Abstract
Circadian expression of the luciferin-binding protein (LBP) from the dinoflagellate Gonyaulax polyedra is regulated at the translational level. A small interval in the lbp 3′-untranslated region, which contains seven UG-repeats, serves as a cis-acting element to which a trans-acting factor (CCTR) binds in a circadian manner. Its binding activity correlates negatively with the circadian expression of LBP. Here I report the identification of a protein in the green alga Chlamydomonas reinhardtii that represents a CCTR analog. It binds both specifically and under control of the circadian clock to the UG-repeat region. The data show for the first time that circadian cis-elements implicated in translational regulation have been conserved during evolution.
Keywords: biological clock, Gonyaulax polyedra, Chlamydomonas reinhardtii, translational control
Circadian rhythms occur in the biological world from microorganisms (including prokaryotes; ref. 1) to humans, and persist under constant conditions of light and temperature with a period of about 24 h. The rhythmicity is generated by an endogenous oscillator, which can be used as a “clock” for the timing of biological events within a solar day. Genes involved in the generation of circadian rhythms have been isolated and characterized in Drosophila melanogaster [period (per) and timeless (tim); refs. 2–4] and Neurospora crassa [frequency (frq); refs. 5 and 6]. The oscillator itself controls the expression of genes/mRNAs participating in a variety of circadian-controlled cellular processes. Examples are the clock-controlled genes (ccg) from N. crassa, some of which are involved in the conidiation rhythm (7), genes coding for certain chlorophyll a/b binding proteins in plants (8, 9), and the mRNA for the luciferin-binding protein (LBP), which is a component of the bioluminescent system in Gonyaulax polyedra (10, 11). The biological clock controls the expression of these genes either at the transcriptional (8, 9, 12–14) or at the translational level (10, 11). Slowly we are beginning to understand which clock-controlled trans-factors interact with the corresponding cis-elements at the DNA or RNA level.
A penetrating insight into the circadian expression of a protein that is regulated at the translational level has been gained recently with the LBP from Gonyaulax. In this case, an RNA-binding protein, designated CCTR (circadian-controlled translational regulator) interacts with the 3′-untranslated region (3′-UTR) of the lbp mRNA (15–17). The binding activity of CCTR changes during the day/night cycle; it begins to decrease at the beginning of the night phase when synthesis of LBP starts and increases again at the end of the night when synthesis of LBP stops, strongly indicating that CCTR functions as a clock-controlled repressor. The binding site of CCTR comprises a 22-nt long region, which is part of a potential hairpin-loop structure and contains seven UG-repeats, including an additional U in front of the fifth repeat (15–17).
It is an open question whether circadian-based elements, which are part of the transduction pathway from the central oscillator to the output rhythms, have been conserved during evolution. The UG-repeat region of the lbp 3′-UTR represents a translational cis-element. If this cis-element has been conserved along with its trans-factor, then it should be possible to use it as a probe for the identification of a CCTR-analog in another organism. For this purpose, Chlamydomonas reinhardtii has been chosen for the following reasons. (i) In this alga, several circadian rhythms are known and physiologically well-characterized (e.g., phototaxis, chemotaxis, cell division; refs. 18–21). (ii) It is phylogenetically different from Gonyaulax. While Gonyaulax is a dinoflagellate, a group of algae that is closely related to ciliates like Tetrahymena or Paramecium, Chlamydomonas belongs to the group of green alga, which are on the same evolutionary branch as higher plants (22, 23). (iii) Chlamydomonas offers many practical advantages for investigations; its genetics are well-understood, and it is easily amenable to biochemical studies (24, 25). Thus, it has been called the “green yeast” (26).
Interestingly, an RNA-binding protein that fulfills both criteria for being named a CCTR analog (specific binding to the UG-repeat and circadian binding activity) has been identified in C. reinhardtii.
MATERIALS AND METHODS
Cell Culture.
Chlamydomonas cells (wild-type strain C137) were grown in high salt acetate medium (27) under a 12-hr light/12-hr dark cycle (LD 12/12) with a light intensity of 300 μE per m2 per sec (1 E = 1 mol of photons) at 24°C. For some experiments, cells were grown up under LD to a cell density of 7 × 105 cells per ml and then put under constant conditions of dim light (LL; 40 μE/m2 per sec) before harvesting. The beginning of the light period is defined as time zero (LD 0 or LL 0), and the end is LD 12.
Preparation of Crude Extracts.
Cells were grown up to a cell density of ca. 2–3 × 106 cells per ml, harvested by centrifugation, and stored frozen in liquid nitrogen. For extracts, cells were resuspended in a buffer of 80 mM NaCl/10 mM Tris·HCl, pH 7.5/0.1 mM EDTA, pH 8.0/2 mM dithiothreitol/5% (vol/vol) glycerol, and lysed by vortexing (highest speed) with glass beads (diameter: 0.25–0.30 mm) for 1× 45 sec and 1× 30 sec. After 45 sec, they were placed on ice for 2 min. Cell debris was removed by centrifugation at 13,000 × g for 12 min.
Preparation of Plasmid Constructs.
The construction of pMM3 has been described previously (15). pMM14 derives from pMM3; it was created by subsequent deletion of a SalI–DraIII vector fragment and a 48-bp long HindIII–BglI fragment of the lbp 3′-UTR, which includes the UG-repeat.
Preparation of RNA Transcripts.
The RNAs containing various lengths of the 3′-UTR of lbp mRNA were transcribed from pMM3 (BglI, 145 nt; XbaI, 263 nt) and pMM14 (XbaI−UG, 215 nt), respectively. The start site of the RNAs was determined by the T7 promotor, and the ends were determined by digestion with BglI (… AG CCC GCA) and XbaI (… CC CCG GGT), respectively. In vitro transcription was carried out by following the procedures of the suppliers (Promega and Ambion, Austin, TX). To the transcript BglI-Ol, a 22-nt antisense oligonucleotide (CTGCACACAACACACACAAAGC) had been attached by heating the samples at 70°C for 10 min and slowly cooling them down to room temperature. The location of all transcripts is shown in Fig. 1A; sequences are published elsewhere (28).
Figure 1.

Chlamy 1 binds specifically to the UG-repeat region of the lbp 3′-UTR. (A) Map of in vitro transcripts covering various parts of the lbp 3′-UTR. The shaded bar shows the location of the UG-repeat. The dotted line indicates the deletion of a 48-nt long region of the lbp 3′-UTR. The ladders show the 22-mer oligonucleotide Ol hybridized to the BglI transcript. (B and C) Autoradiograms of mobility-shift assays using the 32P-labeled BglI transcript or its hybrid (RNA BglI-Ol) in the presence of poly(G) as nonspecific competitor RNA. For the binding reaction, the samples were incubated with Chlamydomonas crude extracts from the night period (LD 14). In some cases, specific cold competitor RNAs (XbaI and XbaI−UG transcripts) were added to the binding reaction up to a 20-fold excess. One lane (RNA BglI) always demonstrates the mobility of the transcript alone.
Mobility-Shift Assay.
Binding assays were performed as described earlier (15). For each probe, 28 μg of protein from a Chlamydomonas crude extract was used.
RESULTS AND DISCUSSION
A Protein from Chlamydomonas (Chlamy 1) Binds Specifically to the UG-Repeat Sequence.
The search for a CCTR analog in Chlamydomonas was carried out with the RNA transcript BglI (Fig. 1A), which includes the front part of the Gonyaulax lbp 3′-UTR comprising the UG-repeat. The BglI transcript contains a hairpin-loop structure that may be relevant to the binding of CCTR in addition to the RNA sequence per se. The BglI transcript and proteins from a Chlamydomonas crude extract were incubated in the presence of a nonspecific competitor RNA [poly(G)] to avoid binding of nonspecific RNA-binding proteins (15). Under these conditions, three distinct RNA–protein complexes could be detected using mobility-shift assays (Fig. 1B). To check if one of these RNA-binding proteins (designated Chlamy 1, 2, and 3) interact specifically with the UG-repeat region of the BglI transcript, two different specific competitors were used. One of these competitors covered the entire lbp 3′-UTR (XbaI) and the other covered the same region except for the UG-repeat region (XbaI−UG) (Fig. 1A). The XbaI transcript competed successfully with the binding of Chlamy 1, 2, and 3 to the radiolabeled lbp 3′-UTR, confirming specific interactions of the three proteins with the lbp 3′-UTR. The XbaI−UG transcript could only compete with Chlamy 2 and 3, while Chlamy 1 could still bind efficiently to its target sequence on the labeled BglI transcript (Fig. 1B). These results indicate that Chlamy 1 is an RNA-binding protein that interacts specifically with the UG-repeat region. To narrow down the binding site further, a different approach was applied. An antisense oligonucleotide (22-mer) that is complementary to the UG-repeat was hybridized to the BglI transcript and used as a template in the mobility-shift assay. This antisense oligonucleotide totally abolished binding of the Gonyaulax CCTR to the lbp 3′-UTR (15). It was also able to inhibit the binding of Chlamy 1, but had no effect on the binding of Chlamy 2 and 3 (Fig. 1C). The results show that Chlamy 1 binds to the same cis-element as the CCTR from Gonyaulax.
The Binding Activity of Chlamy 1 Is Controlled by the Circadian Clock.
To check whether the binding activity of Chlamy 1 changes during the day-night cycle as is the case with the Gonyaulax CCTR, Chlamydomonas cells were grown in a 12-hr light/12-hr dark cycle (LD) and harvested every 4 hr. The binding activity of Chlamy 1, which was tested in crude extracts, indeed changed during the day/night cycle as did the activities of Chlamy 2 and 3 (Fig. 2A). The binding activities of the three proteins changed in phase, but the amplitudes were slightly different, with maximum activity occurring during the night.
Figure 2.
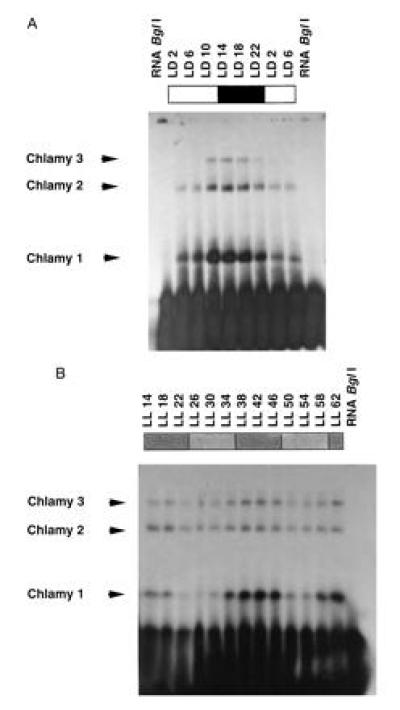
Circadian changes in binding activity of Chlamy 1 and of Chlamy 2 and 3 (with reduced amplitude in the last two cases). (A) Autoradiograms of mobility-shift assays using the 32P-labeled BglI transcript in the presence of poly(G). One lane (RNA BglI) always demonstrates the mobility of the transcript alone. Chlamydomonas crude extracts were prepared from cells grown under LD 12/12 and harvested every 4 hr at the indicated times. (B) Cells were grown up under LD 12/12 and then put under constant dim light (LL: stippled) and harvested at the indicated times. Times are given in hours after lights were switched on. Subjective day phase, less-dense stippling; subjective night phase, dense stippling.
If Chlamy 1 represents a clock-regulated trans-factor, then its binding activity should fluctuate in cells kept under constant conditions of light and temperature. Thus, cells were grown in a 12/12 LD regime and then put under constant conditions of dim light and temperature. Binding activity was monitored over an entire 2-day period. A circadian rhythm of binding activity of Chlamy 1 was clearly visible under these conditions (Fig. 2B). In case of Chlamy 3 and especially of Chlamy 2, the amplitude of the rhythm was reduced. The data show that Chlamy 1 is a clock-controlled trans-factor. Since it recognizes the same cis-element as CCTR, it should be considered as a CCTR analog.
Chlamy 1 may regulate the circadian synthesis of any proteins in Chlamydomonas that are controlled at the translational level and that contain a UG-repeat sequence. Since its main binding activity occurs in the night, it may function as a clock-controlled repressor of day-induced protein(s). It is of special interest that the UG repeat is a target RNA-motif for clock-controlled trans-factors, whose binding activity is out of phase. Thus, the CCTR from Gonyaulax has its maximum binding activity during the day and Chlamy 1 has it during the night. This indicates that the phase relationship of circadian rhythms is not determined by cis-elements or trans-factors involved in the final step of the transduction pathway from the oscillator to the output rhythm.
UG-Repeat Sequences Situated in UTRs Are Present Throughout the Eukaryotic Kingdom.
It is too early to predict if the UG-element is also used as a binding site in RNAs of higher eukaryotes. Indications that this might be indeed the case come from the observation that there are some RNA-binding proteins in reticulocyte lysates that recognize the lbp 3′-UTR (BglI transcript) (unpublished data). One of them might be a CCTR analog. Also, the presence of the UG-repeat sequence in the UTRs of several sequenced genes, including some from higher eukaryotes (Table 1) is consistent with this possibility. Interestingly, one of these genes belongs to Chlamydomonas eugametos and is coding for a Ca2+-dependent protein kinase (CDPK; GenBank accession no. Z498233). It contains six UG repeats in its 3′-UTR and the additional U in front of the fifth repeat, which is present in the lbp 3′-UTR, is conserved at this position. Even though CDPKs are the predominant Ca2+-dependent protein kinases in plants, an understanding of their physiological role has remained elusive up to now (29). However, it is noteworthy that cytosolic and chloroplastic free calcium undergo circadian oscillations in plants (30). Regulation of calcium flux is thought to be fundamental to the organization of circadian systems (30).
Table 1.
Presence of UG-repeat sequences in UTRs and coding regions
| Organism | Gene/protein | No. of identical nucleotides |
|---|---|---|
| 3′-UTR | ||
| G. polyedra | LBP | 15 |
| Mus musculus | Novel protein kinase | a: 15; b: 13 |
| Trypanosoma cruzi | Kinetoplast-associated protein | 15 |
| Human | Wilm tumor (WT 33) | 15 |
| Human kidney | Cyclophilin C | 14 |
| Human | Wilm tumor WT1 | 14 |
| Human | Endothelial membrane glycoprotein III a | 14 |
| Human | Pre-B cell enhancing factor | 14 |
| Saccharomyces cerevisiae | Polymerase I | 14 |
| Medicago sativa | Heat shock protein | 14 |
| T. cruzi | Surface antigen | 14 |
| D. melanogaster | Dras 1 (ras oncogenes) | 14 |
| Drosophila ananassae | OM (1D) | 14 |
| Tradescantia paludosa | tpc 70 | 14 |
| Ciona intestinalis | Ctm 1 (tropomyosin) | 14 |
| Oryza sativa | MADS-box protein (MADS 3) | 14 |
| Rattus norvegicus | Myogen | 14 |
| M. musculus | G-alpha-13-protein | 14 |
| Xenopus laevis | Integrin beta-1 subunit | 14 |
| M. musculus | Mouse developmental kinase | 13 |
| Chlamydomonas eugametos | Ca-stimulating protein kinase | 13 |
| Human | Serum response factor | 13 |
| 5′-UTR | ||
| Human | TF II A-alpha | 14 |
| D. melanogaster | Ethanolamine kinase | 14 |
| Coding sequence | ||
| M. musculus | NPY-1 receptor | 15 |
A GenBank search (via National Center for Biotechnology Information blast; cut off = 60; alignment = 500) with the central part of the UG-repeat region (UGUGUGUGUUGUGUG) was conducted. Genes with known function containing the 15-nt long sequence or a slightly modified version of it in their 3′-UTR (upper section), 5′ UTR (medium section), or coding sequence (lower section) are listed. A consensus of all 15 nt is indicated by 15. A change of 1 and 2 nt is indicated by 14 and 13, respectively. a and b describe the presence of two repeat units.
Further analysis on UG-elements in other organisms along with their trans-factors will shed some light on their ubiquity and the diversity of their biological functions.
Acknowledgments
This paper is dedicated to J. W. Hastings on his 70th birthday. I thank A. Esen, T. Roenneberg, and W. Rüdiger for helpful comments on the manuscript. The work was supported by a habilitation fellowship Mi373/2-1 and Mi373/2-2 to M.M., both from the Deutsche Forschungsgemeinschaft.
Footnotes
Abbreviations: LBP, luciferin-binding protein; CCTR, circadian-controlled translational regulator; LD, light/dark; LL, constant dim light.
References
- 1.Kondo T, Strayer C A, Kulkarni R D, Taylor W, Ishiura M, Golden S S, Johnson C H. Proc Natl Acad Sci USA. 1993;90:5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardin P E, Hall J C, Rosbash M. Molecular Genetics of Biological Rhythms. New York: Dekker; 1993. pp. 155–170. [Google Scholar]
- 3.Myers M P, Wager-Smith K, Wesley C S, Young M W, Sehgal A. Science. 1995;270:805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 4.Zeng H, Qian Z, Myers M P, Rosbash M. Nature (London) 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- 5.Dunlap J C. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- 6.Crosthwaite S K, Loros J J, Dunlap J C. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 7.Loros J J, Denome S A, Dunlap J C. Science. 1989;243:385–588. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen H, Bogorad L. Plant Physiol. 1988;88:1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millar A J, Kay S A. Plant Cell. 1991;3:541–550. doi: 10.1105/tpc.3.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse D, Milos P M, Roux E, Hastings J W. Proc Natl Acad Sci USA. 1989;86:172–176. doi: 10.1073/pnas.86.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse D, Fritz L, Hastings J W. Trends Biochem Sci. 1990;15:262–265. doi: 10.1016/0968-0004(90)90050-l. [DOI] [PubMed] [Google Scholar]
- 12.Loros J J, Dunlap J C. Mol Cell Biol. 1991;11:558–563. doi: 10.1128/mcb.11.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell Pedersen D, Dunlap J C, Loros J J. Mol Cell Biol. 1996;16:513–521. doi: 10.1128/mcb.16.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teakle G R, Kay S A. Plant Mol Biol. 1996;29:1253–1266. doi: 10.1007/BF00020466. [DOI] [PubMed] [Google Scholar]
- 15.Mittag M, Lee D-H, Hastings J W. Proc Natl Acad Sci USA. 1994;91:5257–5261. doi: 10.1073/pnas.91.12.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittag M, Lee D-H, Hastings J W. Evolution of Circadian Clocks. Sapporo, Japan: Hokkaido Univ. Press; 1995. pp. 97–140. [Google Scholar]
- 17.Mittag M, Hastings J W. Physiol Plant. 1996;96:727–732. [Google Scholar]
- 18.Bruce V G. Protozoology. 1970;17:328–330. [Google Scholar]
- 19.Byrne T E, Wells M R, Johnson C H. Plant Physiol. 1992;98:879–886. doi: 10.1104/pp.98.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson C H, Kondo T, Goto K. Circadian Clocks from Cell to Human. Sapporo, Japan: Hokkaido Univ. Press; 1992. pp. 139–155. [Google Scholar]
- 21.Goto K, Johnson C H. J Cell Biol. 1995;129:1061–1069. doi: 10.1083/jcb.129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenaers G, Scholin C, Bhaud Y, Saint-Hilaire D, Herzog M. J Mol Evol. 1991;32:53–63. doi: 10.1007/BF02099929. [DOI] [PubMed] [Google Scholar]
- 23.Morse D, Salois P, Markovic P, Hastings J W. Science. 1995;268:1622–1624. doi: 10.1126/science.7777861. [DOI] [PubMed] [Google Scholar]
- 24.Harris E H. The Chlamydomonas Sourcebook. New York: Academic; 1989. [Google Scholar]
- 25.Rochaix J-D. Annu Rev Genet. 1995;29:209–230. doi: 10.1146/annurev.ge.29.120195.001233. [DOI] [PubMed] [Google Scholar]
- 26.Goodenough U W. Cell. 1992;70:533–538. doi: 10.1016/0092-8674(92)90424-b. [DOI] [PubMed] [Google Scholar]
- 27.Sueoka N, Chiang K S, Kates J R. J Mol Biol. 1967;25:47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee D-H, Mittag M, Sczekan S A, Morse D, Hastings J W. J Biol Chem. 1993;268:8842–8850. [PubMed] [Google Scholar]
- 29.Stone J M, Walker J C. Plant Physiol. 1995;108:451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson C H, Knight M R, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]


