Abstract
Angiotensin-converting enzyme (ACE) inhibitors are frequently used to treat hypertension in children (1). ACE inhibitors alter the balance between the vasoconstrictive, salt-retentive, and cardiac hypertrophic properties of angiotensin II and the vasodilatory and natriuretic properties of bradykinin; they also alter the metabolism of other vasoactive substances (2). Through these mechanisms, ACE inhibitors decrease systemic vascular resistance and promote natriuresis without increasing heart rate. They have proven efficacy in the treatment of hypertension, congestive heart failure, and left ventricular dysfunction and also delay the progression of diabetic nephropathy (2). In adults, ACE inhibitors are less potent in blacks than in whites (3, 4). Although racial differences in response to ACE inhibitors are established in adults, data in children are limited by small sample sizes of individual trials (5, 6). We evaluated results of 6 trials of ACE inhibitors submitted to the US Food and Drug Administration (FDA) for pediatric exclusivity using meta-analytic techniques to estimate the effect of race on blood pressure response.
METHODS
The cohort
Between January 1, 1998, and December 31, 2005, efficacy data from 12 antihypertensive products were submitted to the FDA’s Division of Cardiovascular and Renal Products. We used the randomized, blinded efficacy trials for this analysis (7). One efficacy trial and at least 1 pharmacokinetic-pharmacodynamic (PK-PD) trial were completed for each agent. Summaries of medical and clinical pharmacology reviews of several of the studies are publicly available (8). Except for demonstrating differences in clearance and half-life for benazepril, the PK trials did not indicate differential dosing from adult data and did not suggest differences in absorption, bioavailability, clearance, or differential response by age or development. PK-PD samples were not obtained in the efficacy trials; therefore, we did not include PK-PD results in this analysis. The PK trials were performed in children ages 1 month to 16 years with the exception of the quinapril trial which was performed in children ages 2.5 months to 6 years and the ramipril trial which was performed in children ages 2 years to 6 years (8–11). In our previous analysis, we evaluated data from the dose-response part of the trials (7). For this analysis, we analyze the placebo phase of the trials; therefore the sample sizes are different.
Of the 12 efficacy trials, 6 were conducted with ACE inhibitors. Inclusion and exclusion criteria among trials were similar. The studies enrolled children 6–16 years of age. The trials excluded children with severe hypertension (because of a placebo phase), low glomerular filtration rates (< 30 mL/min/1.73 m2), electrolyte abnormalities, renal disease, and other substantive medical problems including clinically significant cardiac disease (5). The trial design (12) and dosing strata of each study are shown in Table 1.
Table 1.
Trial design and dosing strata
| Drug | Trial design | Dosing |
|---|---|---|
| Benazepril | D | L: 5 mg if < 50 kg |
| L: 10 mg if ≥ 50 kg | ||
| M: 10 mg if < 50 kg | ||
| M: 20 mg if ≥ 50 kg | ||
| H: 20 mg if < 50 kg | ||
| H: 40 mg if ≥ 50 kg | ||
| Enalapril | C | L: 0.625 mg if < 50 kg |
| L: 1.25 mg if≥ 50 kg | ||
| M: 2.5 mg if < 50 kg | ||
| M: 5.0 mg if ≥ 50 kg | ||
| H: 20 mg if < 50 kg | ||
| H: 40 mg if ≥ 50 kg | ||
| Fosinopril | C | L: 0.1 mg/kg |
| M: 0.3 mg/kg | ||
| H: 0.6 mg/kg, not to exceed 40 mg | ||
| Lisinopril | C | L : 0.625 mg if < 50 kg |
| L : 1.25 mg if ≥ 50 kg | ||
| M: 2.5 mg if < 50 kg | ||
| M: 5.0 mg if ≥ 50 kg | ||
| H: 20 mg if < 50 kg | ||
| H: 400 mg if ≥ 50 kg | ||
| Quinapril | A | L: 2.5 mg if 20–40 kg |
| L: 5 mg if > 40 kg | ||
| M: 5 mg if 20–40 kg | ||
| M: 10 mg if > 40 kg | ||
| H: 10 mg if 20–40 kg | ||
| H: 20 mg if > 40 kg | ||
| Ramipril | D | L: 0.625 to 5 mg |
| M: 1.25 to 10 mg | ||
| H: 2.5 to 20 mg |
L denotes low dose, M denotes medium dose, H denotes high dose.
Data management
The data, protocols, and case report forms from these 6 trials were submitted electronically to the FDA. Source data were available in SAS datasets, which we converted to STATA datasets via STAT Transfer. We combined patient-level data to obtain 1 observation per patient.
From each trial, we assembled 30 common variables: antihypertensive product, unique patient identification number, age, sex, race, height, weight, body mass index (BMI), baseline sitting diastolic blood pressure, baseline sitting systolic blood pressure, diastolic and systolic blood pressure at end of the placebo-controlled phase, and amount of drug in milligrams and per dosing stratum (low, medium, or high [Table 1]). We used a categorical variable of white/black/other for race because several trials used this format to report race, and more specific information was not available.
Baseline systolic and diastolic blood pressures were the average of 3 sequential values obtained at the beginning of the randomized placebo-controlled phase. If 1 value was missing, the average of 2 observations was used. Blood pressures taken at the end of the placebo-controlled phase were calculated in similar fashion. If the last observation at the end of the placebo-controlled phase was missing, we used the last observation carried forward as an imputation method. Percent change from baseline blood pressure was used as a primary end point to assess response to antihypertensive therapy. This was calculated by subtracting the end–of–placebo-controlled phase blood pressures from the baseline values.
Statistical analysis
We analyzed the data using 2 methods. In the first analysis, we extracted each variable listed above into a common dataset in which each patient from each trial was represented with 1 observation. We assessed blood pressure response with respect to race in each trial. In each analysis, we used a linear regression evaluating interaction with race as a categorical variable. A significant response was concluded if the slope of the regression line differed from 0 at the 0.05 significance level. In the second analysis, we used meta-analytic techniques to evaluate the data. Least mean squares parameter estimates and their standard errors were obtained for each analysis and then entered into the Borenstein M., Hedges L., Higgins J., Rothenstein H., Comprehensive Meta-analysis Version 2 software (Englewood, NJ). A fixed-effects meta-analysis was employed for comparing the role of dose, race, and dose–race interactions on change in blood pressure across all ACE inhibitors. A random effects meta-analysis was also performed, and the results were identical. The analysis had > 90% power to detect a 6 mm Hg change in blood pressure if the standard deviation is assumed to be 10 mm Hg with a treated sample size of 48 subjects and a placebo sample size of 87 subjects. We used the individual patient data to report the results in Table 2 and the results from the meta-analysis to provide graphic representation of the data.
Table 2.
Baseline demographics
| Patient baseline characteristic | All Drugs
(N=848) |
Benazepril
(n=85) |
Enalapril
(n=101) |
Fosinopril
(n=233) |
Lisinopril
(n=102) |
Quinapril
(n=111) |
Ramipril
(n=216) |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| Median (IQR) | 12 (10,14.5) | 13 (12,15) | 12 (9,14) | 12 (10,14) | 13 (10,14) | 13 (10,15) | 12 (10,14) |
| Sex | |||||||
| Male (%) | 519 (61.2%) | 51 (60.0%) | 57 (56.4%) | 154 (66.1%) | 67 (65.7%) | 66 (59.5%) | 124 (57.4%) |
| Race | |||||||
| White (%) | 370 (43.6%) | 48 (56.5%) | 38 (37.6%) | 138 (59.2%) | 44 (43.1%) | 41 (36.9%) | 61 (28.2%) |
| Black (%) | 228 (26.9%) | 19 (22.4%) | 22 (21.8%) | 48 (20.6%) | 11 (10.8%) | 38 (34.2%) | 90 (41.7%) |
| Other (%) | 250 (29.5%) | 18 (21.2%) | 41 (40.6%) | 47 (20.2%) | 47 (46.1%) | 32 (28.8%) | 65 (30.1%) |
| Placebo-control phase | |||||||
| Placebo | 326 (38.4%) | 19 (22.4%) | 50 (49.5%) | 125 (53.7%) | 51 (50.0%) | 27 (24.3%) | 54 (25.0%) |
| Low | 172 (20.3%) | 24 (28.2%) | 12 (11.9%) | 38 (16.3%) | 15 (14.7%) | 29 (26.1%) | 54 (25.0%) |
| Medium | 163 (19.2%) | 23 (27.1%) | 14 (13.9%) | 34 (14.6%) | 11 (10.8%) | 27 (24.3%) | 54 (25.0%) |
| High | 187 (22.1%) | 19 (22.4%) | 25 (24.8%) | 36 (15.5%) | 25 (24.5%) | 28 (25.2%) | 54 (25.0%) |
| Baseline weight (kg) | |||||||
| Median (IQR) | 66.0 (44.0, 88.7) | 71.0 (50.9,93.0) | 44.5 (33.0,68.5) | 68.9 (51.0,94.8) | 50.0 (37.0,72.6) | 68.2 (51.8,91.7) | 70.5 (47.0,93.0) |
| Baseline BMI z-score | |||||||
| Median (IQR) | 1.8 (0.8, 2.4) | 1.9 (0.8,2.4) | 1.0 (−0.2,2.0) | 2.0 (1.2,2.5) | 1.1 (−0.0,1.9) | 1.9 (1.2,2.5) | 2.1 (1.0,2.5) |
| Baseline diastolic BP | |||||||
| Median (IQR) | 80.0 (70.2, 86.0) | 83.0 (73.0,86.0) | 88.0 (84.0,94.0) | 70.0 (64.7,77.7) | 86.0 (82.7,91.3) | 78.0 (70.0,84.0) | 80.0 (71.0,85.0) |
| Baseline systolic BP | |||||||
| Median (IQR) | 131.0 (124.0, 137.5) | 134.0 (128.0,138.0) | 128.0 (118.0,137.5) | 133.0 (127.0,139.3) | 125.8 (118.3,135.3) | 132.0 (124.7,138.7) | 130.0 (123.0, 136.0) |
BMI = body mass index; BP = blood pressure; IQR = interquartile range
Ethics
We presented the project to the Duke University Medical Center Institutional Review Board and the FDA’s Research Involving Human Subjects Committee and received a waiver of review for this analysis because there were no patient identifiers in the already existing data which were used for this review.
RESULTS
The fraction of male children and the age distribution of enrolled subjects were similar between trials (Table 1). The ramipril trial enrolled a lower proportion of white children (28.2%, compared with 43.6% across all trials). The distribution of race was pre-specified by the protocols because the FDA written request asked for a larger percentage of black children enrolled than that observed in the US population. The distribution of weight and BMI of subjects varied among trials. The median BMI z-score of subjects enrolled in the enalapril and lisinopril trials was 1.0 and 1.1, respectively; median BMI z-score was 2.0 in the fosinopril trial and 2.1 in the ramipril trial.
Systolic blood pressure
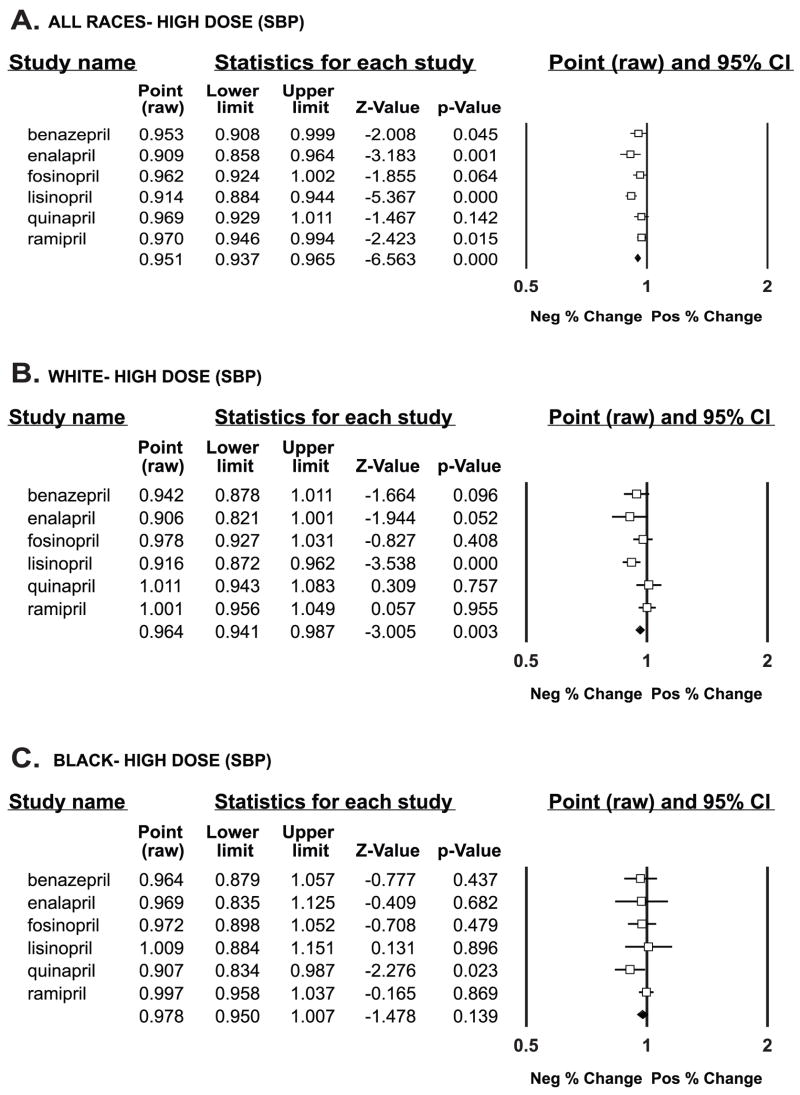
For all races combined, the individual trials and the aggregate result did not show a significant difference from baseline using low-dose drug. Only the benazepril (P = 0.011) and enalapril (P = 0.032) trials showed differences from baseline with medium-dose drug; however, in aggregate across trials, a significant difference from baseline (P < 0.001) was seen. With high-dose drug, benazepril (P = 0.045), enalapril (P = 0.001), lisinopril (P < 0.001), and ramipril (P = 0.015) showed a difference from baseline, and the aggregate response was highly significant (P < 0.001) (Figure 1). Therefore, at the highest dose in the trials, a significant systolic blood pressure response was more likely to be demonstrated.
Figure 1.
Systolic blood pressure response to individual drugs and aggregate response (last line of data) compared with baseline (shown by the vertical line at 1) by % change at high-dose drug. Data are graphically shown as point estimates with 95 % confidence intervals. Estimates that fall between 0.5 and l denote a negative % change, and estimates that fall between 1 and 2 denote a positive % change.
A—All races
B—White race
C—Black race
When systolic blood pressure analysis was stratified by race, children of white race at high doses of drug had significant difference from baseline only in the lisinopril trial (P < 0.001); across trials, there was a significant difference from baseline in systolic blood pressure (P =0.003). In contrast, however, black children showed a significant difference at high-dose drug only in the quinapril trial (P = 0.02) and no significant response across trials (P = 0.139) (Figure 1).
Diastolic blood pressure
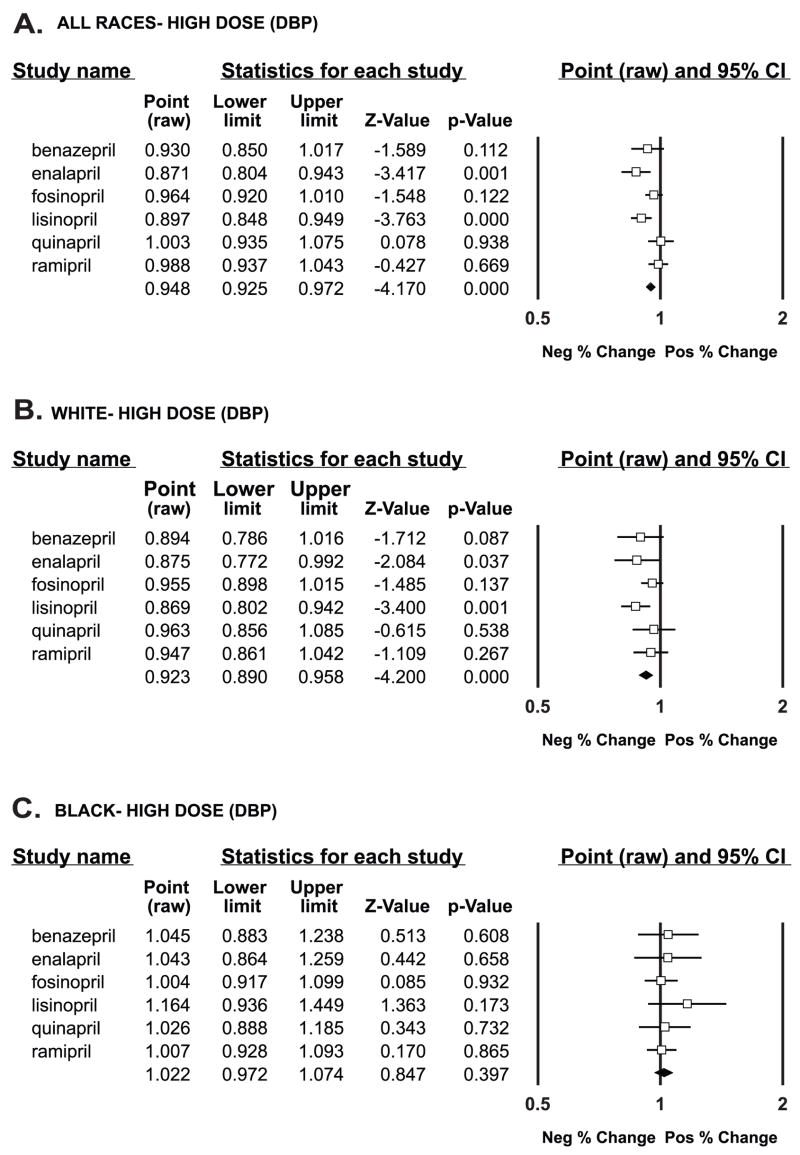
For all children at the high dose of drug, the enalapril (P = 0.001) and the lisinopril (P < 0.001) trials showed a significant difference from baseline, and the aggregate result was highly significant (P < 0.001) (Figure 2).
Figure 2.
Diastolic blood pressure response to individual drugs and aggregate response (last line of data) compared with baseline (shown by the vertical line a 1) by % change at high-dose drug. Data are graphically shown as point estimates with 95 % confidence intervals. Estimates that fall between 0.5 and l denote a negative % change, and estimates that fall between 1 and 2 denote a positive % change.
A—All races
B—White race
C—Black race
When stratified by race, children of white race at high-dose drug showed a significant change from baseline in the enalapril trial (P = 0.037) and the lisinopril trial (P = 0.001); in aggregate, there was a highly significant difference (P < 0.001). Children of other races similarly had a highly significant aggregate difference (P < 0.001). However, black children did not show a difference from baseline in any individual trial or in aggregate (P = 0.397) (Figure 2).
DISCUSSION
We were provided access to individual patient data for each of the ACE inhibitor pediatric antihypertensive efficacy trials submitted for pediatric exclusivity from 1998 to 2005, inclusive. All 6 trials enrolled a substantial proportion of children of black or other race. At the highest dose of drug, children of white or other race demonstrated a significant systolic and diastolic blood pressure response to ACE inhibition. In contrast, at the highest dose of drug, children of black race did not show a response in aggregate to ACE inhibition.
We previously reported a racial difference in response to blood pressure of fosinopril using a different analytic method (slope of dose response in black patients compared with white patients). The present analysis differs in that, here, we have used absolute change from baseline in each trial. Our previous work is the only study to report on racial differences in blood pressure response to antihypertensive drugs in children (13).
Our study demonstrates that black children do not respond as well to ACE inhibitors compared with children of white or other race. This is consistent with the well-described racial differences in responsiveness to ACE inhibitors in adult patients (3, 4). ACE inhibitors are less effective as monotherapy in black adults because of low renin levels and a high degree of salt sensitivity (14). This differential response of the renin angiotensin system in blacks may be due to genetic polymorphisms in the angiotensinogen gene (15, 16). In black adults, the intrarenal renin–angiotensin system is not suppressed as effectively as in whites in response to high salt intake but shows the same level of activation with low salt intake. Black adults on a low-salt diet or a diuretic show a renal vascular response to renin and ACE inhibition identical to whites (17).
The differential blood pressure response to ACE inhibitors according to race seen in this study could not have been demonstrated by evaluating individual trials but was discernible in the meta-analysis. We were only able to do this by having access to the data across trials. Access to protocols, final study reports, and individual patient data for each trial was therefore crucial to our investigation.
Limitations to our study include that trials of beta-blockers and calcium channel blockers were not included. We only had access to 1 trial of beta-blocker and 2 trials of calcium channel blockers compared with 6 trials of ACE inhibitors. In addition, the race group of “other” is non-specific and included children of mixed race; therefore, we could not particularly evaluate other ethnic groups besides white and black. Other limitations of this study are the small age range studied which included mainly children > 10 years of age with adult weights. Also, many patient populations that frequently use ACE inhibitors (e.g., those with cardiac disease, renal disease, or diabetes) were excluded from these studies. Hence, these data may not be applicable to younger children and/or sicker children
We have compiled the results of 6 anti-hypertensive trials in children; our analysis suggests that black children do not have as robust a response to ACE inhibitors as white children. Our analysis is limited to the dosages used in the trials; many of these trials used limited differences in the amount of product given between low- and high-dose groups (7). Moreover, the highest doses of drug used in each trial were up to the maximum adult dose. Therefore, higher dosages of ACE inhibitors may be effective in treating hypertensive black children. The safety implications of using potentially higher doses beyond the adult maximum dose, however, require further study.
Acknowledgments
Dr. Norman Stockbridge of the Division of Cardiovascular and Renal Products, Center for Drug Evaluation and Research, Food and Drug Administration, provided access to study data. The views expressed are those of the authors. No official endorsement by the Food and Drug Administration is provided or should be inferred. The Duke Clinical Research Institute was the coordinating center for the fosinopril trial.
Sources of Funding
J.S.L. and D.K.B. received support from National Institute of Child Health and Human Development grant 1U10-HD45962-04 and the Food and Drug Administration. J.S.L., P.B.S., R.M.C., and D.K.B. received support from the National Center for Research Resources and the National Institutes of Health grant 1UL1RR024128-01.
Footnotes
Conflicts of interest
None.
References
- 1.Li JS, et al. Is the extrapolated adult dose of fosinopril safe and effective in treating hypertensive children? Hypertension. 2004;44:1–5. doi: 10.1161/01.HYP.0000138069.68413.f0. [DOI] [PubMed] [Google Scholar]
- 2.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 3.Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol. 2007;6:67–71. doi: 10.1097/HPC.0b013e318053da59. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, et al. Clinical experience with perindopril in Africa-American hypertensive patients: a large United States community trial. Am J Hypertens. 2004;17:134–138. doi: 10.1016/j.amjhyper.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Wells T, et al. A double-blind, placebo-controlled, dose-response study of the effectiveness and safety of enalapril for children with hypertension. J Clin Pharmacol. 2002;42:870–880. doi: 10.1177/009127002401102786. [DOI] [PubMed] [Google Scholar]
- 6.Soffer B, et al. A double-blind, placebo-controlled, dose-response study of the effectiveness and safety of lisinopril for children with hypertension. Am J Hypertens. 2003;10:795–780. doi: 10.1016/s0895-7061(03)00900-2. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK, Jr, et al. Pediatric antihypertensive trial failures: analysis of endpoints and dose range. Hypertension. 2008;51:834–840. doi: 10.1161/HYPERTENSIONAHA.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. [Accessed April 9, 2008];Summaries of medical and clinical pharmacology reviews of pediatric studies. Available at: http://www.fda.gov/cder/pediatric/Summaryreview.htm.
- 9.Blumer JL, et al. Pharmacokinetics of quinapril in children: assessment during substitution for chronic angiotensin-converting enzyme inhibitor treatment. J Clin Pharmacol. 2003;43:128–132. doi: 10.1177/0091270002239820. [DOI] [PubMed] [Google Scholar]
- 10.Wells T, et al. The pharmacokinetics of enalapril in children and infants with hypertension. J Clin Pharmacol. 41:1064–1074. doi: 10.1177/00912700122012661. [DOI] [PubMed] [Google Scholar]
- 11.Hogg R, et al. A multicenter study of the pharmacokinetics of lisinopril in pediatric patients with hypertension. Pediatr Nephrol. 2007;22:695–701. doi: 10.1007/s00467-006-0399-5. [DOI] [PubMed] [Google Scholar]
- 12.Pasquali SK, Sanders SP, Li JS. Oral antihypertensive trial design and analysis under the pediatric exclusivity provision. Am Heart J. 2002;144:608–614. doi: 10.1067/mhj.2002.125323. [DOI] [PubMed] [Google Scholar]
- 13.Menon S, et al. Racial differences are seen in response to fosinopril in hypertensive children. Am Heart J. 2006;152:394–399. doi: 10.1016/j.ahj.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Brewster IM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614–627. doi: 10.7326/0003-4819-141-8-200410190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Woodiwiss AJ, et al. Functional variants of the angiotensinogen gene determine antihypertensive responses to angiotensin-converting enzyme inhibitors in subjects of African origin. J Hypertens. 2006;24:1057–1064. doi: 10.1097/01.hjh.0000226195.59428.57. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, et al. Angiotensinogen gene polymorphisms at -217 affects basal promoter activity and is associated with hypertension in African Americans. J Biol Chem. 2002;277:36889–36896. doi: 10.1074/jbc.M204732200. [DOI] [PubMed] [Google Scholar]
- 17.Veterans Administration Co-operative Study Group of Antihypertensive Agents. Racial differences in response to low-dose captopril are abolished by the addition of hydrochlorothiazide. Br J Clin Pharmacol. 1982;14:97S–101S. [PMC free article] [PubMed] [Google Scholar]