Abstract
Kidney cysts in autosomal dominant polycystic kidney disease (ADPKD) undergo progressive enlargement together with luminal fluid secretion. This involves active, uphill transcellular Cl− transport which drives passive Na+ and water secretion. Implicit in this mechanism is the assumption that the paracellular permeability of the cyst epithelium to Cl− must be very low. Claudins are tight junction (TJ) transmembrane proteins that determine the ion selectivity of paracellular barriers. The aim of this study was to determine the expression and localization of claudins within renal cysts in a mouse hypomorphic model of ADPKD and in human patients. We found that the majority of cysts were of collecting duct origin. Claudins normally expressed in collecting duct (3, 4, 7, 8 and 10) were found in small cysts. However, only claudin-7 persisted at substantive levels in the dedifferentiated epithelium of large, presumably late-stage cysts, where it was localized both at the TJ and basolaterally. The constititutively expressed TJ proteins, ZO-1 and occludin, were also abundantly expressed and correctly localized, suggesting that the basic infrastructure of the TJ is preserved. A previous study suggested that claudin-7 may function as a paracellular Cl− barrier. We postulate that the role of claudin-7 in ADPKD is to seal the paracellular route in Cl−-secreting cyst epithelium, preventing backleak of Cl−, and that it thereby plays a permissive role in fluid secretion and cyst growth.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) affects 1 in 800 individuals and is a major cause of cardiovascular morbidity and end-stage renal disease. The culprit genes mutated in this disease, PKD1 and PKD2, encode membrane proteins, polycystin-1 and -2, respectively, that function as ion channels and signalling proteins. In the kidneys these proteins are predominantly expressed in tubular epithelia, occupying different subcellular localizations including the primary cilia. In current models, it is postulated that the renal tubule cilia normally sense luminal fluid flow, and initiate calcium and other signalling cascades that regulate epithelial cell differentiation, proliferation, and orientation in the plane of the tissue layer (reviewed in ref. [1]). Polycystic kidney disease mutations are thought to disrupt this mechanism and lead to proliferation of epithelial cells and loss of differentiation, leading to cyst formation.
It has been estimated that approximately 70% of cysts are completely closed and not connected to any renal tubule [2], so the pathogenesis of fluid accumulation within the cysts, and hence cyst enlargement, must involve transepithelial fluid and solute secretion. Early studies suggested that the Na-K-ATPase might be mislocalized to the apical membrane and hence drive active Na+ secretion [3], but several subsequent studies have found that the Na-K-ATPase is predominantly basolateral even in renal cysts [4; 5], and that the transepithelial electrical potential is lumen-negative suggesting that the actively secreted ion is more likely to be Cl− [6]. Grantham and colleagues have suggested a model of active transcellular Cl− secretion [6]. In the current version of this model, Cl− is actively taken up across the basolateral membrane by the Na-K-2Cl cotransporter, NKCC1 [5; 7], driven by the Na+ gradient from the basolateral Na-K-ATPase, and effluxes apically through a cyclic AMP-stimulated cystic fibrosis transmembrane regulator (CFTR) chloride channel [4; 8]. Na+ presumably follows passively, driven by the transepithelial potential, but the route for this is unknown. Active accumulation of NaCl within the cyst lumen drives osmotic water secretion, likely via aquaporin water channels, including AQP1 and AQP2 [9]. Implicit in this model for cyst fluid secretion is the requirement that the paracellular pathway be impermeable to Cl−. If not, the transepithelial Cl− gradient generated by active secretion would be dissipated by backleak of Cl−, eliminating the driving force for Na+ and fluid secretion.
The paracellular barrier in epithelial sheets is sealed by the tight junction, which is composed of a complex of interacting proteins, including scaffolding proteins such as ZO-1, and transmembrane proteins such as occludin and members of the claudin family (reviewed in refs. [10; 11]). It is now believed that claudins are the major proteins responsible for determining the selectivity of the barrier, at least to small ions [12]. There are 24 different claudin genes so multiple isoforms with different selectivity profiles could potentially be generated. Furthermore, claudins are expressed in a nephron segment-specific pattern along the renal tubule [13–18], presumably accounting for the different paracellular permeability properties of different segments.
There is already biochemical evidence that the adherens junction [19; 20], and even desmosomes [21; 22], are abnormal in cystic diseases. Polycystin-1 normally forms a complex at the adherens junction with E-cadherin and β-catenins [19]. This complex is disrupted in ADPKD so that E-cadherin is sequestered internally and replaced at the surface by N-cadherin [20]. E-cadherin is known to play a central role in initiating assembly of the junctional complex [23; 24]. Thus, disruption of normal adherens junction structure in ADPKD might be expected to interfere with tight junction assembly.
Only limited analysis of tight junction structure and function in cystic kidney disease has been undertaken to date. Ultrastructural studies have shown preservation of the lateral junctional complex in cyst epithelium [5]. By contrast, Lee et al. found decreased expression and variable localization of claudin-19 in human polycystic kidneys [25].
The aim of this study was to characterize the claudin isoforms expressed in renal cysts in a mouse model of ADPKD, as well as in humans with ADPKD, with the goal of identifying potential candidate proteins that might form the paracellular Cl− barrier and hence be permissive for cyst fluid secretion and cyst growth.
MATERIALS AND METHODS
Tissue samples of normal and cystic kidneys
Mouse renal cystic tissue was isolated from a mouse model with hypomorphic Pkd1 alleles as a result of the presence of a neomycin resistance marker in intron 1 (Pkd1nl/nl) [26]. These mice, on mixed 129Ola and C57Bl6/J genetic background, develop polycystic kidney disease by 4 weeks of life, with the majority of cysts originating from collecting ducts. Healthy, wild-type, age-matched mice were used as controls.
Human renal tissue from patients with ADPKD and end-stage renal failure was fixed in 4% formalin and embedded in paraffin as described [27]. Control renal tissue was isolated from donor kidneys intended for transplantation but not suitable for technical reasons. In total, two human control renal tissue samples and four renal cystic tissue samples from ADPKD patients (two known PKD1-patients and two with >85% probability of carrying a mutation in PKD1) were analyzed. All human tissue samples were anonymized following procedures approved by the ethical committee (institutional review board) of the Leiden University Medical Center.
Antibodies used for immunodetection
Polyclonal antisera against claudin-8 and -19 were raised in rabbit and affinity-purified (ref. [17; 28]). Antibodies to all the other claudin isoforms, occludin, β-catenin, and the mouse monoclonal ZO-1 antibody were from Invitrogen, Carlsbad, CA. Neither the immunizing peptide nor preimmune serum were available for the commercial antibodies. Rabbit anti-claudin-7 immunofluorescence staining of renal tubule basolateral membranes was conpared to staining with non-immune rabbit serum (Suppl. Fig. S1). Rabbit anti-actin (A2066) was from Sigma-Aldrich, St. Louis, MO, mouse monoclonal anti-Na-K-ATPase α1 subunit (C464.6)[29] was a gift from Dr. Alicia McDonough, and mouse monoclonal anti-H-ATPase (E11) was a gift from Dr. Steve Gluck. Secondary antibodies were anti-rabbit, mouse, or rat IgG, conjugated to Alexa Fluor 488 or 555 (Invitrogen) for immunofluorescence, or to horseradish peroxidase (GE Healthcare Biosciences) for immunoblots.
Immunofluorescence staining
Mouse and human kidney sections were deparaffinized and washed with water. Microwave antigen retrieval was performed by boiling in 1 mM EDTA, pH 8, for 10 min followed by cooling for 20 min. Sections were then washed in phosphate-buffered saline and immunostained as described previously [17]. Images were acquired at the USC Center for Liver Diseases using a Nikon PCM confocal microscopy system with argon and helium-neon lasers.
Cyst stage was classified according to the scheme of Thomson et al. [5]: small cysts were defined as having less than 50 cyst-lining epithelial cells per section, intermediate 51–200 cells, and large if greater than 200 cells. For quantitation of claudin-7 signal intensity, multiple random fields were acquired at the same exposure settings and the unprocessed images were opened in ImageJ software (NIH). For each individual tubule or cyst, 5 different regions were selected. The tight junction and basolateral membrane were traced by drawing a freehand line, and the mean pixel intensity of the line was measured and the 5 measurements were averaged. Differences in the mean intensity of claudin-7 staining between mice of different age, genotype and cyst stage were compared for overall statistical significance by one-way ANOVA, and post-hoc comparisons with Tukey’s test.
RESULTS
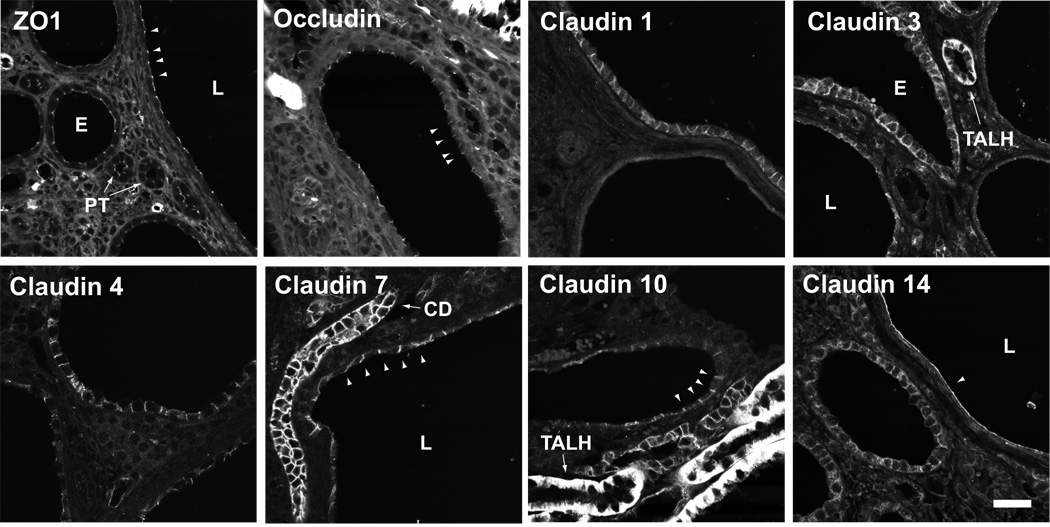
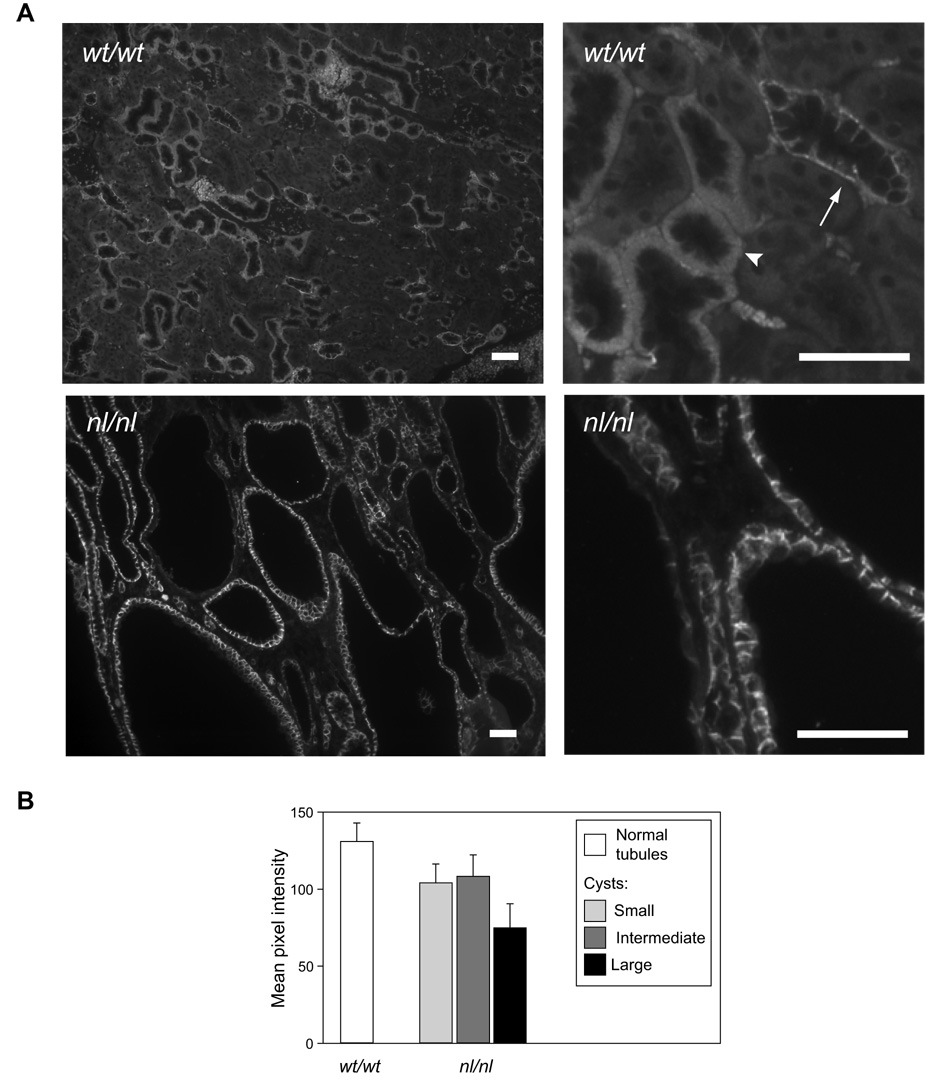
To determine the expression and localization of tight junction proteins in polycystic kidney disease, we first performed immunofluorescence staining in kidney sections from a hypomorphic Pkd1 mouse model (Pkd1nl/nl) and compared it to sections from control, wild-type mice (Fig. 1, and summarized in Table 1). Because we observed that the staining pattern of tight junction proteins varied depending on cyst size, we also compared staining between cysts stratified according to their size. Small cysts had small lumens and cuboidal epithelium, intermediate-stage cysts had larger lumens, and large cysts had very large lumens, flattened epithelium and surrounding fibrosis. ZO-1, a ubiquitous tight junction plaque protein that is constitutively expressed in all epithelia, was detected at the tight junction at approximately equal intensity in wild-type tubules, non-cystic tubules of Pkd1nl/nl mice, and in cysts of all stages. Occludin, a tight junction membrane protein that is also likely constitutively expressed, was similarly found to be expressed uniformly throughout epithelia of control and cystic kidneys. By contrast, of the claudin isoforms that were detectable in cysts, most were expressed predominantly in small cysts at levels similar to or lower than levels in normal tubule epithelium, were progressively dowregulated in intermediate cysts, and were almost (claudin-3 and -4) or completely absent (claudin-10) in large cysts. The exception to this was claudin-7, which appeared to be abundantly expressed in small and intermediate cysts at levels similar to that of normal tubules, as well as in the majority of large cysts. This impression was confirmed when we examined low-magnification digital images and quantitated the claudin-7 signal intensity (Fig. 2), using objective criteria for cyst stage based on cell number.
Fig 1.
Expression of tight junction proteins in Pkd1 nl/nl mouse kidney. Immunofluorescence staining was performed on paraffin-embedded sections using antibodies against the indicated proteins, and images acquired by confocal microscopy. Scale bar represents 30 µm. ZO-1: Tight junction staining (arrowheads) of approximately equal intensity is observed in normal, non-cystic tubules such as proximal tubules (PT), and in small (S) and large (L) cysts. Occludin: Occludin is expressed in tight junctions of all cysts, similar to ZO-1. Claudin-1: The upper cyst is a rare cyst staining basolaterally for claudin-1. Most cysts (like the lower cyst in this image) showed no staining at all. Claudin-3: Weak basolateral staining was observed in most cysts, more in small than in large cysts. By comparison, strong claudin-3 staining of a non-cystic thick ascending limb (TALH) is shown. Claudin-4: Basolateral staining was observed in most cysts, more in small than in large cysts. Claudin-7: All cysts showed strong staining for claudin-7, even large cysts such as the one shown here. Note the flattened epithelium and distorted lateral membrane. An intact collecting duct (CD) is also shown. Claudin-10: Occasional small cysts showed weak basolateral claudin-10 staining (arrowheads). By comparison, adjacent non-cystic tubules of the thick ascending limb showed much stronger staining. Claudin-14: Apparent claudin-14 staining of uncertain specificity was observed at the apical membrane (arrowhead) predominantly in very large cysts.
Table 1.
Pattern of expression of tight junction proteins in normal mouse renal tubule and in cysts of Pkd1 nl/nl mice
| Tight junction1 protein | Normal wt/wt | Pkd1 nl/nl | |||
|---|---|---|---|---|---|
| Tubule segments2 | Subcellular location3 | Cyst type4 | Subcellular location2 | Expression level5 | |
| ZO-1 | All | TJ | S = I = L | TJ | 2 |
| Occludin | All | TJ | S = I = L | TJ | 2 |
| Claudin-1 | BC | ? | Very rare L | Bl | 2 |
| Claudin-2 | PT, uTDL | TJ | - | - | 0 |
| Claudin-3 | TALH, DCT, CD | Bl | S ≫ I, L | Bl | +/− |
| Claudin-4 | ThAL, CD | TJ | S ≫ I, L | Bl | 1 |
| Claudin-7 | lTDL, DCT, CNT, CD | TJ, Bl | S = I ≥ L | Bl | 2 |
| Claudin-10 | PT, TALH | TJ, Bl | Few S | Bl | +/− |
| Claudin-14 | CD | TJ | L? | Ap? | 1 |
Antibodies to claudins 8, 11, 16 and 19 failed to stain mouse kidney paraffin sections satisfactorily.
Tubule segment localization in normal kidney: summary of data from control (wt/wt) mice staining in this study and from published reports [13; 14; 16; 17]. For examples of claudin-7 localization in wild-type mice, please refer to Supplemental Fig. S2 BC, Bowman's capsule; PT, proximal tubule; uTDL, upper thin descending limb; lTDL, lower thin descending limb; ThAL, thin ascending limb; TALH, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct.
TJ, tight junction; Ap, apical plasma membrane; Bl, basolateral plasma membrane
S, small cyst; I, intermediate cyst; L, large cyst
Comparison of staining intensity in positive small or intermediate cysts of nl/nl mice, relative to tubules in wild-type mice: 2, similar intensity; 1, slightly weaker intensity; +/−, much weaker intensity; 0, no staining.
Fig 2.
Relative claudin-7 expression in tubules and cysts. A. The images show low (left) and high (right) magnification views of claudin-7 antibody staining in wild-type (wt/wt), and homozygous (nl/nl) PKD1 hypomorphic mice. Claudin-7 is expressed on the basolateral membrane. In wild-type renal tubules, this appears as a diffuse staining in the thick ascending limb/distal tubule (arrowhead) due to extensive basal membrane invaginations in these segments, and as a more discrete delineation of the base and sides of each cell in the collecting ducts (arrow). The section of cystic kidney overall appears darker but this is simply due to the large area occupied by fluid-filled cyst lumens. The cyst walls, particularly of the small and intermediate cysts, in the nl/nl mice, stain similarly to the walls of normal tubules in the wt/wt mice. Scale bar represents 50 µm. B. Quantitation of claudin-7 immunofluorescence intensity in tight junctions and basolateral membranes of tubule and cyst epithelium. Columns represent mean ± S.E (n = 8 tubules or cysts) and is representative of two such experiments. The p value for comparison between groups is non-significant by one-way ANOVA.
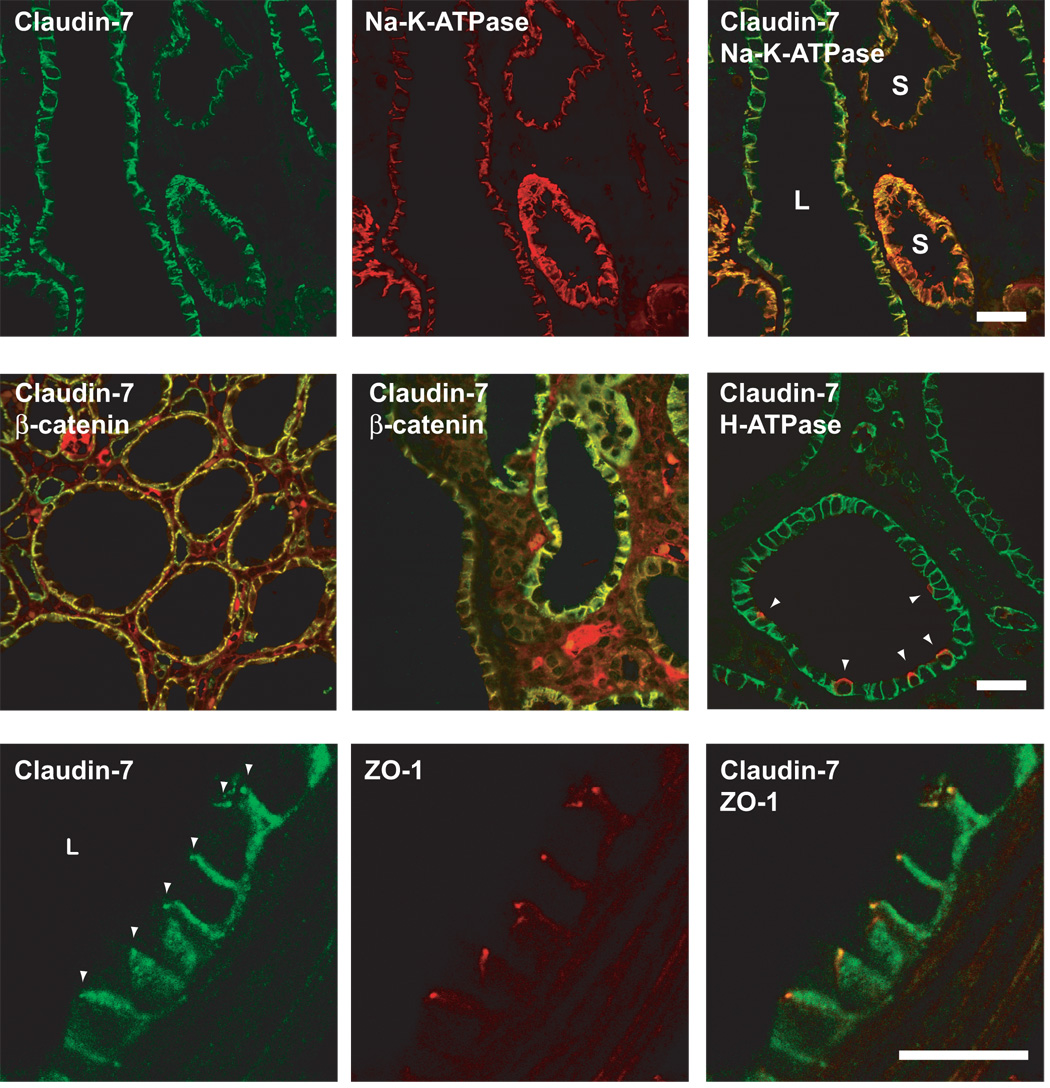
Cyst epithelial cells are hypothesized to undergo progressive dedifferentiation as cysts enlarge, and a good marker for this is the level of Na-K-ATPase expression [5; 30]. In our Pkd1 nl/nl mice, the α1 subunit of the Na-K-ATPase was strongly expressed at the basolateral membrane in small cysts, but markedly downregulated in intermediate and large cysts (Fig. 3, top row). By double-staining the same sections, claudin-7 expression was found to be maintained at high levels even in intermediate and large cysts with low levels of Na-K-ATPase, suggesting that claudin-7 expression is preferentially preserved despite cyst epithelial dedifferentiation.
Fig 3.
Double immunofluorescence localization of claudins in Pkd1 nl/nl mouse renal cysts. Scale bars represent 30 µm. Upper row: Double staining for claudin-7 (green) and the α1 subunit of Na-K-ATPase (red) show that both are expressed at the basolateral membrane. Note that the expression of Na-K-ATPase is much lower in large cysts (L) compared to small cysts (S), while claudin-7 expression is well-preserved. This is most obvious in the merged image, where the color of the basolateral membrane in small cysts (orange) clearly differs from that of large cysts (yellow-green). Middle row: Double staining for claudin-7 (green) and β-catenin (red) also shows colocalization to the lateral membrane in cysts of varying size. The apparent interstitial staining is due to non-specific binding of the anti-mouse IgG used as a secondary antibody to detect mouse anti-β-catenin. In the rightmost panel, claudin-7 (green) is demonstrated in a small cyst in which a minority of cells stain at the apical membrane (arrowhead) for H+-ATPase expression (red), representing α-intercalated cells of cortical collecting duct origin. Bottom row: Double staining for claudin-7 (green) and ZO-1 (red) shows that a subset of claudin-7 does localize at the tight junction (arrowheads).
In small and intermediate cysts, double-staining with segment-specific markers showed that the majority of claudin-7-expressing cysts are of collecting duct origin. Fig. 3 (middle row) shows vacuolar H+-ATPase at the apical membrane in a subset of claudin-7-expressing cells within a small cyst, indicating the presence of collecting duct α-intercalated cells. This was rarely observed in large cysts, presumably either because they are overgrown by other cells, or because the intercalated cells undergo dedifferentiation. We also used the lectins, Lotus tetraglonobulus agglutinin (LTA), and Dolichus biflorus agglutinin (DBA) to analyze the segments of origin (Supplemental Fig. S4). LTA, which stains the brush border membranes of proximal tubules in normal mouse kidney [31], was almost completely absent from Pkd1nl/nl cysts, indicating that very few cysts are of proximal tubule origin. Rare cysts showed intermittent cells with intracellular LTA staining, but the significance of this is unclear. DBA is known to be a marker of the collecting duct in normal mice [31]. In our hands, it stained basolaterally and intracellularly in principal cells of the collecting duct. In Pkd1nl/nl mice, approximately half the cysts were positive for DBA. Furthermore, the majority of cysts expressing claudin-7 were DBA-positive, confirming their collecting duct origin (Fig. 4).
Fig 4.
Double fluorescence images of claudin-7 (left panel and green in merged image) and DBA (right panel and red in merged image) staining. Claudin-7 is expressed at the basolateral membrane of most cysts, many of which (asterisks) also stain with DBA, indicating that they probably originated from principal cells of the collecting tubule. Scale bar represents 50 µm.
As in normal collecting duct, claudin-7 within cyst epithelium is predominantly expressed at the basolateral membrane, where it colocalizes with the Na-K-ATPase and β-catenin (Fig. 3, middle row). In most cysts, just like in normal tubules, claudin-7 also extends apically into the tight junction, as evidenced by colocalization with ZO-1 (Fig. 3, bottom row; see also superimposed DIC images in Supplemental Fig. S3).
To determine if protein abundance was changed, we performed Western blots on whole kidney lysates from control (wt/wt) mice, and from Pkd1 heterozygous (wt/nl) and homozygous (nl/nl) mice (Supplemental Fig. S5). These showed that Na-K-ATPase, claudin-10 and claudin-14 protein were reduced in the nl/nl mice, compared to the other two groups. However, the majority of proteins, including occludin, claudin-3, claudin-7 and ZO-1 appeared no different in all the mice.
Although genetic mouse models provide a powerful system to test experimental hypotheses, they do not necessarily fully reproduce the human disease. We therefore also examined human nephrectomy specimens from patients with ADPKD (known or likely to be due to PKD1 mutations) and from control transplant donors (Table 2). We found that Na-K-ATPase was strikingly downregulated in cystic epithelium, compared to normal tubules, and was essentially absent from large cysts, suggesting that the same process of dedifferentiation observed in Pkd1 mice also occurs in humans. Similarly, expression of claudin-3, -4 and -8 were downregulated; antibodies to other claudin isoforms did not work in human tissue. By contrast, ZO-1, occludin and claudin-7 expression were very well-preserved in small and large cysts. Fig. 5 shows examples of large cysts with strong expression of ZO-1 and claudin-7 but complete absence of Na-K-ATPase. Unlike in the mice, our human ADPKD kidney sections also occasionally included parts of very large cysts. These cysts, which we term "advanced cysts", were surrounded by a thick circumferential layer of material, presumably basement membrane and/or fibrosis, from which we were unable to discern a discrete epithelial layer. Advanced cysts did not stain with any of our epithelial markers.
Table 2.
Summary of ADPKD human kidney tissue staining intensity
| Normal tubules | Cysts | |||
|---|---|---|---|---|
| Small | Large | Advanced | ||
| Claudin-3 | 1+ | 1+ | - | - |
| Claudin-4 | 1+ | 1+ | +/− | - |
| Claudin-7 | 2+ | 2+ | 2+ | - |
| Claudin-8 | +/− | - | - | - |
| Na-K-ATPase | 2+ | +/− | - | - |
| ZO-1 | 1+ | 1+ | 1+ | - |
| β-catenin | 2+ | 1+ | 1+ | - |
| Occludin | 1+ | 1+ | 1+ | - |
Fig 5.
Double immunofluorescence localization of claudin-7 in kidney sections from normal human adult transplant donors (WT) and ADPKD patients (PKD). Note that claudin-7 and ZO-1 are present in cystic epithelium but Na-K-ATPase is absent. Scale bar represents 20 µm.
DISCUSSION
We examined, in ADPKD models, the expression of constitutive proteins in the epithelial cell junctional complex and found that ZO-1, occludin, and β-catenin were abundantly expressed in renal cysts, showed correct subcellular localization, and persisted even in large, and presumably late-stage cysts. ZO-1 is constitutively expressed at the tight junction and functions as a scaffolding protein, recruiting and stabilizing claudins, so that in their absence tight junction strands fail to form [32]. Occludin is an integral membrane protein that is also constitutively expressed at tight junctions but its function remains elusive. Mice with occludin knockout [33; 34] and renal epithelial cells with occludin knockdown [35] still form tight junctions and show only subtle alterations in paracellular permeability. Thus, the significance of occludin expression in advanced cysts is unclear. Nevertheless the preservation of both ZO-1 and occludin suggest that the basic biochemical infrastructure of the tight junction remains intact even in late-stage cysts. Our findings are consistent with electron microscopy studies showing that the lateral junctional complex is structurally preserved even in large cysts [5]. β-catenin is a key component of the adherens junction that anchors cadherins to the cytoskeleton, but is also expressed along the basolateral membrane. Interestingly, polycystin-1 and -2 have been shown to exist in a complex with E-cadherin and β-catenin [19; 20]. In PKD1 cells, this complex is disrupted; E-cadherin is lost and compensated for by N-cadherin expression while β-catenin is preserved, consistent with our finding that β-catenin is preserved in large cysts. The integrity of the adherens junction is required to maintain an intact epithelial monolayer lining the cyst.
We also performed a comprehensive survey of the expression of multiple members of the claudin family, in renal cysts of mice and patients with ADPKD. We are confident that the antibodies we used were specific for each claudin isoform. All of them were raised against peptides from the C-terminus, which shares little or no homology between different claudin isoforms. Furthermore, all of these claudin antibodies have had testing for specificity documented in product data sheets (Invitrogen) and/or published research reports [13; 16–18; 36; 37].
In our study, most of the cysts appeared to be derived from the collecting duct, as evidenced by staining for the vacuolar H+-ATPase and DBA. Consistent with this, most of the claudin isoforms (3, 4, 7, 10, and 14) that are known to be expressed in the collecting duct [14; 16–18; 37] were present in at least small cysts, except for claudin-14 which showed equivocal staining. Claudin-2, which is normally expressed in the proximal tubule [13; 14], was absent from the cysts, as was LTA, consistent with the idea that renal cysts derived from proximal nephron segments are rare [5].
All of the claudins that were detectable in cysts stained in a predominantly basolateral pattern. Data that we have previously published [17; 18], as well as our current observations in wild-type mice, indicate that all of these claudins are found basolaterally in at least some nephron segments in normal tubules. We speculate that the role of basolateral claudins may be interaction with the basement membrane, or represent an inactive pool in transit to the tight junction. Importantly, we show clearly that claudin-7 expression extends apically into the tight junction itself, thus supporting a role in the formation of the paracellular barrier.
The most provocative finding of our study is that claudin-7 is highly expressed in renal cysts, and that its expression is well-preserved even in large and presumably late-stage cysts, in which the epithelium is dedifferentiated (as evidenced by loss of Na-K-ATPase α1 subunit expression) and other isoforms such as claudin-3, -4, and -10 are downregulated or absent (as determined by immunofluorescence). Interestingly, the expression of claudin-7 appears to be predominantly basal in normal tubules and becomes more lateral in location in cyst epithelium, supporting a possible functional role in the paracellular barrier. Although our Western blots of kidney lysates showed few gross changes in protein expression levels, this is hardly surprising since these were done using whole kidney samples, which are therefore very insensitive to subtle changes in regional protein expression in individual nephron segments or in subpopulations of cysts.
Claudin-7 is normally expressed predominantly in the aldosterone-sensitive distal nephron [17; 37], which has a low permeability both to cations and anions. When overexpressed in LLC-PK1 cells, claudin-7 augmented the Cl- permeability barrier [37]. A caveat to this is that a recent study using RNA interference to knockdown claudin-7 in MDCK cells reached the opposite conclusion about the role of claudin-7 [38]. If claudin-7 does indeed function to reduce Cl− permeability, we postulate that the role of persistent expression of claudin-7 at the tight junction of ADPKD renal cysts as they enlarge may be to maintain an intact paracellular Cl− barrier. By preventing passive, paracellular backleak of Cl−, this would allow active transcellular secretion of Cl− to generate a stable transepithelial Cl− and potential gradient, thus providing the driving force for Na+ and water transport into the cyst lumen. Other claudins such as claudin-3 and -4 may also be important during the earlier stages of cyst growth when they are highly expressed, but their exact role is unclear. If our model is correct, then claudin-7 might be a good target for inhibitory drugs designed to retard cyst growth in ADPKD.
Supplementary Material
ACKNOWLEDGEMENTS
We thank A. van de Wal (Dept. of Pathology, LUMC) for technical assistance. This work was supported in part by National Institutes of Health grants DK062283 (to ASLY) and DK48522 (to the USC Center for Liver Diseases, for the Microscopy Sub-core), by a Polycystic Kidney Disease Research Foundation Grant-in Aid (to ASLY), by a Dutch Kidney Foundation grant (C05-2132 to DJMP) and by the Netherlands Organization for Scientific Research grant (NWO/ZonMw 016.036.353 to DJMP).
REFERENCES
- 1.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70(5):854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31(5):1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na(+) -K(+)-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol. 1991;260(3 Pt 2):F420–F430. doi: 10.1152/ajprenal.1991.260.3.F420. [DOI] [PubMed] [Google Scholar]
- 4.Brill SR, Ross KE, Davidow CJ, Ye M, Grantham JJ, Caplan MJ. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc Natl Acad Sci U S A. 1996;93(19):10206–10211. doi: 10.1073/pnas.93.19.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson RB, Mentone S, Kim R, Earle K, Delpire E, Somlo S, et al. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/- mouse model of ADPKD. Am J Physiol Renal Physiol. 2003;285(5):F870–F880. doi: 10.1152/ajprenal.00153.2003. [DOI] [PubMed] [Google Scholar]
- 6.Grantham JJ, Ye M, Gattone VH, 2nd, Sullivan LP. In vitro fluid secretion by epithelium from polycystic kidneys. J Clin Invest. 1995;95(1):195–202. doi: 10.1172/JCI117638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebeau C, Hanaoka K, Moore-Hoon ML, Guggino WB, Beauwens R, Devuyst O. Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflugers Arch. 2002;444(6):722–731. doi: 10.1007/s00424-002-0880-3. [DOI] [PubMed] [Google Scholar]
- 8.Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. Am J Physiol. 1996;270(1 Pt 1):C389–C399. doi: 10.1152/ajpcell.1996.270.1.C389. [DOI] [PubMed] [Google Scholar]
- 9.Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol. 1996;271(1 Pt 2):F169–F183. doi: 10.1152/ajprenal.1996.271.1.F169. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 11.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16(4):181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Van Itallie CM, Anderson JM. Claudins and Epithelial Paracellular Transport. Annu Rev Physiol. 2005 doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 13.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281(5):F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 14.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13(4):875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 15.Reyes JL, Lamas M, Martin D, Del Carmen Namorado M, Islas S, Luna J, et al. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62(2):476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;23(16):2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 17.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286(6):F1063–F1071. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 18.Van Itallie CM, Rogan S, Yu AS, Seminario-Vidal L, Holmes J, Anderson JM. Two Splice Variants of Claudin-10 in the Kidney Create Paracellular Pores with Different Ion Selectivities. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 19.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104(10):1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell. 2004;15(3):1334–1336. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffers MS, van der Bent P, Prins F, Spruit L, Breuning MH, Litvinov SV, et al. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum Mol Genet. 2000;9(18):2743–2750. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- 22.Russo RJ, Husson H, Joly D, Bukanov NO, Patey N, Knebelmann B, et al. Impaired formation of desmosomal junctions in ADPKD epithelia. Histochem Cell Biol. 2005;124(6):487–497. doi: 10.1007/s00418-005-0055-3. [DOI] [PubMed] [Google Scholar]
- 23.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107(4):1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18(1):189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee NP, Tong MK, Leung PP, Chan VW, Leung S, Tam PC, et al. Kidney claudin-19: localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS Lett. 2006;580(3):923–931. doi: 10.1016/j.febslet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13(24):3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 27.Peters DJ, Spruit L, Klingel R, Prins F, Baelde HJ, Baelde PC, et al. Adult, fetal, and polycystic kidney expression of polycystin, the polycystic kidney disease-1 gene product. Lab Invest. 1996;75(2):221–230. [PubMed] [Google Scholar]
- 28.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal Localization And Function Of The Tight Junction Protein, Claudin-19. Am J Physiol Renal Physiol. 2007 doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 29.Van Why SK, Mann AS, Ardito T, Siegel NJ, Kashgarian M. Expression and molecular regulation of Na(+)-K(+)-ATPase after renal ischemia. Am J Physiol. 1994;267(1 Pt 2):F75–F85. doi: 10.1152/ajprenal.1994.267.1.F75. [DOI] [PubMed] [Google Scholar]
- 30.Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest. 2005;115(4):910–918. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laitinen L, Virtanen I, Saxen L. Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem. 1987;35(1):55–65. doi: 10.1177/35.1.3794309. [DOI] [PubMed] [Google Scholar]
- 32.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126(4):741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, et al. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669(1):34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288(6):C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 36.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120(2):411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 37.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl-conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005 doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 38.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281(47):36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.