SCCHN cell lines. However, a low proportion of NK cells in IPP expanded PBMC and the CD56+ γδTCR+ isolate suggest that NK cells are not the major effectors in SCCHN killing.
γδ T cells are required for IPP+IL-2-induced cytotoxicity against SCCHN
The above data demonstrate that both CD56+ NK cells and CD56+ γδ T cells activated in the presence of IPP are capable of killing SCCHN cell lines. It has been previously shown that IPP directly activates γδ T lymphocytes (30). To determine the effects of IPP and/or IL-2 on the activation of NK cells we compared the cytotoxic activity of freshly isolated NK cells, γδ T cells and PBMC expanded with IPP + IL-2 against SCCHN cell lines. As expected freshly isolated NK cells killed NK-sensitive K562 tumor cell lines (Fig 4A). In contrast, γδ T cell-sensitive Daudi and SCCHN 012SCC target cells were lysed only by IPP expanded PBMC (Fig. 4A). Neither γδ T cells nor NK cells isolated from fresh PBMC were able to lyse Daudi and 012SCC cells. These data indicate that IPP + IL-2 activation is essential for the generation of cytotoxicity in both NK and γδ T cells against SCCHN cell lines.
In order to assess whether IPP and/or IL-2 can directly stimulate NK cells to kill SCCHN targets, we depleted γδ T cells from PBMC using magnetic beads and stimulated these cells with IPP + IL-2 for 14 days. Whole PBMC stimulated by IPP + IL-2 were cytotoxic towards both Daudi and 012SCC cell lines. The depletion of γδ T cells completely abrogated the ability of IPP + IL-2 to stimulate remaining PBMC, containing NK cells, to destroy SCCHN cell lines (Fig. 4B). However, purified γδ T lymphocytes cultured with γδ T cell-depleted PBMC separated by a membrane permeable for soluble factors, restored the ability of NK cell-containing PBMC to kill SCCHN (Fig 4B). Purified γδ T cell stimulated with IPP + IL-2 killed SCCHN (data not shown). Therefore, we confirmed that in our experimental system IPP and/or IL-2 do not stimulate NK cells directly. The above data indicate that γδ T lymphocytes are important effectors in SCCHN killing and are crucial for the IPP induced-generation of cytotoxicity against SCCHN tumors mediated by NK cells.
CD56+ γδ T cells utilize perforin-granzyme pathway for SCCHN killing
We next sought to understand the mechanisms underlying killing of SCCHN cell lines by CD56+ γδ T lymphocytes. We evaluated the expression of CD107a (lysosome-associated membrane protein-1), a marker associated with the degranulation of cytotoxic T lymphocytes and NK cells. The incubation of IPP expanded PBMC with PMA and Ionomycin for 4 h resulted in the upregulation of CD107a in 68% of CD56+ γδ T cells, while only 21% of CD56− cells expressed CD107a (Fig. 5B). As can be seen in Fig 5C, incubation of γδ T lymphocytes with Daudi induced the expression of CD107a in CD56+ γδ T lymphocytes. CD56+ γδ T lymphocytes cultured with SCCHN cell lines for 15 h demonstrated increased CD107a expression, though not as robust as when cultured with Daudi (data not shown). These results indicate that upon stimulation CD56+ γδ T cells show more intensive granule release, as compared to CD56− γδ T lymphocytes.
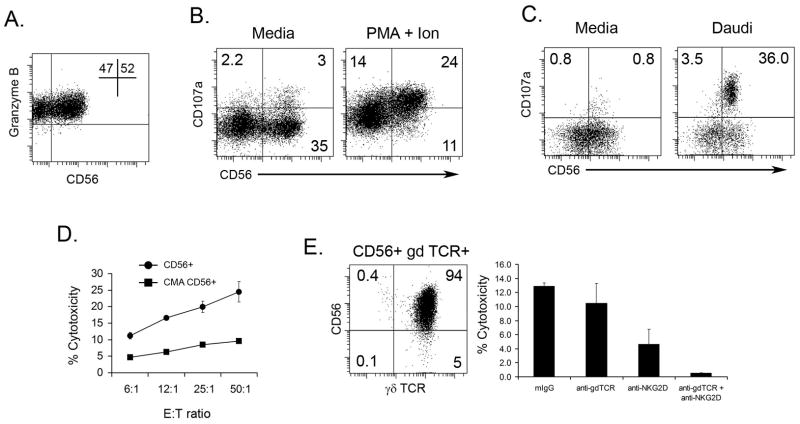
Figure 5. Expression of degranulation marker (CD107a) is increased in CD56+ γδ T cells after stimulation.
γδ T cells were generated by expanding PBMC (see Materials and Methods). A. Cells were stained with anti-CD56 and anti-Granzyme B and analyzed by flow cytometry. B. Expanded γδ T cells were cultured with either media or PMA and Ionomycin in the presence of CD107a-FITC mAB for 4 hours (see Materials and Methods). The cells were then stained for anti-CD3, anti-γδTCR, and anti-CD56 and analyzed by flow cytometry. C. Expanded γδ T cells were separated for CD56+ and CD56− populations using magnetic beads. The populations were then cultured with media or Daudi cells in the presence of CD107a-FITC mAB anti-CD107a. After 15 hours, the cells were stained with anti-CD3, anti-γδTCR, and anti-CD56 and analyzed by flow cytometry. A representative dot blot from one of three experiments is shown. D. Expanded γδ T cells were incubated with CMA during the standard 4 h 51Cr-release assay against TU159 SCCHN cell line. One of three independent experiments is shown. E. The cytotoxic activity against TU159 of highly purified CD56+ γδ T cells (dot plot) was measured in standard 4 h 51Cr-release assay. Blocking anti-γδ TCR antibodies were added into the wells containing CD56+ γδ T cell effectors and TU159 HNSCC cell line for the duration of the cytotoxicity test. Cytotoxicity is shown for 12:1 Effector:Target ratio.
To confirm the involvement of cytolytic granule release in SCCHN lysis by CD56+ γδ T lymphocytes, effectors cells were pre-incubated with CMA, which inhibits acidification of organelles and perforin-mediated cytotoxicity. The lytic potential of IPP expanded CD56+ γδ T cells was significantly abrogated (~70% inhibition) after CMA treatment, indicating that cytotoxic activity of CD56+ γδTCR+ effectors chiefly involves the perforin-granzyme pathway (Fig. 5D).
It has been shown that cytotoxicity of total γδ T cell population can be partially or completely blocked, depending on tumor type, by a combination of anti- γδ TCR and anti-NKG2D mAb (32). We have determined that SCCHN cell lines used in our experiment express NKGD2 ligands such as ULBP-1, ULBP-2 and ULBP-3 (data not shown). To verify if cytotoxicity mediated by CD56+ γδ T cells involves the γδ TCR and NKG2D molecules, blocking antibody against both γδ TCR and NKG2D were added to wells containing highly purified CD56+ γδ T cells (Fig 5E) and TU159 target cells. As shown in Fig. 5E lysis of TU159 cells was partially blocked by anti-γδ TCR and anti-NKG2D mAb alone while a combination of these Abs resulted in complete inhibition of target cells lysis. We have also observed that the combination of anti-γδ TCR and anti-NKG2D mAb was more efficient in blocking lysis of other SCCHN cell lines by CD56+ γδ T cells from various donors. The addition of blocking anti-CD56, anti-TRAIL or anti-FasL Ab did not affect CD56+ γδ T cell mediated cytotoxicity (data not shown). The CD56− fraction of IPP expanded PBMC did not kill SCCHN cell lines and therefore was not used in the above experiments.
Differential expression of surface markers and cytokines in CD56+ and CD56− γδ T cell populations
Since we observed that NKG2D is involved in the killing of SCCHN targets by CD56+ γδ T cells we measured the expression of NKG2D and other surface molecules on γδ T cell subsets. We observed that both CD56+ and CD56− γδ T cell subsets express the same levels of NKG2D, CD94, CD85 and CD158b while neither expressed CD158a, CD158e, CD244, FasL or TRAIL (Fig 6A and data not shown). CD56+ and CD56− γδ T cells expressed the same levels of activation markers such as CD69 (Fig 6A) suggesting that both subsets of γδ T cells are equally activated by IPP and IL-2. Strikingly we found that 66% of CD56+ γδ T cell express the CD16 receptor on their surface, while only 27% of CD56− γδ T cell were CD16 positive. In addition we determined that all γδ T cell subsets expressed same levels of intracellular TNF-α after stimulation with PMA and Ionomycin. In contrast we showed a small but reproducible increase in IFN-γ positive cells within CD56+ γδ T lymphocytes after similar stimulation. IPP + IL-2 expanded, PMA + Ionomycin stimulated γδ T cells stained negative for IL-15, IL-12, IL-18 and IL-21.
Figure 6. Phenotype of γδ T cell subsets.
A. IPP + IL-2 expanded PBMC were stained with anti-CD56, anti-γδTCR, anti-CD16, anti-CD69, anti-NKG2D and CD94 mAb. Histograms of gated γδTCR+CD56+ and γδTCR+CD56− are presented. One of three independent experiments is shown. B. Expanded γδ T cells were cultured with either media or PMA and Ionomycin for 4 hours (see Materials and Methods). The cells were then stained for anti-CD3, anti-γδTCR, and anti-CD56 and analyzed by flow cytometry. One of five independent experiments is shown.
Resistance of CD56+ γδ T cells to apoptosis
It has been shown that tumors can induce apoptosis in T lymphocytes (33, 34). The efficacy of tumor killing by T cells may rely on the ability of the effector cells to resist apoptosis. To assess if CD56+ and CD56− γδ T lymphocytes differ in their ability to tolerate apoptosis, we first evaluated the expression of the Fas receptor (CD95) on their surface. All CD56+ and CD56− γδ T cells expanded in the presence of IPP and IL-2 expressed CD95 on their surface (Fig. 7A).
Figure 7. CD95 expression and apoptosis in γδ T cells.
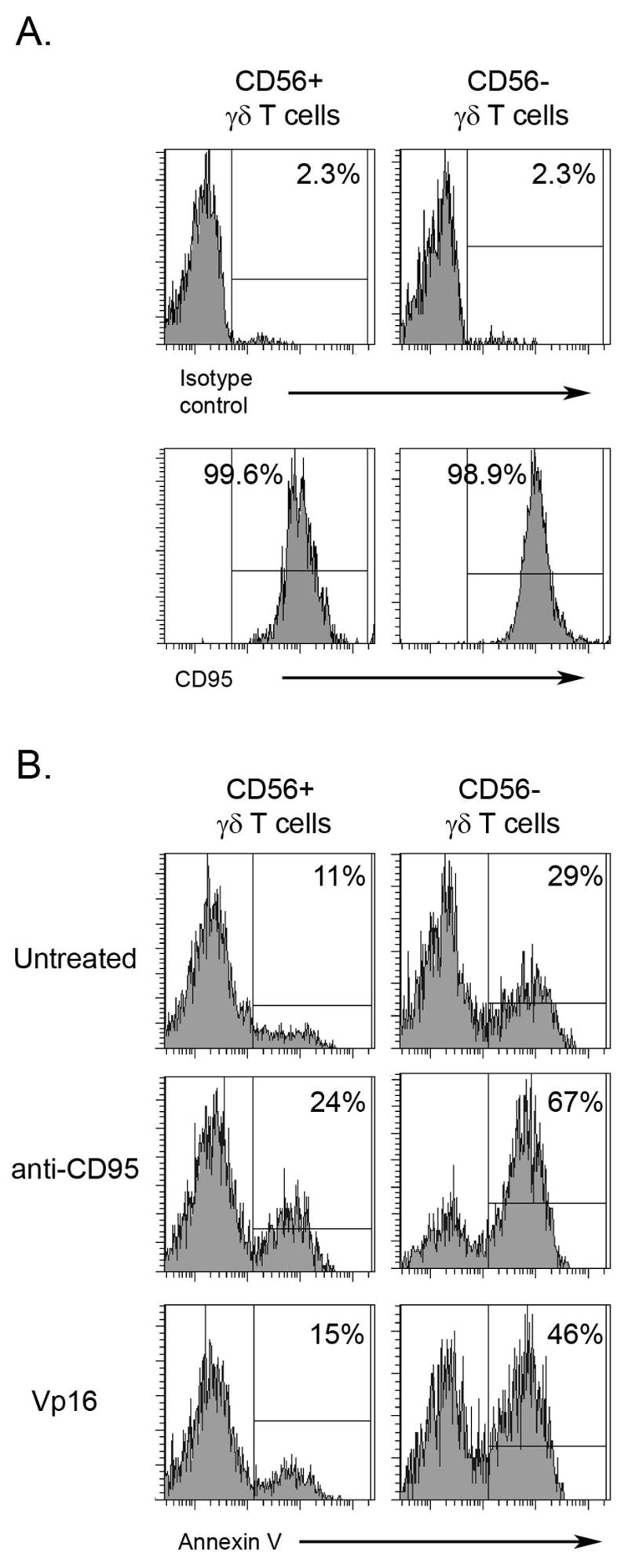
A. IPP + IL-2 expanded PBMC were stained with anti-CD56, anti-γδTCR and anti-CD95 mAb. Histograms of gated γδTCR+CD56+ and γδTCR+CD56− are presented. One of three independent experiments is shown. B. IPP + IL-2 expanded PBMC were treated with anti-CD95 mAb or Vp16 for 18 h. Cells were stained with anti-CD56, anti-γδTCR and Annexin V. Histograms of gated γδTCR+CD56+ and γδTCR+CD56− are presented. One of three independent experiments is shown.
Given the expression of CD95 on both CD56+ and CD56− gd T cells, we evaluated the sensitivity of these γδ T cell populations to undergo Fas-mediated apoptosis. IPP expanded PBMC were treated with anti-CD95 Ab and the apoptosis of CD56+ γδTCR+ and CD56− γδTCR+ cells was measured by annexin V staining. The data presented in Fig 7B indicate increased annexin V binding to CD56− γδ T cells treated with anti-Fas Ab. In addition, more annexin V positive cells were found among CD56− γδ T lymphocytes treated with a chemotherapeutic agent, Vp16 (Fig. 6B). Moreover, higher annexin V binding was also observed in the untreated CD56− fraction of γδ T cells (Fig. 6B). These data indicate that a significantly higher proportion of CD56− γδ T cells bind Annexin V, as compared to CD56+ cells, suggesting that CD56+ γδ T cells are more resistant to apoptosis.
Discussion
Our study provides solid evidence supporting the ability of stimulated CD56+ γδ T cells to kill SCCHN tumors. Consistent with previous reports, we determined that γδ T cells can be expanded from PBMC of healthy donors by IPP stimulation in the presence of exogenous IL-2 (30) with approximately 30–50% of IPP expanded γδ T cells expressing CD56 on their surface (35, 36). While previous reports have shown that depletion of CD56+ cells abrogates cytotoxicity of both αβ and γδ T lymphocytes (36), a direct comparison of cytolytic activity of CD56+ and CD56− γδ T cell fractions has not been performed. Since the expression of the CD56 molecule on stimulated conventional T lymphocytes is associated with a differentiated effector function (23–25), we compared the cytotoxic characteristics of IPP expanded CD56− and CD56+ γδ T cell populations. Our results confirmed that many tumor targets, including multiple SCCHN cell lines, are susceptible to γδ T cell mediated lysis (32). However, our data indicate that only CD56+ γδ T cells are able to kill SCCHN tumor cells. In addition, CD56+ γδ T cells showed higher levels of cytotoxicity against Daudi lymphoma cells as compared to CD56− γδ T cells. These findings indicate that IPP activated CD56+ γδ T cells are more potent anti-tumor effectors than their CD56− counterpart.
Because of the heterogeneous nature of PBMC, we next evaluated the relative contribution of various cell populations to the killing of SCCHN. We determined that only NK cells and CD56+ γδ T lymphocytes isolated from IPP + IL-2 expanded PBMC can kill SCCHN cell lines. It has been shown that NK cells activated with high doses of IL-2 can be cytotoxic towards SCCHN (37). However, in our experiments, PBMC stimulated with IL-2 alone (100 U/ml) did not show the increased cytotoxicity against SCCHN, suggesting no direct effects of IL-2 on NK cell activation. Furthermore, we determined that depletion of γδ T lymphocytes from PBMC, prior to IPP + IL-2 expansion, completely diminished the cytotoxic activity of NK cells. This functional activity was restored by exposure of NK cells to γδ T cells through a Transwell. These data imply that IPP and IL-2 do not stimulate NK cells directly. Rather, IPP + IL-2 induced soluble factors (e.g. cytokines) from γδ T lymphocytes can stimulate NK cell mediated cytotoxicity towards SCCHN. These observations are consistent with other reports indicating that γδ T cells produce TNF-α and IFN-γ after activation (10) which are known to stimulate cytolytic NK cells.
Two recognized mechanisms, cell-cell contact and release of cytolytic factors are involved in γδ T cell anti-tumor activity. For example, it is known that tumor recognition and cytotoxicity of γδ T cells depend on γδ TCR and NKG2D (32). In order to understand the mechanism of CD56+ γδ T cell anti-tumor function, we first explored the relative contribution of the γδ TCR and NKG2D in SCCHN killing. First we determined that SCCHN cell lines express NKG2D ligands. Furthermore, addition of a combination of anti-γδ TCR and anti-NKG2D mAbs to cytotoxicity assays induced complete inhibition of cytotoxic activity against SCCHN targets by CD56+ γδ T cells. Each of the Abs alone induced marginal but significant inhibition of CD56+ γδ T cell mediated cytotoxicity. These data indicate that both the γδ TCR and NKG2D are involved in recognition and killing of SCCHN by CD56+ γδ T cells. Importantly, in blocking experiments we used highly purified CD56+ γδ T cell fractions (<1% NK cell contamination) which argues against major NK cell contribution in SCCHN killing by IPP + IL-2 expanded PBMC.
Because both CD56− and CD56+ γδ T cell populations have similar levels of γδ TCR and NKG2D, the expression of these receptors can not explain the increased cytolytic activity of CD56+ γδ T cells. The main mechanism of cytotoxicity by γδ T cells involves the release of granules containing granzyme B and perforin (38). Therefore, in next set of experiments we assessed the presence of granzyme B in CD56+ and CD56− populations of γδ T lymphocytes. Interestingly, both cytotoxic CD56+ γδ T cells and non-cytotoxic CD56− γδ T cells have equivalent amounts of Granzyme B. These data indicate that both CD56+ and CD56− γδ T cells have the same killing machinery. However, after co-culture with Daudi cells CD56+ γδ T lymphocytes express significantly higher levels of CD107a, a marker demonstrating the intensity of killer cell degranulation (39). We also confirmed the involvement of granzyme and perforin in SCCHN killing by CD56+ γδ T cells using CMA, a granzyme-perforin pathway inhibitor. Therefore, our data indicate that CD56 is expressed on activated effector γδ T lymphocytes that are capable to lyse SCCHN by releasing increased amount of cytolytic granules.
We determined that CD56 expression itself does not generate the increased cytotoxicity, since a subset of fresh, unstimulated γδ T lymphocytes expressing CD56 does not kill Daudi or SCCHN cell lines. Only after activation with IPP + IL-2 the CD56+ γδ T cells become potent anti-tumor effectors. We have also noted the increased expression of CD16, the low affinity type 3 receptor for the Fc portion of IgG, on IPP + IL-2 expanded CD56+ γδ T cells. The expression of functional CD16 on activated γδ T cells has been reported previously (40, 41). Expression of CD16 was associated with the increased direct cytotoxicity of γδ T cells (40). Additional experiments are required for understanding the relative contribution of CD56 and CD16 molecules in the augmented γδ T cell cytotoxicity. However, combination of these two markers can be useful for detection of most potent anti-tumor effector γδ T cell subsets in vitro and in vivo. This may be critical for the design and evaluation of a new anti-cancer therapeutics.
The expression of Fas ligand on tumors raises the possibility that malignant cells could induce apoptosis in effector cells and decrease their cytotoxicity (33). We have determined that 100% of both CD56− and CD56+ IPP activated γδ T cells express CD95, suggesting that FasL expressing tumor cells can eliminate these important anti-tumor effector cells. However, our data indicate that CD56+ γδ T cells were more resistant to FasL mediated apoptosis compared to CD56− γδ T cells. It is not clear whether relative apoptosis resistance of CD56+ γδ T cells can explain the enhanced anti-tumor activity of these cells. Nevertheless, this observation correlates with previously published findings that ex vivo expanded CD3+CD56+ T lymphocytes are resistant to Fas-mediated apoptosis (42). Importantly, preferential apoptosis of NK cells expressing low (dim) levels of CD56 was observed in cancer patients, while only a small subset of CD56bright NK cells underwent apoptosis (43). It remains to be determined if direct signaling through the CD56 molecule can protect effector cells from apoptosis.
Our findings indicate that activated CD56+ γδ T cells can efficiently kill SCCHN in a γδ TCR dependant fashion. While the mechanisms underlying the observed functional differences between CD56+ and CD56− cells are not completely understood, it is clear that effector function correlates with higher levels of granzyme release upon stimulation, and higher resistance to apoptosis. In addition we observed that γδ T lymphocytes are important not only for tumor lysis, but also play a role in enhancing the cytotoxic potential of NK cells. Our study describes a subset of CD56+ γδ T cells with potent anti-tumor effector function and provides optimism that these cells may be harnessed for the immunotherapy of SCCHN and other malignances.
Acknowledgments
We thank Cheryl Armstrong for technical assistance.
This work was supported by grants from University of Maryland to AC, NIH grant CA113261 to DP and Marlene and Stewart Greenebaum Cancer Center to DP, AC and BG.
References
- 1.Strome SE, Weinman EC. Advanced larynx cancer. Curr Treat Options Oncol. 2002;3:11–20. doi: 10.1007/s11864-002-0037-9. [DOI] [PubMed] [Google Scholar]
- 2.Albers AE, Ferris RL, Kim GG, et al. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–81. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai T, Storkus WJ, Mueller-Berghaus J, et al. In vitro generated cytolytic T lymphocytes reactive against head and neck cancer recognize multiple epitopes presented by HLA-A2, including peptides derived from the p53 and MDM-2 proteins. Cancer Immun. 2002;2:3. [PubMed] [Google Scholar]
- 4.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 5.Strome SE, Chen L. Costimulation-based immunotherapy for head and neck cancer. Curr Treat Options Oncol. 2004;5:27–33. doi: 10.1007/s11864-004-0003-9. [DOI] [PubMed] [Google Scholar]
- 6.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 7.Weinman EC, Roche PC, Kasperbauer JL, et al. Characterization of antigen processing machinery and Survivin expression in tonsillar squamous cell carcinoma. Cancer. 2003;97:2203–11. doi: 10.1002/cncr.11311. [DOI] [PubMed] [Google Scholar]
- 8.Falini B, Flenghi L, Pileri S, et al. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989;143:2480–8. [PubMed] [Google Scholar]
- 9.Kunzmann V, Wilhelm M. Anti-lymphoma effect of gammadelta T cells. Leuk Lymphoma. 2005;46:671–80. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- 10.Corvaisier M, Moreau-Aubry A, Diez E, et al. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–8. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 11.Viey E, Fromont G, Escudier B, et al. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–47. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 12.Guo BL, Liu Z, Aldrich WA, Lopez RD. Innate anti-breast cancer immunity of apoptosis-resistant human gammadelta-T cells. Breast Cancer Res Treat. 2005;93:169–75. doi: 10.1007/s10549-005-4792-8. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Kimura S, Segawa H, et al. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–9. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 14.Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–76. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 15.Bas M, Bier H, Schirlau K, et al. Gamma-delta T-cells in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:691–7. doi: 10.1016/j.oraloncology.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human gammadelta T lymphocytes. Int Arch Allergy Immunol. 2000;122:1–7. doi: 10.1159/000024353. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Morita CT, Tanaka Y, et al. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 18.Gober HJ, Kistowska M, Angman L, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–46. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 21.Rincon-Orozco B, Kunzmann V, Wrobel P, et al. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–51. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 22.Lafarge X, Pitard V, Ravet S, et al. Expression of MHC class I receptors confers functional intraclonal heterogeneity to a reactive expansion of gammadelta T cells. Eur J Immunol. 2005;35:1896–905. doi: 10.1002/eji.200425837. [DOI] [PubMed] [Google Scholar]
- 23.Cohavy O, Targan SR. CD56 marks an effector T cell subset in the human intestine. J Immunol. 2007;178:5524–32. doi: 10.4049/jimmunol.178.9.5524. [DOI] [PubMed] [Google Scholar]
- 24.Casado JG, Soto R, DelaRosa O, et al. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005;54:1162–71. doi: 10.1007/s00262-005-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 26.Lanier LL, Chang C, Azuma M, et al. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56) J Immunol. 1991;146:4421–6. [PubMed] [Google Scholar]
- 27.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 28.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 29.Kelly-Rogers J, Madrigal-Estebas L, O’Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum Immunol. 2006;67:863–73. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- 30.Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual Vgamma2-Jgamma1. 2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56:819–29. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 32.Wrobel P, Shojaei H, Schittek B, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–8. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 33.Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006;28:462–70. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 35.Fujimiya Y, Suzuki Y, Katakura R, et al. In vitro interleukin 12 activation of peripheral blood CD3(+)CD56(+) and CD3(+)CD56(−) gammadelta T cells from glioblastoma patients. Clin Cancer Res. 1997;3:633–43. [PubMed] [Google Scholar]
- 36.Satoh M, Seki S, Hashimoto W, et al. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–92. [PubMed] [Google Scholar]
- 37.Whiteside TL, Chikamatsu K, Nagashima S, Okada K. Antitumor effects of cytolytic T lymphocytes (CTL) and natural killer (NK) cells in head and neck cancer. Anticancer Res. 1996;16:2357–64. [PubMed] [Google Scholar]
- 38.Mohamadzadeh M, McGuire MJ, Smith DJ, et al. Functional roles for granzymes in murine epidermal gamma(delta) T-cell-mediated killing of tumor targets. J Invest Dermatol. 1996;107:738–42. doi: 10.1111/1523-1747.ep12365634. [DOI] [PubMed] [Google Scholar]
- 39.Marcenaro S, Gallo F, Martini S, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13–4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 40.Angelini DF, Borsellino G, Poupot M, et al. FcgammaRIII discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood. 2004;104:1801–7. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 41.Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol. 2001;166:7190–9. doi: 10.4049/jimmunol.166.12.7190. [DOI] [PubMed] [Google Scholar]
- 42.Verneris MR, Kornacker M, Mailander V, Negrin RS. Resistance of ex vivo expanded CD3+CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49:335–45. doi: 10.1007/s002620000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–24. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]