Abstract
Objective
Microbial biofilms are communities of sessile microorganisms formed by cells that are attached irreversibly to a substratum or interface or to each other and embedded in a hydrated matrix of extracellular polymeric substances. Microbial biofilms have been implicated in >80% of human infections such as periodontitis, urethritis, endocarditis, and device-associated infections. Thus far, intra-amniotic infection has been attributed to planktonic (free-floating) bacteria. A case is presented in which “amniotic fluid sludge” was found to contain microbial biofilms. This represents the first report of a microbial biofilm in the amniotic cavity.
Study Design
“Amniotic fluid sludge” was detected by transvaginal sonography, and retrieved by transvaginal amniotomy. Bacteria were identified using scanning electron microscope and fluorescence in situ hybridization for conserved regions of the microbial genome; and the exopolymeric matrix was identified by histochemistry using the wheat germ agglutinin lectin method. The structure of the biofilm was imaged with confocal laser scanning microscopy.
Results
“Amniotic fluid sludge” was imaged with scanning electron microscopy, which allowed identification of bacteria embedded in an amorphous material and inflammatory cells. Bacteria were demonstrated using fluorescent in situ hybredization using a eubacteria probe. Extracellular matrix was identified with the wheat germ agglutinin lectin stain. Confocal microscopy allowed three-dimensional visualization of the microbial biofilm.
Conclusion
Microbial biofilms have been identified in a case of intra-amniotic infection with “amniotic fluid sludge.”
Keywords: amniocentesis, amniotic fluid sludge, chorioamnionitis, intra-amniotic infection, microbial invasion of the amniotic cavity, preterm labor
Microbial invasion of the amniotic cavity has been detected in the mid-trimester of pregnancy in apparently healthy women,1–5 and in the amniotic fluid of women with cervical insufficiency,6;7 preterm labor with intact membranes, preterm prelabor rupture of membranes, idiopathic vaginal bleeding,8 preterm rupture of membranes at term,9;10 spontaneous labor at term with intact membranes11 and clinical chorioamnionitis. Intra-amniotic infection is a common mechanism of disease in obstetrics and bacteria can attack the fetus and cause systemic inflammation (a fetal inflammatory response syndrome12;13) and multiple organ damage.14
The microbiologic diagnosis of infection has been based upon the use of cultivation techniques in which bacteria are recovered from amniotic fluid. Growth of microorganisms in the laboratory depends on the provision of suitable nutritive and environmental conditions. This practice was developed after the seminal observations of Robert Koch, who established the time-honored isolation and pure culture techniques. However, there are two major limitations of cultivation-based techniques. First, approximately 99% of bacteria in aquatic and soil ecosystems resist cultivation (a phenomenon called “the great plate count anomaly”15); second, bacteria grow in communities called “biofilms” which typically adhere to surfaces.16 Molecular microbiologic techniques have been used recently to detect microorganisms in the amniotic cavity.17–21
The biofilm theory states that most of bacteria that grows in matrix-enclosed biofilms differ from their planktonic counterparts22 (isolated bacteria seen in Gram stain examinations of biologic fluids or of pure cultures). It is now recognized that biofilms play a major role in human disease. Periodontitis, otitis media, endocarditis, prostatitis, biliary tract infections and many other infections in which there is a device (e.g., prosthetic valves, catheters) involve bacterial biofilms.23
Microbial biofilms are important because bacteria in these communities are more resistant to antimicrobial agents and to the host response to infection.24–27 Moreover, since bacteria in biofilms are more difficult to isolate in culture, these infections are often overlooked.
The presence of particulate matter in the amniotic fluid in close proximity to the cervix was recognized recently as “amniotic fluid sludge”.28 This finding was associated with microbial invasion of the amniotic cavity, impending preterm delivery and histologic chorioamnionitis.28 Subsequently, these results were confirmed in asymptomatic patients with sludge in mid-trimester of pregnancy29 and in those with cervical cerclage.30 The finding that “amniotic fluid sludge” represents the presence of bacteria and intra-amniotic inflammation has been recently reported.31
Because of the original description of “amniotic fluid sludge”, it has been speculated that this material may reflect the presence of biofilms in the amniotic fluid,28 which is a hypothesis with biological, diagnostic and therapeutic implications. The evidence in support of the contention that microorganisms can form a biofilm in the amniotic cavity is the subject of this case report.
MATERIALS AND METHODS
To determine whether amniotic fluid sludge (Figure 1) represents a biofilm, the material was aspirated by transvaginal needle amniotomy under ultrasound guidance in a patient at 28 weeks of gestation with spontaneous preterm labor and clinical chorioamnionitis. This was done in accordance with an Institutional Review Board-approved protocol; the patient provided written informed consent at the time of enrollment, before to the collection of the amniotic fluid samples. Amniotic fluid studies indicated that the glucose concentration of <10 mg/dL a blood cell count was 19,650 cells/mm3, and the presence of Gram-positive cocci. The patient was treated with Ampicillin and Gentamycin for the clinical diagnosis of chorioamnionitis, and labor progressed quickly to a spontaneous vaginal delivery of a female infant weighing 1,135 g, with Apgar scores at 1 and 5 minutes of 8 and 8. The amniotic fluid culture obtained from the sludge material was positive for Mycoplasma hominis, Streptococcus mutans and Aspergillus flavus. Histological examination of the placenta revealed marked acute necrotizing chorioamnionitis and acute funisitis. The details of this case have been reported previously.31 The newborn infant was admitted to the neonatal intensive care unit and experienced metabolic acidosis and respiratory distress syndrome that resolved in the first week of life. There was no evidence of pneumonia; a neurosonogram was normal, and microbial cultures of the cerebrospinal fluid and blood were negative. However, the newborn was treated with ampicillin and gentamycin because of suspected sepsis. After 45 days the infant was discharged home in good condition.
Figure 1.
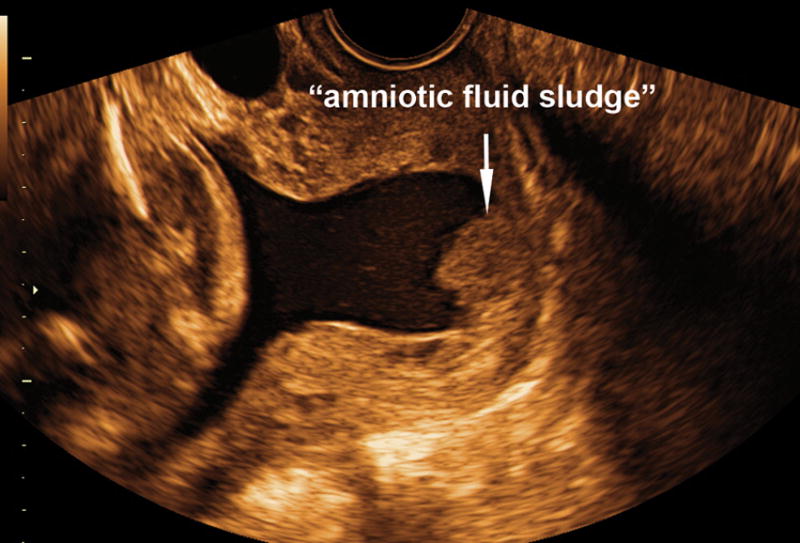
Two-dimensional transvaginal ultrasound image shows the presence of “amniotic fluid sludge”.
Scanning electron microscopy
Amniotic fluid was dehydrated in a graded ethanol line, critical point dried (EMS 850), mounted on a stub, sputter coated with 8-nm platinum and examined with a scanning electron microscope with 5 kV in the secondary electron mode (XL30 SFEG, FEI Inc., Hillsboro, OR, USA).
Fluorescent in situ hybridization (FISH) and confocal laser scanning microscopy
Amniotic fluid was transferred to 100% ethanol for 24 hours and washed with phosphate-buffered saline solution. Volumes of 100 μL of the amniotic fluid were hybridized with the probe EUB338- Cy3 (final concentration, 5 ng μL−1; Integrated DNA Technologies, Coralville, Iowa, USA) for 90 minutes at 46ºC in the dark. The samples were washed with buffer for 20 minutes at 46ºC in the dark.
Wheat germ agglutinin lectin stain
Histochemistry was used to detect the presence of extracellular matrix that is characteristic of a biofilm. This was performed by the wheat germ agglutinin method (final concentration 20 ng μL−1) as described in the following website: Vector Laboratories, Burlingame, California, USA; www.vectorlabs.com. Staining was performed at room temperature for 20 minutes which was followed by three washing steps with double distilled water. The amniotic fluid was mounted on a slide and examined with confocal laser scanning microscopy (LSM 5 PASCAL, Carl Zeiss, Thornwood, NY, USA). These techniques have been extensively used in the study of biofilms and published elsewhere in detail.32;33
RESULTS
Scanning electron microscopy showed flocs of “amniotic fluid sludge” that consist of bacterial cells and the exopolymeric matrix material that are typical of a biofilm (Figure 2). Cocci are resolved among a fibrous mass of matrix material. Figure 3A shows a microbial biofilm with neutrophils. Figure 3B shows bacterial cells (coccoid) in the form of individual cells or as a chain of cocci on the surface of a fetal epithelial cell and partially covered in amorphous slime. Figure 3C shows large aggregates of biofilm and most of the bacterial cells that were enclosed in amorphous matrix material. Figure 3D shows the presence of well defined bacterial cells on the surface of an epithelial cell; and bacteria in this surface have formed amorphous matrix material.
Figure 2.
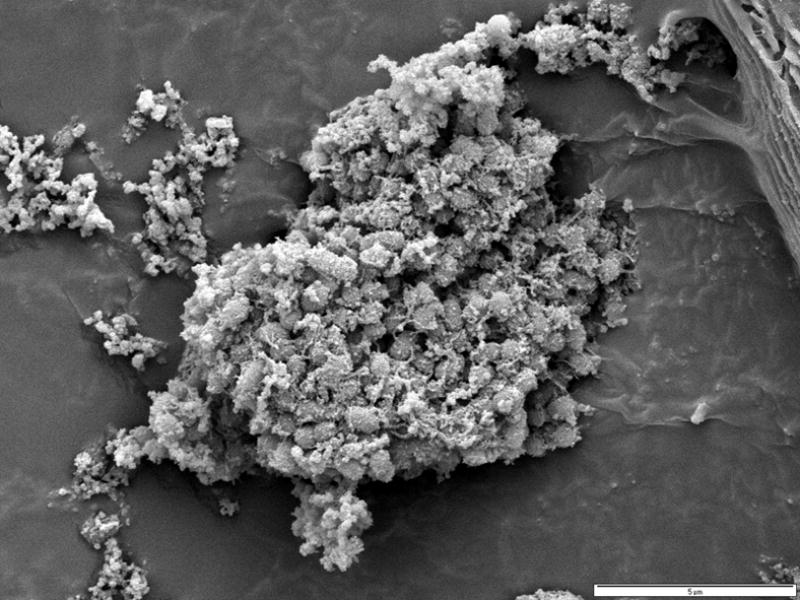
Scanning electron micrograph of a floc of “amniotic fluid sludge” showing the bacterial cells and the exopolymeric matrix material which constitute a biofilm. In the center of the image, cocci are resolved among a fibrous mass of matrix material. The lectin-based evidence for a matrix and a molecular evidence for bacterial presence are demonstrated in Figure 4.
Figure 3.
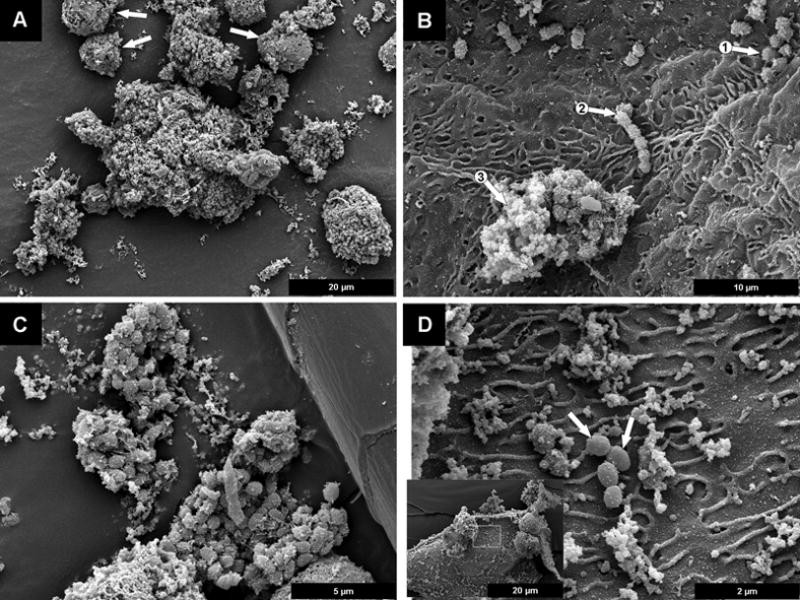
Scanning electron micrograph of “amniotic fluid sludge”. A The biofilm, in the center of the image, and several neutrophils which are identified by arrows. B The coccoid bacteria are on the surface of a fetal epithelial cell, these bacterial cells are seen in the form of individual cells (arrow 1), a chain of cocci (arrow 2), and a biofilm aggregate in which they are partially “buried” in amorphous slime (arrow 3). C Several large aggregates of biofilm, are next to a hair shed from the fetus; most of the bacterial cells are enclosed in amorphous matrix material. D The presence of four very well defined bacterial cells (arrows) on the rugose surface of a fetal epithelial cell, although other bacteria that have colonized this surface have begun to accrete amorphous matrix material.
The evidence that “amniotic fluid sludge” is a biofilm is presented in Figure 4. Figure 4A is a confocal scanning laser micrograph of a floc. The presence of bacteria or bacterial fragments is demonstrated by Figure 4B using FISH using the Eubac338 probe. The presence of an extracellular matrix is demonstrated in Figure 4C with the use of wheat germ agglutinin lectin, which reacts with the N-acetylglucoseamine of the matrix exopolymer. Figure 4D is the composite image of the previous three and illustrates bacteria within the biofilm. Figure 5 is a three-dimensional reconstruction of the biofilm with the use of confocal laser scanning microscopy. (A videoclip of the bacterial biofilms is available online).
Figure 4.
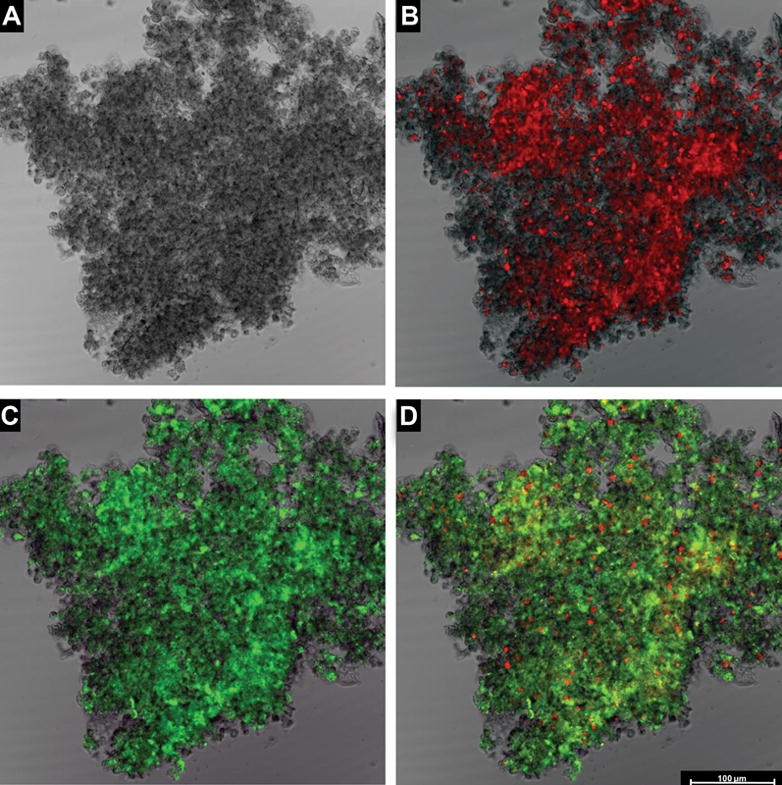
Demonstration that “amniotic fluid sludge” is a biofilm. These images were generated using confocal laser scanning microscopy. A The structure of a floc of “amniotic fluid sludge” without staining of any component. B The same structure that has been stained with the EUB338-Cy3 probe for eubacteria which reacts with the 16S rRNA component of bacteria. Bacteria and bacterial fragments are seen in red. C The same floc that has been stained with wheat germ agglutinin, which reacts with the N-acetylglucosamine of the component of the matrix material that forms the structural framework of the biofilm. D A superposition of the three images (A, B and C) showing bacteria (red dots), matrix material (green), and some unstained material which is likely to represent host components trapped by the biofilm. The bar represents 100 microns.
Figure 5.
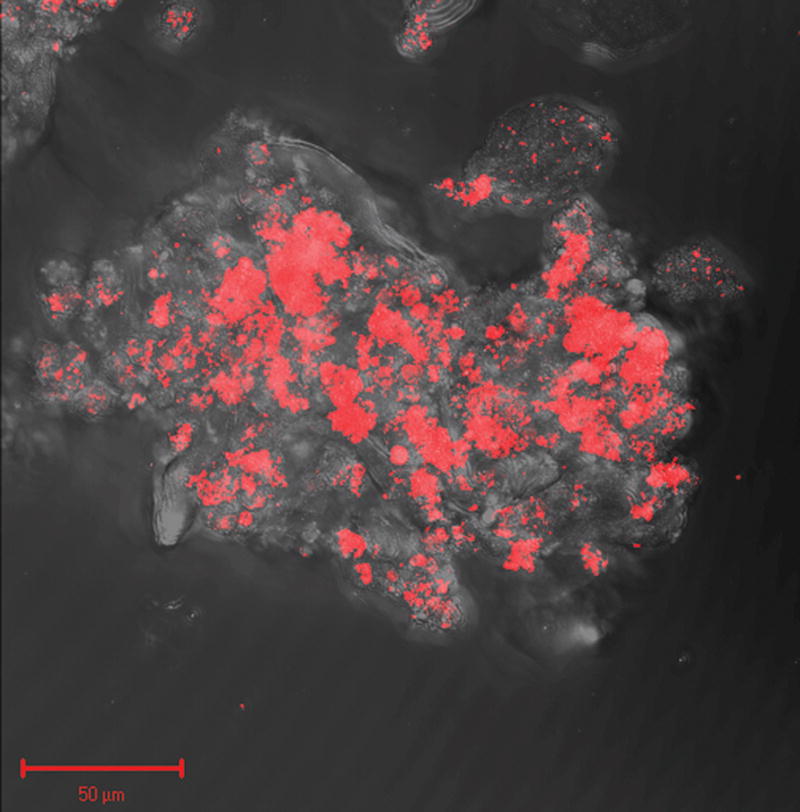
Three dimensional reconstruction of sequential Z stack images of a bacterial biofilm in amniotic fluid by confocal laser scanning microscopy. Bacteria are stained in red due to the hybridization with the probe EUB338-Cy3. (A video clip is available online).
COMMENT
The observations reported herein represent the first evidence that bacteria can form a microbial biofilm within the amniotic cavity and that such biofilm was found in a case with “amniotic fluid sludge”.
The following evidence determines that the material retrieved from the amniotic cavity represents a biofilm is: 1) bacteria were detected with FISH, with the use of a probe against the conserved sequence of prokaryotes; 2) bacterial aggregates were separated by material that resembled a matrix; and 3) lectin-based identification of exopolymeric matrix that stained with wheat germ agglutinin.
Biofilms are defined as communities of sessile organisms characterized by cells that are attached to a substratum or interface or to each other, that are embedded in a hydrated matrix of extracellular polymeric substances which they have produced, and that exhibit an altered phenotype with respect to growth rate and gene transcription in comparison to planktonic cells.22 It has been postulated that most bacteria grow in matrix enclosed-biofilms adherent to surfaces in all nutrient-sufficient aquatic ecosystems, and that these sessile bacterial cells differ profoundly from their planktonic counterparts, which account for most physiological processes in these ecosystems.16;22 Indeed, based on direct microscopic observations and quantitative recovery techniques, it has been demonstrated that > 99.9% of the bacteria grow in biofilms on a wide variety of surfaces.22
Bacterial biofilms have been shown to play a major role in many chronic infections. Indeed, a public announcement of the National Institute of Health has stated that biofilms account for > 80% of microbial infections of the body. Biofilms have been implicated in vaginitis, conjunctivitis, otitis, colitis and gingivitis. Moreover, they are also important in colonizing medical devices such as urinary, venous and arterial catheters. Biofilms have been found in pacemakers, heart valves, vascular grafts and stents, artificial joints and pins, and even breast implants.
This communication is based on the observations of a single case. The prevalence of microbial biofilms in intra-amniotic infection is unknown. However, the recognition that bacteria can form biofilms within the amniotic cavity is important for both diagnostic and therapeutic reasons. First, the diagnosis of microbial invasion in the presence of biofilms is extremely challenging and current cultivation techniques are inadequate to detect such infections. The consequence is that the frequency of infection of the amniotic cavity may be underestimated and that molecular microbiologic techniques will be required to improve diagnosis.17–21 Second, the optimal treatment of biofilm-related infections represents a challenge in clinical medicine. Antimicrobial agents appear to be inactivated or fail to reach bacteria within a biofilm. Interestingly, bacteria within biofilms have increased resistance to antimicrobial compounds even though the bacteria can be sensitive to the same agent if grown under standard conditions.24–27 Thus, the difficulties in the treatment of intra-amniotic infection may be due to the refractoriness of biofilms to conventional antibiotic treatment. Third, biofilms in the amniotic fluid may represent a unique form of these structures which can be dislodged by fetal movement resulting in seeding of planktonic bacteria and the eliciting of an inflammatory response. This study also demonstrates that biofilms in the amniotic fluid can be formed by multiple organisms. Further research is required to characterize, with specific probes, the microbial constituents of amniotic fluid biofilms. The mechanisms responsible for the formation of a microbial biofilm in amniotic fluid are unknown and may involve a combination of microbial and host factors.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
CONDENSATION Bacteria in intra-amniotic infection could from a microbial biofilm in amniotic fluid.
Reference List
- 1.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294–302. [PubMed] [Google Scholar]
- 2.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–17. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375–79. [PubMed] [Google Scholar]
- 4.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen DP, Gerber S, Hohlfeld P, Sandrine G, Witkin SS. Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome. J Perinat Med. 2004;32:323–26. doi: 10.1515/JPM.2004.060. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 7.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–55. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 8.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129–33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Gomez R, Galasso M, Salafia C, Yoon BH, Behnke E, et al. Is oligohydramnios a risk factor for infection in term premature rupture of membranes? Ultrasound Obstet. Gynecol. 1994;4:95–100. doi: 10.1046/j.1469-0705.1994.04020095.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–48. [PubMed] [Google Scholar]
- 12.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 14.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 15.Staley JT, Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–46. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 16.Costerton JW, Geesey GG, Cheng KJ. How bacteria stick. Sci Am. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 17.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–37. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–52. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 21.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319–23. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 22.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 24.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46:S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 25.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–38. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 26.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–13. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 27.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–22. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 28.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–52. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 29.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007 doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusanovic JP, Romero R, Espinoza J, Goncalves LF, Gotsch F, Camacho N, Lee W, Erez O, Schoen ML, Hassan S. Clinical significance of the presence of amniotic fluid ‘sludge’ in patients with cervical cerclage. Ultrasound Obstet Gynecol. 2007;30(4):411. doi: 10.1002/uog.4081. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet. Gynecol. 2007;30:793–98. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gersdorf H, Meissner A, Pelz K, Krekeler G, Gobel UB. Identification of Bacteroides forsythus in subgingival plaque from patients with advanced periodontitis. J Clin Microbiol. 1993;31:941–46. doi: 10.1128/jcm.31.4.941-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gersdorf H, Pelz K, Gobel UB. Fluorescence in situ hybridization for direct visualization of gram-negative anaerobes in subgingival plaque samples. FEMS Immunol Med Microbiol. 1993;6:109–14. doi: 10.1111/j.1574-695X.1993.tb00312.x. [DOI] [PubMed] [Google Scholar]


