Abstract
The hypothesis that vasopressin (VP) becomes the main mediator of pituitary corticotroph responsiveness during chronic hypothalamic pituitary adrenal (HPA) activation, was tested by examining the effect of pharmacologic VP receptor blockade on the ACTH and corticosterone responses of 14-day repeatedly restrained rats. In spite of the increased vasopressinergic activity, repeatedly restrained rats showed lower ACTH and corticosterone responses to 10 min white noise compared with handled controls. These responses were unchanged by the non-peptide selective V1b receptor antagonist, SSR149415, i.v., 1h before noise application. In contrast to noise stress, plasma ACTH responses to i.p. hypertonic saline injection were enhanced in the repeatedly restrained rats compared with handled controls but responses were also unaffected by SSR149415 administered orally, daily 1 h before restraint. Since SSR149415 effectiveness was low, we used minipump infusion of the peptide V1 receptor antagonist, dGly[Phaa1,D-Tyr(et), Lys, Arg]VP (V1-Ant) for 14 days, which effectively blocked ACTH responses to exogenous VP. Chronic V1-Ant infusion reduced plasma ACTH responses to i.p. hypertonic saline in handled controls but not in repeatedly restrained rats. These data suggest that the increased vasopressinergic activity characteristic of chronic stress plays roles other than mediating the hypersensitivity of the HPA axis to a novel stress.
Keywords: Stress, vasopressin, HPA axis, vasopressin antagonists, vasopressin V1b receptor
INTRODUCTION
The nonapeptide vasopressin (VP), well known for its antidiuretic and vasopressor actions in the periphery, is also a recognized regulator of pituitary corticotroph function 1-3. While VP secreted into the peripheral circulation from hypothalamic magnocellular paraventricular (PVN) and supraoptic (SON) neuronal terminals in the neural pituitary lobe is responsible for the antidiuretic effects of the peptide, VP released from parvocellular neurons secreted into the pituitary portal circulation stimulates ACTH secretion2. In contrast to magnocellular vasopressin, which is stimulated by osmotic stimulation, parvocellular VP levels respond to stress and changes in plasma glucocorticoid levels but not to osmotic changes 4. Although there is evidence suggesting that magnocellular VP can stimulate ACTH secretion under acute osmotic stimulation conditions 1,2,5, prolonged activation of magnocellular VP inhibits rather than stimulates the HPA axis 2,5-8.
In humans and rodents, VP alone is a weak stimulus of ACTH secretion. However, by synergizing with the stimulatory effect of CRH, VP is believed to contribute to the maintenance of corticotroph responsiveness despite the elevated glucocorticoid levels during chronic stress2,9,10. A number of studies have shown that inhibition of vasopressinergic activity by the use of VP antibodies or receptor antagonists can decrease ACTH and corticosterone responses to acute stress. In support of a role for VP in HPA axis responses to stress are reports showing rapid release of VP from parvocellular terminals in the median eminence and upregulation of VP receptor binding and mRNA levels in the pituitary following stress 11,12. Even more compelling are the marked and sustained increases in VP expression in parvocellular neurons of the PVN during prolonged stimulation of the HPA axis in conditions such as chronic stress and adrenalectomy 11-16. In several chronic stress conditions the increases in CRH expression are transient either returning to basal levels or even being inhibited 1,13 with the repeated homotypic stress. Similar to the changes in VP expression, upregulation of V1b receptor content in the pituitary during chronic stress is usually accompanied by CRH receptor downregulation 1,11-18. This predominant increase in vasopressinergic activity during chronic stress has lead to the hypothesis that during chronic stimulation of the HPA axis, mediation of pituitary ACTH regulation switches from CRH to VP. However, studies using genetic models of VP or V1b R deficiency do not fully support this view. For example, the VP deficient, Brattleboro rat shows normal responses to acute stress and only a transient reduction in ACTH responses during repeated restraint 19. Studies in V1b receptor knock out mice have shown reduced responses to some stressors such as lipopolysaccharide and ethanol administration, insulin hypoglycemia 20,21, and swim stress 22, but normal basal ACTH levels and responses to restraint 21. Though this evidence suggests that VP contributes to the ACTH responses in some acute stress paradigms, the role of VP mediating the changes in responsiveness of the HPA axis during chronic stress is unknown.
A selective V1b receptor antagonist, SSR149415, has been recently developed 23. This compound, which is non-peptide in structure and orally active has been shown to effectively block VP-induced ACTH secretion offers an excellent resource to study the relative participation of VP on HPA axis regulation during chronic stress. The availability of SSR149415 encouraged us to examine its effect on plasma ACTH and corticosterone responses to a novel stress in chronically stressed rats and handled controls. Since the experiments using the selective V1b receptor antagonist failed to yield conclusive results, we extended the study and employed chronic minipump infusion of the peptide non-selective V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP, recently shown to prevent the elevation of plasma ACTH and corticosterone following VP injection 24. The results suggest that VP contributes to the acute stress response, but it does not mediate the hypersensitivity of the HPA axis to a novel stress typically found in chronically stressed animals.
MATERIALS AND METHODS
Animals and in vivo procedures
Adult male Sprague Dawley rats weighing 250−300g were housed 3 per cage with a 14−10 h light-dark cycle with food and water available ad libitum, for at least 5 days prior to the study. All procedures were performed according to the NIH guidelines, and experimental protocols were approved by the NICHD Animal Care and Use Committee and the UK Home Office Animal Inspectorate.
Experiment I Effect of a single administration of SSR149415
Rats were initially divided in 2 groups of 14 rats; one was subjected to repeated restraint for 14 days (1h daily beginning at 9−10 AM), by placing them in plastic cylinders (2.5 × 8 inches), and a control group subjected to daily handling. Three days before study, rats were anaesthetized, the right jugular vein was exposed and a silastic-tipped polythene cannula (i.d. 0.58 mm, Portex, Hythe, UK) filled with 10 U/ml heparinized isotonic saline was inserted into the vessel. The free end of the cannula was exteriorized through a scalp incision and then tunneled through a protective spring attached to a mechanical swivel. For collection of blood samples animals were attached to an automated blood sampling system, as previously described 25. The collection of 60 μl samples for the measurement of corticosterone and ACTH commenced at 08.00 h on the day of study (day 15) and continued every 10 min for 3 h. At 8AM, one half of the rats in each group received an i.v. injection of the non-peptide V1b receptor antagonist SSR149415, (3 mg/kg), and one h later a white noise generator was activated and rats exposed to 114 dB for 10 min 25. The other half of the rats received an injection of vehicle (5% DMSO, 5% Cremophor, 90% saline) at 8AM.
Experiment II. Effect of chronic administration of SSR149415
Effect of chronic administration of SSR149415 To determine the effect of chronic administration of the V1b receptor antagonist on stress responsiveness, groups of rats subjected to daily handling or repeated restraint for 14 days received daily doses of SSR149415, 30 mg/kg, mixed in peanut butter, or peanut butter alone one h prior to restraint. On the day of the experiment (day 15) rats were killed by decapitation at 0, 15 or 30 min after receiving an i.p. injection of hypertonic saline (1.5 N NaCl, 1.5 ml/100g), a painful stress model (xx), or the same volume of normal saline (controls). Groups receiving chronic SSR149415 were given an i.p injection of 10 mg SSR149415 dissolved in 5% DMSO, 5% Cremophor and 90% saline, 1h before the i.p. saline injection. Trunk blood was collected in 50 ml polypropylene tubes containing 5 mg EDTA for ACTH and corticosterone measurements.
Experiment III. Effect of chronic administration of a peptide V1 antagonist
Rats were divided into 4 experimental groups: handled controls; handled controls plus VP V1 antagonist; repeated restraint, 1h/daily for 13 days, and repeated restraint plus VP V1 antagonist. Prolonged infusion of the V1 antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP, (Bachem, Torrance, CA) was performed using Alzet osmotic mini-pumps, (Durect Corporation, Cupertino, CA), model 2004, (delivery rate 250ng/hr, for 14days) implanted subcutaneously. On day 13, rats were fitted with intra-atrial cannulae, filled with 0.9% NaCl containing 50U/ml heparin, through the right jugular vein (Braintree Scientific Inc, MA) in order to facilitate repeated blood sampling with minimal stress. The catheter was exteriorized in the neck of the rat, protected by metal springs and connected to swivels fixed at the top of the cage by means of a counterbalanced beam. Catheters were flushed in the evening with 200μl of 50 units/ml of heparin to keep the lines open. On day 14, after collection of basal blood samples for ACTH and corticosterone rats received an i.p. hypertonic saline injection (1.5N NaCl, 1.5ml/100g) before continuing blood collections at 15, 30 and 60 min. Five hundred μl blood samples were collected from the catheters in ice-cold 1.5 ml microfuge tubes containing 0.5 mg EDTA. After 30 min centrifugation at 2,000 × g, plasma transferred to additional tubes and stored at −20 C until assayed.
To determine the effect of the V1 antagonist on blood pressure and heart rate, groups of 6 rats receiving osmotic minipump infusion of V1 antagonist or vehicle were anesthetized on day 13 of infusion, and PE-50 catheters were implanted in the left carotid artery . Twenty four hours later, blood pressure and heart rate were recorded by connecting the arterial catheter to a Statham Db pressure transducer linked to a Gould processor and recorder. After 10 min basal recording, rats received and i.p hypertonic saline injection before continuing recording for an additional 30 min.
Radioimmunoassays
Blood samples for ACTH and corticosterone measurement were collected in EDTA-coated tubes and kept on ice. Blood was centrifuged (10 min, 1500g*/4,000 rpm) within 1 h of collection, and plasma was stored at −80°C until time of assay. Plasma levels of ACTH were measured using a commercially available ACTH IRMA Immunoradiometric Assay (Nichols Institute Diagnostics, San Clemente, CA, for experiment I, and USA DiaScorin, Stillwater, Minnesota for experiments II and III) according to the manufacturer's instructions. Corticosterone levels were measured using an in-house radioimmunoassay in whole blood 25 for Experiment I, or a commercially available Rat Corticosterone Coat-A-Count (Diagnostic Products Corporation (DPC), Los Angeles,CA) according to the manufacturer's instructions.
Statistical analysis
Differences between groups was determined by t-test or two-way analysis of variance (ANOVA) followed by Fisher's least significant difference procedure (PLSD) test to assess statistical significance between control and experimental groups at each time point, as specified in results and Figure legends. P < 0.05 was considered to be statistically significant.
RESULTS
Effect SSR149415 on ACTH and corticosterone responses to a novel stress in repeatedly restrained rats
To determine whether increased hypothalamic VP and pituitary V1b receptor expression observed following repeated restraint stress mediate the changes in responsiveness of the HPA axis to a novel stress, we first examined the effect of a single i.v. injection of the non-peptide selective V1b receptor antagonist, SSR149415, on ACTH and corticosterone responses to white noise.
Plasma ACTH levels increased at 10 min white noise exposure in handled controls. Unlike the enhanced responses to i.p. hypertonic saline injection reported in this chronic stress model, ACTH responses to noise were attenuated and not significantly different from basal in repeatedly restrained rats. SSR149415 injection, 1h before noise exposure, had no effect on ACTH responses in handled control rats but tended to enhance responses in repeatedly restrained rats (Fig 1-A). The difference between ACTH responses to noise in the repeated restraint group and repeated restraint plus SSR149415 was not significantly different in the overall analysis. However, t-test analysis shows ACTH levels significantly higher at 10 min noise in the group receiving SSR149415 injection compared with repeated restrained controls (P< 0.01).
Fig. 1.
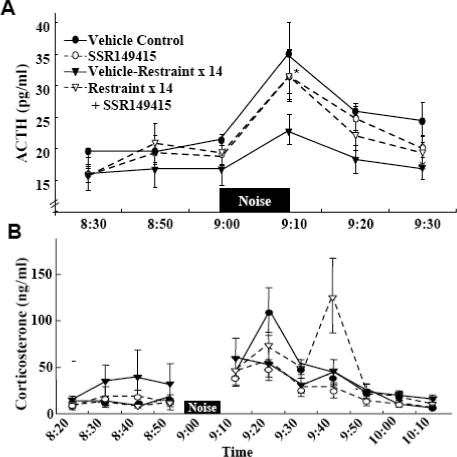
The effect of the selective V1b receptor antagonist, SSR149415, on plasma ACTH (A) and corticosterone (B) responses to noise stress in conscious naïve control rats, or rats subjected to restraint stress for 1h daily for 14 days. SSR149415, 1mg, was injected through the jugular catheter 1 h before noise application. Data are the mean and SE of the values obtained in 6 to 9 rats per experimental group. 2 way ANOVA revealed an effect of noise stress for all groups. *, p<0.05 ACTH response to noise at 10 min in repeated restraint compared with repeated restraint plus SSR149415.
Plasma corticosterone levels tended to be higher in repeatedly restrained rats than in handled controls (Fig 1-B). However, similar to ACTH, corticosterone responses to noise stress showed a tendency to be smaller in repeatedly restrained rats compared with responses in handled controls. Again, SSR149415 injection 1h before stress had no significant effect on basal levels or responses to noise stress (Fig 1-B).
To determine the effectiveness of the iv injection of SSR149415 we measured plasma corticosterone responses to exogenous VP (100 ng, i.v.). The biological activity of SSR149415 injected intravenously was short, showing a 42% reduction in the area under the curve for VP-stimulated corticosterone when VP was injected 10 min after the antagonist (AUC 11,878±1,735 and 6,877±934, for vehicle and SSR149415, respectively) (Fig. 2-A). Only a 32% reduction (AUC 9742 ± 1367 and 6624 ± 1994, for vehicle and SSR149415, respectively) was observed when SSR129145 injection was given 60 min prior to VP, time point at which noise was applied (Fig 2-B), and the antagonist was totally ineffective when injected 120 min before VP (AUC, 9,453±1,232 and 10,777±1,432, for vehicle and SSR149415, respectively) (Fig 2-C).
Fig. 2.
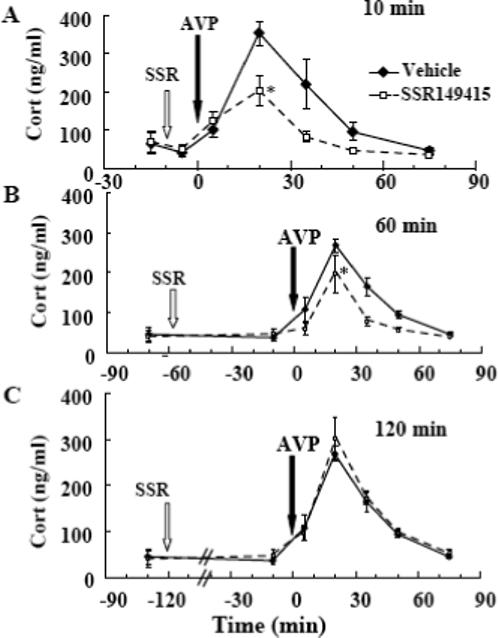
Effect of SSR149415 (1mg, i.v.) on plasma corticosterone responses to exogenous VP (100 ng, i.v.) injected, either 10 (A), 60 (B) or 120min (C) after the SSR149415 injection Data points are the mean and SE of the values obtained in 4 (60 min) to 8 rats per group. *, p< 0.05 between areas under the curve in SSR149415-injected rats compared with vehicle-injected controls.
In a second experiment, we attempted to block V1b receptors for the duration of the experiment by administering the selective V1b receptor antagonist, SSR149415 (30 mg/kg/day), orally, 1h prior to daily restraint. Repeated restraint stress had significant effects on body weight and food intake, leading to significantly smaller increments in body weight and decreased food intake in repeatedly restrained rats compared with handled controls. Chronic administration of the V1b antagonist had no significant effect on body weight or food intake in either handled controls or in repeatedly restrained rats. Repeated restraint had no significant effect on water intake. However, there was a significant stimulatory effect of SSR149415 on water intake in repeatedly restrained rats but not in handled controls (Table 1).
Table 1.
Effect of repeated restraint and chronic administration of the V1bR antagonist SSR149415 on body weight, food intake and water intake in rats
| Control | Restraint x14 | SSR149415 | Restraint x 14 + SSR149415 | |
|---|---|---|---|---|
| Weight gain (g x14 days) | 51.8±2.3 | 35.3±2.1* | 56.9±1.8 | 30.1±1.5*# |
| Food intake (g/day) | 23.7±0.5 | 20.5±0.4* | 24.6±0.4 | 20.1± 0.6*# |
| Water intake (ml/day) | 41.9±0.8 | 45.4±1.0 | 38.9±0.8 | 49.8±1.5#& |
Values are the mean and SE of data obtained from 12 rats per experimental group.
, p<0.001 vs controls
, p< 0.001 vs SSR149415
, p<0.04 vs restraint x 14.
On day 15, rats were killed in basal conditions or 15 or 30 min after an i.p. injection of hypertonic saline, a painful stressor used as a novel stimulus. Basal ACTH levels were similar in handled controls and repeatedly restrained rats. Consistent with previous observations, a single i.p. hypertonic saline injection caused marked increases in plasma ACTH at 15 and 30min and this response was significantly higher in repeatedly restrained rats compared with their handled controls. Daily administration of SSR149415 for the duration of the experiment had no significant effect on plasma ACTH levels in either handled controls or repeatedly restrained rats (Fig 3-A). Basal plasma corticosterone levels and the increases in response to i.p. hypertonic saline injection were similar in repeatedly restrained rats and handled controls (Fig 3-B). As with plasma ACTH levels, daily administration of SSR149415 failed to modify plasma corticosterone responses to i.p. hypertonic saline either in handled controls or chronically stressed rats. As shown in Fig 4, assesment of the effectiveness of this regime of administration of SSR149415 to block V1b receptors revealed a significant but only partial reduction in ACTH responses to i.v. injection of 100 ng VP (AUC, 832 ± 245 and 428 ± 135, for vehicle an SSR149415, respectively). Plasma corticosterone responses to VP showed a tendency to be lower in rats receiving SSR149415, compared with vehicle fed controls but the difference was not statistically significant (AUC, 8,356 ± 936 and 7988 ± 1045, for vehicle and SSR149415, respectively).
Fig. 3.
The effect of chronic oral administration of the selective V1b receptor antagonist, SSR149415, on plasma ACTH (A) and corticosterone (B) responses to i.p. hypertonic saline injection in conscious naïve control rats, or rats subjected to restraint stress for 1h daily for 14 days. SSR149415 (10 mg/rat) was given mixed in peanut butter, daily, for 14 days about one h before restraint, and as an i.p. injection on the day of the experiment. Data are the mean and SE of values obtained in 8 rats per group. 2-way ANOVA revealed an effect of i.p. hypertonic saline injection for all groups (p, <0.01). *, p<0.05 ACTH response to i.p. hypertonic saline injection in repeated restraint compared with repeated restraint plus SSR149415.
Fig. 4.
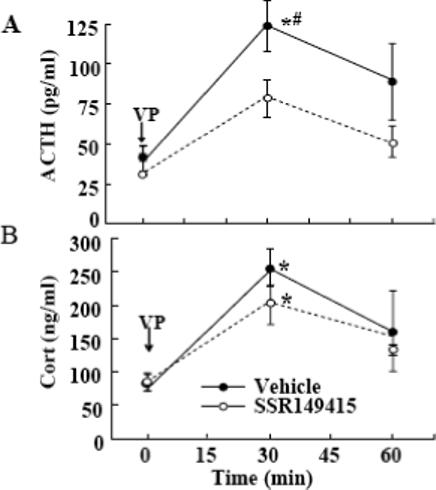
Effect of chronic oral administration of SSR149415 (10 mg/day for 10 days) on plasma (A) ACTH, and (B) corticosterone (Cort) responses to exogenous VP (100 ng, i.v.) VP injections and blood collections were performed through jugular catheter. Data points are the mean and SE of values obtained from data in 6 rats per group. *, p< 0.05 between areas under the curve in SSR149415 treated rats compared with vehicle (peanut butter) ingesting controls.
Effect of a peptide V1 antagonist on ACTH and corticosterone responses to a novel stress in repeatedly restrained rats
Since the selective V1b receptor antagonist only partially blocked VP responses under the above experimental conditions, a third experiment was conducted in a further attempt to block the effects of VP using chronic administration of the non-selective V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP in handled controls and repeatedly restrained rats. As previously reported, osmotic minipump administration of the V1 antagonist was effective in blocking ACTH and corticosterone responses to an iv injection of 100 ng of VP (Subburaju and Aguilera, 2007).
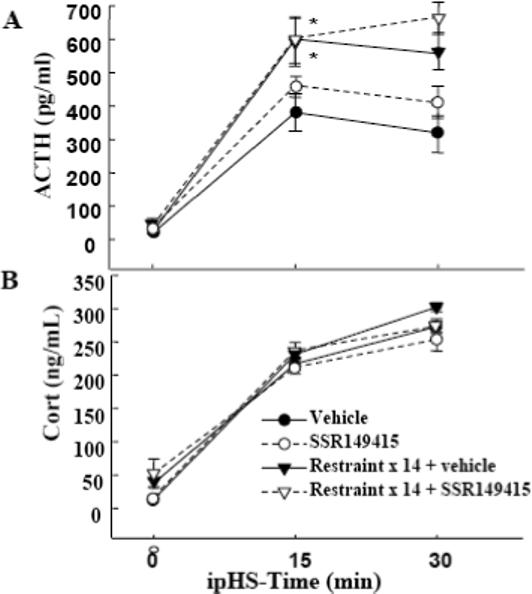
As shown in Fig 5, plasma ACTH responses to i.p. hypertonic saline injection were significantly higher in repeatedly restrained rats compared with handled controls. Chronic blockade of V1 receptors by osmotic minipump infusion of the V1 antagonist significantly reduced plasma ACTH responses to i.p. hypertonic saline injection in handled control rats. However, chronic VP receptor blockade failed to reduce plasma ACTH responses to i.p. hypertonic saline injection in repeatedly restrained rats (Fig 5-A). Plasma corticosterone responses were similar in handled control rats and repeatedly restrained rats and these responses were completely unaffected by V1 receptor blockade (Fig 5-B).
Fig 5.
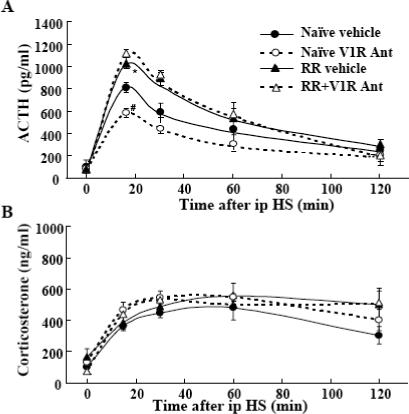
The effect of chronic osmotic minipump administration of the non-selective V1 receptor antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP (V1R Ant), or vehicle for 14 days on plasma ACTH (A) and corticosterone (B) responses to i.p. hypertonic saline injection (ipHS) in conscious naïve control rats, or rats subjected to restraint stress for 1h daily for 14 days (RR). Data are the mean and SE of values obtained in 8 to 10 rats per group. All groups showed a significant effect of i.p. hypertonic saline on both plasma ACTH and corticosterone levels (p<0.001). *, p<0.04 ACTH responses in repeated restraint vs. vehicle-infused naïve controls; #, p<0.02 ACTH responses to i.p. hypertonic saline injection in V1R Ant vs. responses in naïve controls.
Since the peptide antagonist blocks equally pituitary V1b receptors and vascular V1a receptors, to assess the cardiovascular participation on the effects on HPA axis, we examined the changes in heart rate and blood pressure after i.p. hypertonic saline injection in vehicle and V1 antagonist infused rats. I.p. hypertonic saline injection caused significant increases in heart rate (p<0.01), an effect which was of similar magnitude in vehicle and V1 antagonist infused groups. Blood pressure showed a tendency to increase after i.p hypertonic saline injection in both groups vehicle and V1 antagonist infused groups. Again, there was no significant differences in blood pressure between groups either under basal conditions or after i.p. hypertonic saline injection (Fig 6).
Fig 6.
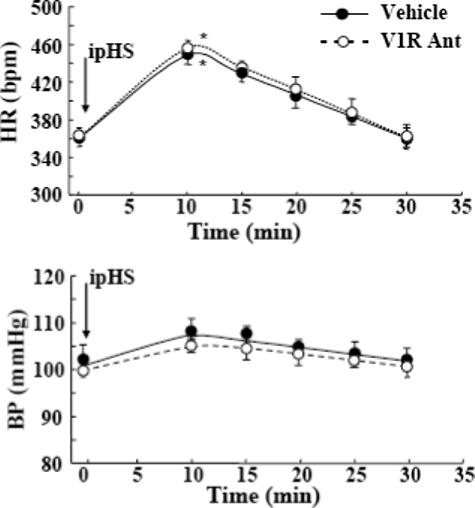
Effect of the V1 antagonist, dGly[Phaa1,D-tyr(et), Lys, Arg]VP (V1R Ant) on (A) heart rate (HR) and (B) blood pressure (BP) following an i.p. injection of hypertonic saline (ipHS). Data points are the mean and SE of the values in 4 to 5rats per group. Two-way ANOVA revealed a significant effect of ipHS (p<0.01) but not of V1 Ant (p<0.13) on heart rate.
DISCUSSION
The selective increase in parvocellular VP and pituitary V1b receptors over CRH and CRH receptor expression during chronic stress has led to the hypothesis that VP becomes the major regulator of ACTH secretion and that the peptide mediates the hyperresponsiveness of the HPA axis to a subsequent novel stress 26,27. The availability of the selective V1b receptor antagonist, SSR149415 23, prompted us to test this hypothesis in rats subjected to repeated restraint, a chronic stress paradigm previously shown to induce enhanced ACTH responses to a novel stressor 18,26,28,29. Despite the fact that the doses of SSR149415 used in the present experiments have been shown to reduce VP-stimulated ACTH levels to basal for at least 3 h 30, in our experimental conditions SSR149415 was only partially effective in blocking the pituitary effects of exogenous VP. The different responses in the present study could reflect effects of the mode of oral administration (peanut butter vs gavage), source of the rats, or some other unidentified variables. The present data show a very rapid decrease in effectiveness of SSR149415 following i.v. administration, with only 32% inhibition of the response to VP remaining at 1 h, time at which rats were exposed to the novel stress. However, we may have underestimated the effectiveness of the antagonist after i.v. administration based solely on corticosterone measurements. It should be noted that consistent with previous reports, there is a dissociation between pituitary and adrenal responses and that small elevations of ACTH can elicit full corticosterone responses.
The finding that ACTH and corticosterone responses to white noise were attenuated in rats subjected to repeated restraint is at variance with usual hyper-responsiveness to a novel stress, such as i.p. hypertonic saline injection, deoxy glucose administration or exposure to a novel environment, previously reported using this chronic stress paradigm 18,26,28,29. This could result from activation of different neural circuitry by diverse acute stressors, and suggests that responsiveness to a novel stress varies according to the nature of the novel stress. In these experiments, the short biological half life of i.v. administration of the V1b receptor antagonist prevents us from drawing any conclusions concerning the role of VP in stress responses. However, the fact that repeatedly restrained rats displayed reduced responses to the novel stress of white noise indicate that increased parvocellular VP and V1b receptor expression, which is characteristic of repeated stress 14,26,29, does not necessarily result in enhanced ACTH responses. A similar opposite correlation with high parvocellular VP expression and low HPA axis responsiveness has been observed during chronic immune challenge in experimental models of autoimmune disease 13,31 or repeated lipopolysaccharide injection 31. Thus, it appears that responsiveness to a novel stressor depends on the nature of the novel stressor rather than adaptive changes resulting from exposure to the chronic stressor. It is noteworthy that a single administration of SSR149415 tended to prevent the attenuation of the responses to the novel stress of white noise in the chronically retrained rats. The mechanisms of this effect are unclear but they may involve inhibition of V1b receptor-mediated repression of central pathways involved in the activation of the HPA axis by noise stress.
In contrast to noise stress and consistent with previous studies 29, ACTH responses to i.p. hypertonic saline injection were significantly higher in repeatedly restrained rats compared with responses in handled control rats (experiments II and III). The lack of any significant effect of chronic administration of the non-peptide selective V1b receptor antagonist in experiment II is difficult to interpret because of the inability of the antagonist to completely block the stimulatory effect of exogenous VP. We therefore sought alternative ways to block pituitary V1b receptors by administering a non-selective peptide antagonist by constant minipump infusion. As shown in these experiments and a previous report 24, minipump administration of the V1 receptor antagonist prevented the rise in plasma ACTH and corticosterone elicited by VP, indicating that V1b receptors in the corticotroph were effectively blocked. In contrast to the lack of effect of the orally active V1b receptor antagonist, chronic administration of the non-selective V1 antagonist caused a small but significant reduction of ACTH responses to i.p. hypertonic saline injection. This suggests that, at least in this acute stress paradigm, VP contributes to the ACTH response. Although no additional acute stressors were tested in this study, studies in V1b receptor knock out mice and Brattleboro rats have shown that the VP dependence of the responses to acute stress depend largely on the stress paradigm. For example, V1b receptor knock out mice show normal ACTH responses to restraint and social stress but reduced responses to insulin-induced hypoglycemia 21, LPS injection or ethanol administration 20 and swim stress 22. On the other hand, the inability of chronic administration of both antagonists to prevent the enhanced ACTH responses to i.p. hypertonic saline injection in repeatedly stressed rats, strongly suggests that VP is not responsible for the hypersensitivity to the novel stress. In addition, the fact that partial (with SSR149415) or complete pituitary V1b receptor blockade (peptide non-selective V1 antagonist) fails to inhibit acute stress responses in repeatedly restrained rats suggests that the VP binding up-regulation observed following repeated restraint is not required for the sensitization of ACTH responses to a novel stress. This study also suggests that rather than stimulation of ACTH secretion, the marked increases in parvocellular-pituitary vasopressinergic activity may have other roles in the pituitary and perhaps in the brain. In this regard we have recently shown that VP mediates mitogenic responses during adrenalectomy 24, as well as antiapoptotic actions in neurons 24,32.
It should be noted that the enhancing effect of repeated restraint on the responses to the novel stressor of i.p. hypertonic saline injection were observed only on plasma ACTH but not on corticosterone responses. This dissociation between ACTH and glucocorticoid responses is commonly observed 33,34 and is likely to reflect maximal stimulation of adrenal receptors with the plasma ACTH levels achieved by strong stressors. On the other hand, experiment 1, using a milder stressor and optimal stress free conditions for blood collection, a good correlation between changes in ACTH and corticosterone could be observed.
Overall, the study provides confirmatory evidence that VP can contribute to the full ACTH responses during acute stress. The prominence of the increases in vasopressinergic activity during chronic stress suggests that VP must have an important role on the adaptation of the HPA axis to chronic stress. However, the ineffectiveness of the peptide V1 antagonist to modify ACTH responses during chronic stress indicates that VP does not mediate the hypersensitivity of ACTH responses to a novel stress and suggests alternative roles for the peptide during stress adaptation.
Footnotes
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH.
References
- 1.Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- 2.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 3.Mccann SM, Brobeck JR. Evidence for a role of the supraopticohypophyseal system in regulation of adrenocorticotrophin secretion. Proc. Soc. Exp. Biol. Med. 1954;87:318–324. doi: 10.3181/00379727-87-21368. [DOI] [PubMed] [Google Scholar]
- 4.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul. Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 5.Irvine CH, Alexander SL, Donald RA. Effect of an osmotic stimulus on the secretion of arginine vasopressin and adrenocorticotropin in the horse. Endocrinology. 1989;124:3102–3108. doi: 10.1210/endo-124-6-3102. [DOI] [PubMed] [Google Scholar]
- 6.Aguilera G, Lightman SL, Kiss A. Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology. 1993;132:241–248. doi: 10.1210/endo.132.1.8380375. [DOI] [PubMed] [Google Scholar]
- 7.Chowdrey HS. Altered adrenocorticotropin, corticosterone and oxytocin responses to stress during chronic salt load. Neuroendocrinology. 1991;54:635–638. doi: 10.1159/000125971. [DOI] [PubMed] [Google Scholar]
- 8.Dohanics J, Kovacs KJ, Folly G, Makara GB. Long-term salt loading impairs pituitary responsiveness to ACTH secretagogues and stress in rats. Peptides. 1990;11:59–63. doi: 10.1016/0196-9781(90)90110-q. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Samra AB, Harwood JP, Manganiello VC, Catt KJ, Aguilera G. Phorbol 12-myristate 13-acetate and vasopressin potentiate the effect of corticotropin-releasing factor on cyclic AMP production in rat anterior pituitary cells. Mechanisms of action. J. Biol. Chem. 1987;262:1129–1136. [PubMed] [Google Scholar]
- 10.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 11.Berkenbosch F, De Goejj DC, Tilders FJ. Hypoglycemia enhances turnover of corticotropin-releasing factor and of vasopressin in the zona externa of the rat median eminence. Endocrinology. 1989;125:28–34. doi: 10.1210/endo-125-1-28. [DOI] [PubMed] [Google Scholar]
- 12.De Goejj DC, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJ. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- 13.Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, Eckland DJ, Lightman SL. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. British journal of pharmacology. 1995;116:2417–2424. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 15.Ma XM, Aguilera G. Transcriptional responses of the vasopressin and corticotropin- releasing hormone genes to acute and repeated intraperitoneal hypertonic saline injection in rats. Brain Res Mol Brain Res. 1999;68:129–40. doi: 10.1016/s0169-328x(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 16.Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc. Natl. Acad. Sci. U. S. A. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- 18.Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Research. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- 19.Zelena D, Foldes A, Mergl Z, Barna I, Kovacs KJ, Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res. Bull. 2004;63:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Lolait SJ, Stewart LQ, Roper JA, Harrison G, Jessop DS, Young WS, O'Carroll AM. Attenuated Stress Response to Acute Lipopolysaccharide Challenge and Ethanol Administration in Vasopressin V1b Receptor Knockout Mice. Journal of neuroendocrinology. 2007;19:543–551. doi: 10.1111/j.1365-2826.2007.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lolait SJ, Stewart LQ, Jessop DS, Young WS, III, O'Carroll AM. The Hypothalamic-Pituitary-Adrenal Axis Response to Stress in Mice Lacking Functional Vasopressin V1b Receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J. Clin. Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serradel-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, Barberis C, Brossard G, Soubrie P, Nisato D, Pascal M, Pruss R, Scatton B, Maffrand JP, Lefur G. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl) -2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J. Pharmacol. Exp. Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- 24.Subburaju S, Aguilera G. Vasopressin Mediates Mitogenic Responses to Adrenalectomy in the Rat Anterior Pituitary. Endocrinology. 2007;148:3102–3110. doi: 10.1210/en.2007-0103. [DOI] [PubMed] [Google Scholar]
- 25.Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. Ultradian Rhythm of Basal Corticosterone Release in the Female Rat: Dynamic Interaction with the Response to Acute Stress. Endocrinology. 1998;139:443–450. doi: 10.1210/endo.139.2.5721. [DOI] [PubMed] [Google Scholar]
- 26.Aguilera G. Corticotropin Releasing Hormone, Receptor Regulation and the Stress Response. Trends in Endocrinology and Metabolism. 1998;9:329–336. doi: 10.1016/s1043-2760(98)00079-4. [DOI] [PubMed] [Google Scholar]
- 27.Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. The Journal of endocrinology. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- 28.Harris RBS, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiology & Behavior. 2004;81:557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–32. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 30.Serradeli-Le Gal C, Wagnon J, Tonnerre B, Roux R, Garcia G, Griebel G, Aulombard A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS drug reviews. 2005;11:53–68. doi: 10.1111/j.1527-3458.2005.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinevich V, Ma XM, Herman JP, Jezova D, Akmayev I, Aguilera G. Effect of Repeated Lipopolysaccharide Administration on Tissue Cytokine Expression and Hypothalamic-Pituitary-Adrenal Axis Activity in Rats. Journal of neuroendocrinology. 2001;13:711–723. doi: 10.1046/j.1365-2826.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Volpi S, Aguilera G. Neuroprotective and Antiapoptotic Actions of Vasopressin in the Hypothalamic Cell Line H32 [abstract].. The Endocrine Society's 89th Annual Meeting; Toronto, Canada abst.. 2007. [Google Scholar]
- 33.Jezova D, Ochedalski T, Glickman M, Kiss A, Aguilera G. Central corticotropin-releasing hormone receptors modulate hypothalamic-pituitary-adrenocortical and sympathoadrenal activity during stress. Neuroscience. 1999;94:797–802. doi: 10.1016/s0306-4522(99)00333-4. [DOI] [PubMed] [Google Scholar]
- 34.Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction Between Oestrogen and Oxytocin on Hypothalamic-Pituitary-Adrenal Axis Activity. Journal of neuroendocrinology. 2007;19:189–197. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]


