Abstract
Purpose
In order to examine the roles of MMP-2 and MMP-9 in retinal neovascularization, the efficacy of three matrix metalloproteinase (MMP) inhibitors with varying selectivity, (Ro-31-9790, AG3340 and DPC-A37668) was investigated in a rat model of retinopathy of prematurity. The susceptibilities of MMP-2−/− and -9−/− mice to pre-retinal neovascularization were investigated in a mouse model of oxygen-induced retinopathy.
Methods
Sprague-Dawley newborn rats were exposed to alternating episodes of 50% and 10% oxygen (variable-oxygen exposure) to induce retinal neovascularization. Three MMP inhibitors with varying selectivity profiles were administered to variable oxygen-exposed rats via local or systemic routes. Anti-neovascular efficacy was determined in drug-treated versus vehicle-treated rat pups by computerized imaging of adenosine diphosphatase (ADPase) stained retinal flatmounts. Wild-type C57BL/6J and isogenic MMP-2−/− and MMP-9−/− mice were exposed to 75% oxygen followed by normoxia. The mice were sacrificed immediately prior to or after the normoxic exposure and eyes were either harvested for retinal dissection and flatmounting or were paraffin embedded and sectioned. Retinal vascular area and retinal neovascularization were assessed by adenosine diphosphatase staining of retinal flatmounts and by counting pre-retinal nuclei of haematoxylin and eosin stained retinal sections, respectively.
Results
Ro-31-9790, AG3340, and DPC-A37668 had no effect on normal development of the rat retinal vasculature regardless of dose or route of administration. Intravitreal injection of Ro-31-9790 (broad-spectrum) immediately after variable-oxygen exposure and 2 days post-exposure resulted in 78% and 82% inhibition of retinal neovascularization, respectively. AG3340 (MMP-2 and MMP-9 selective inhibitor) and DPC-A37668 (MMP-2 selective inhibitor) resulted in 65% and 52% inhibition when administered by intravitreal injection immediately after variable-oxygen exposure. Intraperitoneal injection of 5, 15 and 50 mg/ml AG3340 or DPC-A37668 for 6 days after variable oxygen exposure resulted in 22%–39% and 0–31% inhibition of neovascularization, respectively. AG3340 and DPC-A37668 administered by oral gavage at doses of 3, 10 or 30 mg/ml provided up to 42% and 86% inhibition of neovascularization, respectively. The average vascular areas of retinas from MMP-2−/− or –9−/− mice at post-natal day 12 were not significantly different from wild-type controls. There was a 75% (p<0.001) and 44% (p<0.01) reduction in pre-retinal neovascularization in oxygen-exposed MMP-2−/− and -9−/− mice at post-natal day 19, respectively, compared to wild-type controls.
Conclusions
The results of this study suggest that MMP-2 plays a predominant role in retinal angiogenesis in both the mouse and rat models of oxygen-induced retinopathy. Furthermore, MMP-2 inhibition may be a viable therapeutic approach for ocular diseases characterized by retinal neovascularization.
Introduction
The term angiogenesis refers to the growth of new capillaries from pre-existing blood vessels. The initial events of angiogenesis involve proteolytic basement membrane degradation; extracellular matrix remodeling; and endothelial cell (EC) proliferation, migration and differentiation. The integration of these events in physiologic angiogenesis involves complex interactions between cells, growth factors, cytokines and extracellular matrix components.1
Matrix metalloproteinases (MMPs) comprise a family of proteolytic enzymes of more than 20 members that are zinc- and calcium dependent. Most MMPs are secreted in the inactive pro-enzyme form, some of them by endothelial cells of the angiogenic phenotype.2 MMP pro-enzymes are activated, in part, by the plasminogen activator (PA) system, giving rise to active forms that digest and remodel the basement membrane and extracellular matrix.1,3 Plasminogen is processed by urokinase-type plasminogen activator (uPA) in tissues to produce plasmin. Once formed, plasmin can cleave inactive MMP pro-enzymes, giving rise to active forms that are regulated by tissue inhibitors of metalloproteinases (TIMPs).4 The regulation of plasmin formation is multifactoral and tissue-dependent. Tissue-type plasminogen activator (tPA) also facilitates the formation of plasmin3,5 and is responsible for the fibrinolytic activity that promotes the dissolution of blood clots.6 Although tPA is secreted by established vessels,7 studies using a guinea pig corneal neovascularization (NV) model demonstrated that endothelial cells in new vessel sprouts secrete uPA exclusively.8 uPA and tPA activities are rigidly controlled by plasminogen activator inhibitor (PAI-1).9
MMP-2 and -9 degrade gelatin; elastin; and collagens IV (a major basement membrane component), V, VII, and X.2 MMP-2 and -9 are likely involved in tumor angiogenesis10–12 and recent studies indicate that MMP-2 and -9 are critical for NV in the posterior segment of the eye. For example, experiments with mouse MMP-2 deletion mutants suggest that MMP-2 plays a role in choroidal NV.2
Retinopathy of prematurity (ROP) is a potentially blinding disease of premature infants and results from exposure to the elevated oxygen levels used to compensate for underdeveloped lung function.13 ROP has been linked to variable systemic oxygen levels during the course of oxygen therapy and relative retinal hypoxia after oxygen therapy.13, 14 The pathogenesis of ROP involves dysregulated angiogenesis. Pre-retinal NV arises from the retinal capillary bed and penetrates the internal limiting membrane extending into the vitreous to form “neovascular tufts”, where tuft number, size and location reflect the severity of disease.15 The growth of neovascular tufts predispose affected infants to the sight-threatening complications of vitreous hemorrhage and tractional retinal detachment.16
Surgical intervention with laser or cryotherapy is a treatment option for ROP; however, functional outcomes are often sub-optimal.17, 18 To enhance the management of ROP, the development of a pharmaceutical agent that prevents pathologic angiogenesis and simultaneously allows continued development of the intra-retinal vasculature becomes an important objective.19 In addition, successful therapies developed to manage ROP might be applicable to other ocular conditions in which NV plays a critical role.
Our laboratory has successfully developed a rat model of oxygen-induced retinopathy (OIR) that mimics human ROP by exposing newborn rat pups to alternating episodes of hyperoxia and hypoxia.20 The advantages of this model are: 1) the retinal pathology has been extensively characterized; 2) the severity of pathology can be manipulated by adjusting the oxygen exposure profiles; 3) the severity of the pathology is reproducible between rats treated with the same exposure protocol; and 4) the pattern of preretinal NV is similar to that exhibited by infants in whom ROP developed.
In a previous study, intravitreal injection of recombinant PAI-1 provided remarkable efficacy against retinal NV in the rat, presumably by the direct inhibition of uPA.21 Although therapeutic strategies directed at upstream angiogenic targets are being pursued clinically, this approach can have the disadvantage of lack of specificity, thereby, affecting a broad range of MMP activities related to physiologic processes. We sought to improve potential clinical utility of proteinase inhibition by targeting MMPs that are likely to play a role in pathologic retinal angiogenesis. Therefore, the efficacies of the following compounds were evaluated in the rat model of ROP: 1) a broad-spectrum MMP inhibitor, Ro 31-9790; 2) a MMP-2- and MMP-9-selective inhibitor, AG3340; and 3) a MMP-2-selective inhibitor, DPC-A37668. Different routes of administration and different doses were investigated. One of these compounds, AG3340, has been evaluated in human clinical trials for both oncologic and ophthalmic indications. Although early data appeared promising, oral administration studies using AG3340 in patients with subfoveal choroidal neovascularization associated with age-related macular degeneration were halted during phase II due to joint swelling and stiffness in the patients receiving the drug.22
There are uncertainties associated with the specific and/or non-specific effects of pharmacological inhibitors tested in animal models of disease. To address this issue, the susceptibilities of MMP-2−/− and -9−/− mice to pre-retinal NV were investigated in a mouse model of OIR. The results of these experiments were compared to those obtained from the MMP inhibitor experiments conducted with the rat model of ROP in this study.
Materials and Methods
Oxygen Treatment
The Vanderbilt University School of Medicine Animal Care and Use Committee approved experiments with rats and mice, and they were conducted according to the principles expressed in the ARVO statement for the use of animals in ophthalmic and vision research.
Rats
Randomized litters of Sprague-Dawley rats were maintained in variable-oxygen (VO) or room air (RA) environments. VO rats were placed with mothers into infant incubators within 4 hours after birth. VO rats were cycled between alternating periods of 24 hrs at 50% oxygen followed by 24 hrs at 10% oxygen, for 14 days. After the 14-day oxygen treatment protocol, the VO rats were moved to room air.
Mice
Seven days after birth (P7), litters of C57BJ/6L wild-type mice and isogenic MMP-2−/− and MMP-9−/− mice (generous gifts from Dr. Lynn Matrisian, Vanderbilt University) were exposed to 75% oxygen for 5 days until post-natal day 12 (P12). Immediately after exposure, they were brought to room air for 7 days until P19.23
Intravitreal Injections
Rats were anesthetized by methoxyflurane (Pitman-Moore; Mundelein, IL) inhalation and a single drop of 0.5% proparacaine (Allergan; Hormigueros, PR) was topically applied to the cornea immediately prior to intravitreal injection. During all intravitreal injections, the globe was penetrated posterior to the ora ciliaris retinal using a 30-gauge needle with a 19° bevel and a 10 μl syringe (Hamilton Co.; Reno, NV). The needle was advanced to the posterior vitreous while maintaining a steep angle to avoid contact with the lens. The injection bolus (5μl) was delivered near the trunk of the hyaloid artery proximal to the posterior pole of the retina. Following injection, a topical antibiotic suspension (neomycin and polymyxin B sulfates and gramicidin; Monarch Pharmaceuticals; Bristol, TN) was applied. Non-injected eyes were also treated with topical proparacaine and antibiotic to control for the potential of these agents to influence retinal vessel growth.
Drug Treatment
Ro 31-9790: Broad spectrum MMP inhibitor
Eyes from VO rats were injected with 5 μl of Ro 31-9790 (Roche Diagnostics Corporation, Indianapolis, IN) at a 150 μg dose immediately upon removal from the oxygen exposure chamber or two days after removal to room air, which is the time of peak VEGF expression (hereafter referred to as days 14(0) and 14(2), respectively). This dose was determined from a preliminary, dose-response experiment (neovascular areas: vehicle = 2.17 mm2, 0.03 mg/ml = 1.90 mm2, 0.3 mg/ml = 1.63 mm2, 3.0 mg/ml = 1.0 mm2, 30.0 mg/ml = 0.5 mm2). The chemical structure of Ro 31-9790 is shown in Figure 1 with the Ki values in Table 1.25 Control eyes from VO rats were either not injected or were injected with vehicle [0.2% carboxymethylcellulose (CMC; Sigma Chemical Co., St. Louis Mo.) and 0.01% Tween 20 (Sigma Chemical Co., St. Louis Mo.)] on 14(0) or 14(2).
FIGURE 1.
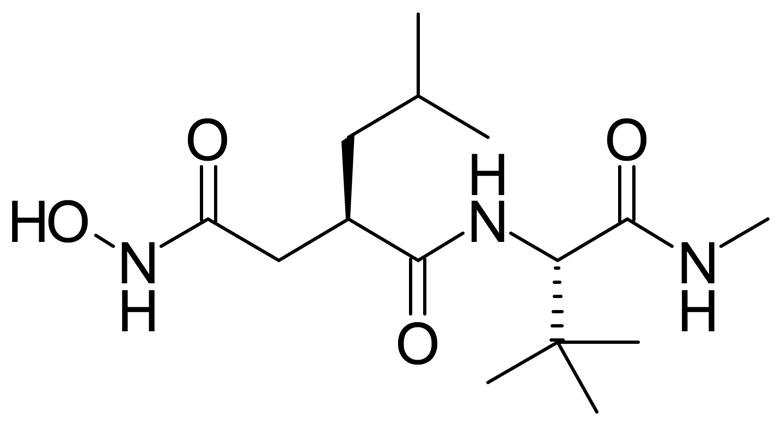
Chemical structure of Ro-31-9790
Table 1.
Ki values for the MMP inhibitors.
| Compound: | Ki values (nM)
|
Selectivity for MMP2 over MMP9 (fold) | ||
|---|---|---|---|---|
| MMP1 | MMP2 | MMP9 | ||
| Ro 31-9790 | 3 | 5.2 | 10.4 | 1 |
| AG 3340 | 8.3 | 0.05 | 0.26 | 5.2 |
| DPC-A37668 | 6500 | 0.48 | 255 | 53 |
AG3340: MMP-2- and -9-selective inhibitor
Eyes from VO rats were injected with 5μl of AG3340 (Alcon Laboratories, Inc., Fort Worth, Texas) at a dose of 150 μg on 14(0). Control eyes were either not injected or were injected with vehicle (0.2% CMC, 0.1% Tween 20).
Drug doses administered by intraperitoneal (IP) injections and oral gavage were calculated on a per kilogram body weight basis. VO rats received IP injections of either vehicle (0.4% CMC) or AG3340 at doses of 5 mg/kg, 15 mg/kg or 50 mg/kg. IP injections were administered daily for 6 days [14(0) –14(5)]. Some VO rats were not treated and served as controls.
For the oral gavage study, VO rats received either vehicle (0.4% CMC) or AG3340 at doses of 3 mg/kg, 10 mg/kg or 30 mg/kg per OS twice a day for 6 days [14(0) –14(5)]. Some VO rats were not treated in order to serve as controls. The chemical structure and MMP selectivity of AG3340 are presented in Figure 2 and Table 1.25
FIGURE 2.
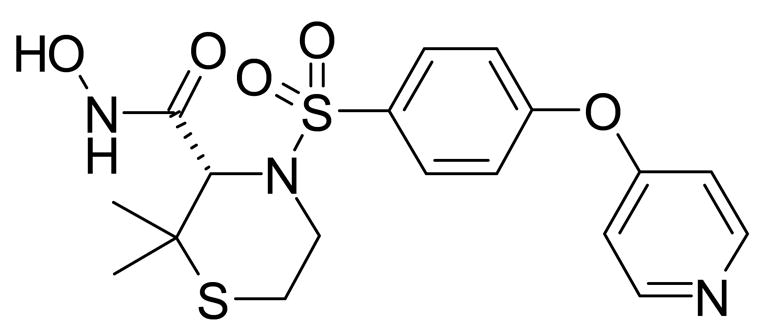
Chemical structure of AG3340
DPC-A37668: MMP-2-selective inhibitor
DPC-A37668 (Bristol-Myers Squibb Company, Princeton, New Jersey) or vehicle was administered by intravitreal injection (vehicle; 0.2% CMC, 0.1% Tween 20), intraperitoneal injection (vehicle; 0.4% CMC) and oral gavage (vehicle; 0.4% CMC) at identical doses and times as described for AG3340. Again, some VO rats served as non-treated controls. The chemical structure and MMP activity of DPC-A37668 is presented in Figure 3 and Table 1.26
FIGURE 3.
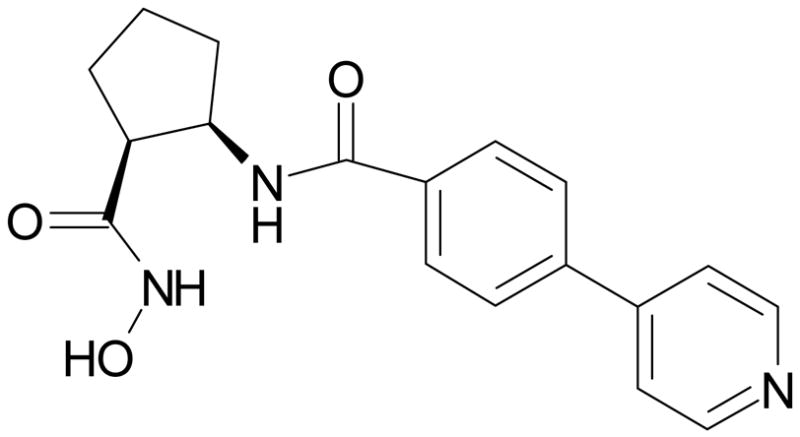
Chemical structure of DPC-A37668
Quantification of Retinopathy
VO rats were euthanized by decapitation at postnatal day 20 (P20), [ie, 6 days following removal to room air - 14(6)]. After hyperoxia treatment, mice were either euthanized by decapitation on P12 or on P19 after 7 days of normoxic exposure. Rats and mice were enucleated and the neural retinas were dissected and placed in cmf-PBS with 10% formaldehyde solution (37% formaldehyde solution; Fisher Scientific; Fair Lawn, NJ) also referred to as 10% neutral buffered formaldehyde (NBF), overnight at 4°C. The vasculature of retinas were stained using a histochemical method for detecting ADPase, according to a previously described method27 adapted for use here.28, 29
Images of ADPase-stained retinas were digitized, captured and displayed at 20x magnification. The total retinal area and the retinal area containing blood vessels was traced on the monitor face with an interactive stylus pen (FTG Data Systems; Stanton, CA).19 The number of pixels within this area was converted to mm2. Measurements of this parameter were recorded.
To determine the effect of the various treatments on pathological angiogenesis, the extent of retinal NV was assessed in flattened rat retinas stained for adenosine diphosphatase (ADPase) activity. Representative retinal flatmounts of vehicle and drug-treated rats dosed with DPC-A37668 by oral gavage are shown in Figure 4. Images of ADPase-stained retinas were digitized, captured and displayed at 65x magnification. Vessel tufts were then outlined directly on the monitor face with the stylus pen. The pixels contained within an encircled area were counted, the total number of pixels from all areas was summed and this value was converted to mm2. The pre-retinal nature of the tufts was confirmed by simultaneous assessment with a microscope at 200x magnification using the plane of focus. This method of estimation correlates well (r2 = 0.947) with the clock hour method of estimation20 and yields normally distributed data that allows for statistically significant differences between treatment groups to be determined by analysis of variance.
FIGURE 4.
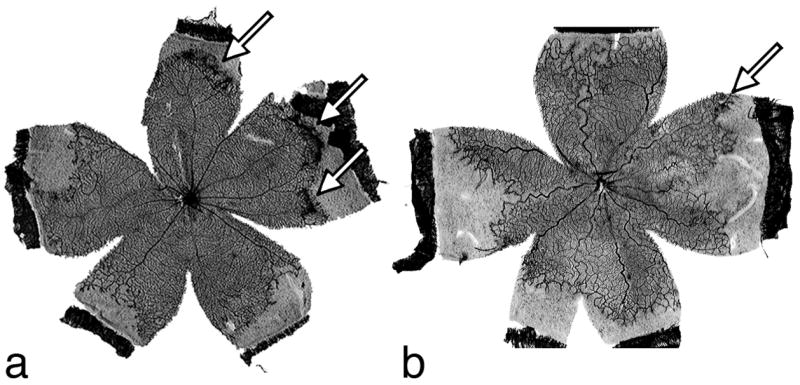
Representative retinal flatmounts from VO rats dosed with (A) vehicle and (B) DPC-A37668 by oral gavage. Arrows indicate areas of neovascularization.
Pre-retinal NV in mice was evaluated according to the method described by Smith et. al.23 Transverse meridianal retinal sections were prepared from paraffin embedded eyes and stained with periodic acid-Schiff (PAS) and hematoxylin. Pre-retinal NV was assessed by counting nuclei within the vitreous cavity of sections that were systematically chosen based on the distance from the optic nerve.
Gel Zymography
MMP-2−/−, MMP-9−/−, and wild type mice were exposed to the oxygen model described earlier. Mice were sacrificed at days P12 and P19, and two retinas were pooled for these mice as well as age-matched control mice raised in room air. The two retinas were then homogenized in 150 μl of extraction buffer (40 mM Tris-HCl, 110 mM Tris base (pH. 7.4), 150 mM NaCl, 5 mM CaCl, 5 mM MgCl2, and 1% Triton X-100) before flash-freezing. Samples were thawed and centrifuged at 20,800g for 8 minutes at 4°C. Protein concentration was measured in all samples with the BCA protein assay kit (Pierce), and an equivalent volume of each was affinitiy purified with an 8:1 ratio of sample volume to gelatin Sepharose 4B beads (Amersham Pharmacia Biotech, Piscataway, NJ) by incubation at 4°C with rocking for 1 hour. Samples were eluted in 40 μl of 2X Bio-rad zymogram sample buffer (Richmond, CA) plus 10% dimethyl sulfoxide (DMSO). A 20-μl aliquot of each sample was loaded on a 10-well, 10% gelatin zymography gel (Ready Gel; Bio-rad) with appropriate markers and controls. The gel was run for 90 minutes at 100 V in 1X Tris-glycine-SDS (20 mM Tris base, 200 mM glycine, 3 mM SDS). After incubation with shaking at 25°C in 1X zymogram renaturation buffer (Bio-Rad; 2.5% Triton X-100) for 45 minutes, the gel was left overnight (16–20 hours, optimally) at 37°C in 1X zymogram development buffer (50 mM Tris-HCl [pH 7.5], 200 mM NaCl, 5 mM CaCl2, 0.02% Brij-35; Bio-Rad). The gel was stained for 20 min in Coomassie blue stain (0.5% Coomassie blue R-250, 40% methanol, 10% acetic acid in distilled water), rinsed briefly in distilled water, and then destained for up to 2 hours in 40% methanol plus 10% acetic acid. Zones of clearing that corresponded to the presence of proteinases in the gel were quantified using image-analysis software (ImageJ, NIH, Bethesda, MD). The data are expressed as pixels per microgram protein (relative gelatinase activity).
Gelatinase Activity Assay
Upon removal from oxygen, rat pups were given an intraocular injection with one of the MMP inhibitors or vehicle and then sacrificed the following day. Retinas from these animals were collected and the protein was isolated in a lysis buffer solution (150 mM NaCl, 50 mM Tris HCl, 1 mM Sodium Orthovanidate, 1% Triton X-100, 0.1% SDS, Complete Mini proteinase inhibitor cocktail EDTA-free [Roche]). The proteins were then assayed for gelatinase activity using to an MMP gelatinase activity assay kit (Chemicon).
Statistical Analysis
Statistically significant differences in average MMP activities, average vascular and average neovascular areas between the treatment groups and control groups were determined by analysis of variance with a Bonferroni/Dunn post hoc procedure. p ≤ 0.05 was considered significant.
Results
Ro-31-9790: Broad spectrum inhibitor
Eyes from VO rats injected with Ro 31-9790 at a dose of 150 μg on 14(0) or 14(2), showed a 78% or 82% reduction in average retinal NV on 14(6), respectively, when compared to vehicle-injected control eyes (Table 2). There was no significant difference in average retinal neovascular areas between non-injected control eyes and vehicle-injected eyes at 14(6) for vehicle injections at 14(0); however, there was a significant difference between non-injected control eyes and vehicle-injected eyes at 14(6) for vehicle injections at 14(2) (p<0.01).There was no significant difference in average retinal vascular area between Ro 31-9790-injected, vehicle-injected and non-injected eyes at 14(6), for either of the injection times. These data are summarized in Table 2.
Table 2.
Summarized data for the 150 μg intravitreal injection of Ro 31-9790 into rats.
| Treatment | n | Treatment Day | Area of Neovascularization
Mean ± S.D.2 (mm2) |
% Inhibition | P1 | Vascular Area
(% of total) |
|---|---|---|---|---|---|---|
| None | 12 | - | 2.5 ± 0.7 | - | - | 73 |
| Vehicle | 13 | 14(0) | 2.3 ± 0.6 | 70 | ||
| Ro 31-9790 | 13 | 14(0) | 0.5 ± 0.5 | 78 | <0.0005 | 70 |
| Vehicle | 8 | 14(2) | 1.7 ± 0.5 | 78 | ||
| Ro 31-9790 | 8 | 14(2) | 0.3 ± 0.4 | 82 | <0.0005 | 79 |
Compared to vehicle;
S.D.=standard deviation
AG3340: MMP-2- and -9-selective inhibitor
Intravitreal injection of VO rats with AG3340 at a dose of 150 μg on 14(0) showed a 65% reduction in average retinal neovascular area at 14(6) when compared to vehicle-injected control eyes (Table 3). There was no significant difference in average retinal neovascular area between the non-injected and vehicle-injected eyes. There was also no significant difference in average retinal vascular area between AG3340-injected, vehicle-injected and non-injected eyes at 14(6).
Table 3.
Summarized data for the treatment of rats with AG3340.
| Treatment | Route of administration | n | Area of Neovascularization
Mean ± S.D.2 (mm2) |
% Inhibition | P1 | Vascular Area
(% of total) |
|---|---|---|---|---|---|---|
| None | Intravitreal injection | 12 | 2.3 ± 1.2 | - | - | 72 |
| Vehicle | 12 | 1.8 ± 0.9 | 74 | |||
| AG3340 (150 μg) | 12 | 0.8 ± 0.5 | 65 | <0.005 | 70 | |
| None | Intraperitoneal injection | 7 | 2.5 ± 1.0 | - | - | 75 |
| Vehicle | 8 | 2.3 ± 0.5 | - | - | 75 | |
| 5 mg/ml AG3340 | 7 | 1.8 ± 0.8 | 22 | N.S. | 77 | |
| 15 mg/ml AG3340 | 5 | 1.5 ± 0.4 | 35 | <0.05 | 73 | |
| 50 mg/ml AG3340 | 5 | 1.4 ± 0.6 | 39 | <0.05 | 78 | |
| None | Oral gavage | 8 | 2.6 ± 1.2 | - | - | 73 |
| Vehicle | 6 | 2.6 ± 0.8 | - | - | 76 | |
| 3 mg/ml AG3340 | 6 | 2.5 ± 1.4 | 0 | N.S. | 72 | |
| 10 mg/ml AG3340 | 8 | 2.2 ± 0.8 | 15 | N.S. | 71 | |
| 30mg/ml AG3340 | 8 | 1.5 ± 0.7 | 42 | <0.02 | 72 |
Compared to vehicle;
S.D.=standard deviation
Intraperitoneal injection of VO rats with AG3340 daily for six days [14(0) through 14(5)] at doses of 5, 15 or 50 mg/ml resulted in no significant differences in average body mass and no significant reduction in average retinal vascular areas when compared to VO rats injected with vehicle by the same route or non-injected rats. There was also no significant difference in average retinal vascular area and average body mass between the non-injected and vehicle-injected groups. The average neovascular areas of the drug-injected groups on 14(6) correspond to a 22%, 35% and 39% inhibition of the vasoproliferative response for the low, mid and high doses, respectively, compared to vehicle-injected groups. No difference in average retinal neovascular area was observed between the vehicle-injected group and the non-injected group.
Administration of AG3340 by oral gavage twice a day for six days [14(0) through 14(5)] at doses of 3, 10 or 30 mg/ml to VO rats resulted in no significant differences in average body mass or average retinal vascular area when compared to vehicle and untreated groups. There was also no significant difference in average retinal vascular area and average body mass between the vehicle and untreated groups. The average neovascular areas of the drug-treated groups at 14(6) corresponded to a 0%, 15% and 42% inhibition of the vasoproliferative response for the low, mid and high doses, respectively, compared to the vehicle group. There was no significant difference in average retinal neovascular area between the untreated and the vehicle group. These data are summarized in Table 3.
DPC-A37668: MMP-2-specific inhibitor
Intravitreal injection of VO rats with DPC-A37668 at a dose of 150μg on 14(0) provided a significant 52% inhibition of the vasoproliferative response, compared to vehicle-injected eyes. There was also a significant difference in average retinal neovascular area between the non-injected eyes and vehicle-injected eyes (p<0.05). Intravitreal injection of DPC-A37668 on 14(0) did not result in a significant difference in retinal vascular area between groups of drug-injected, vehicle-injected and non-injected eyes (Table 4).
Table 4.
Summarized data for the treatment of rats with DPC-A37668.
| Treatment | Route of administration | n | Area of Neovascularization
Mean ± S.D.2 (mm2) |
% Inhibition | P1 | Vascular Area
(% of total) |
|---|---|---|---|---|---|---|
| None | Intravitreal injection | 8 | 2.8 ± 0.5 | 68 | ||
| Vehicle | 10 | 2.3 ± 0.6 | 74 | |||
| DPC-A37668 (150 μg) | 10 | 1.1 ± 0.4 | 52 | <0.005 | 72 | |
| None | Intraperitoneal injection | 7 | 2.6 ± 1.0 | - | 75 | |
| Vehicle | 9 | 2.6 ± 0.8 | - | 74 | ||
| 5 mg/ml DPC-A37668 | 8 | 2.6 ± 0.9 | 0 | N.S | 75 | |
| 15 mg/ml DPC-A37668 | 5 | 2.1 ± 0.5 | 17 | N.S | 73 | |
| 50 mg/ml DPC-A37668 | 5 | 1.8 ± 0.4 | 31 | <0.05 | 74 | |
| None | Oral gavage | 7 | 2.2 ± 1.0 | 70 | ||
| Vehicle | 9 | 2.1 ± 0.7 | 69 | |||
| 3 mg/ml DPC-A37668 | 7 | 2.0 ± 0.9 | 5 | N.S. | 69 | |
| 10 mg/ml DPC-A37668 | 7 | 1.4 ± 0.9 | 33 | <0.05 | 67 | |
| 30mg/ml DPC-A37668 | 9 | 0.3 ± 0.3 | 86 | <0.0005 | 71 |
Compared to vehicle;
S.D.=standard deviation
Intraperitoneal injection of DPC-A37668 daily for six days [14(0)–14(5)] at doses of 5 mg/ml, 15 mg/ml or 50 mg/ml resulted in no significant reduction in average retinal vascular area and no significant difference in average body mass when compared to the vehicle-injected and non-injected groups. There was also no significant difference in retinal vascular area and average body mass between the non-injected and the vehicle-injected groups. There was a 17% or 31% inhibition of the vasoproliferative response for the groups receiving the 15 or 50 mg/ml doses, respectively, when compared to the vehicle-injected group. The low dose had no effect on the vasoproliferative response, and there was no significant difference in the average retinal neovascular area between the non-injected and the vehicle-injected groups.
DPC-A37668 administered by oral gavage twice a day for 6 days [14(0)–14(5)] at doses of 3 mg/ml, 10 mg/ml or 30 mg/ml resulted in no significant reduction in average retinal vascular area and no significant differences in average body mass when compared to the vehicle and the non-treated groups. There was also no difference in average retinal area and average body mass between the non-treated and the vehicle group. The retinal neovascular areas of the groups receiving the 3 mg/ml, 10 mg/ml or 30 mg/ml doses corresponded to a 5%, 33% or 86% inhibition of the vasoproliferative response, respectively, when compared to the vehicle group. There was no significant difference in the average retinal neovascular area between the untreated group and the vehicle group. These data are summarized in Table 4.
MMP-2−/− and MMP-9−/− mice
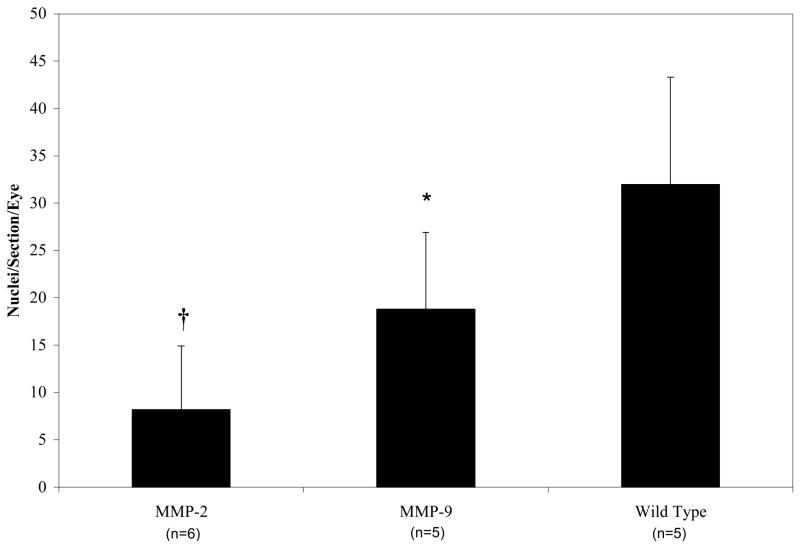
The average percent vascular areas (mean ± S.D.) of ADPase-stained mouse retinas at P12 were: 1) C57BJ/6L wild type (n=5), 78.03 ± 4.52%; 2) MMP-2−/− (n=12), 78.67 ± 3.52%; and 3) MMP-9−/− (n=4), 78.91 ± 4.52%. There were no significant differences in percent avascular area between any of the groups. Pre-retinal NV on P19, as measured by pre-retinal nuclei counts, is reported as follows: (mean ± S.D.): 1) wild type C57BJ/6L (n=5), 32.0 ± 11.3; 2) MMP-2−/− (n=6), 8.2 ± 6.7 (p<0.001); 3) MMP-9−/− (n=5), 18.8 ± 8.1(p<0.01). MMP-2 and MMP-9 gene deletion resulted in a 75% and 44% reduction in pathology respectively (Figure 5).
FIGURE 5.
Pre-nuclear counts of oxygen-treated mice (mean±standard deviation). *p<0.01, †p<0.001.
The zymography of the gelatinase deficient mice demonstrated that there was indeed a compensatory effect of the non-deficient MMP in room air mice. However, in the oxygen treated mice, any compensatory effect was absent or greatly reduced (as seen by the difference in MMP-2 of the MMP-9 deficient mice at P12 compared to wild type mice, which resolved by P19), strengthening support of the notion that the observed graded neovascular response of MMP-2 vs. MMP-9 non-knockouts is directly related to the deficient MMP. In these zymograms, activity of the gelatinase is indicated by the amount of the lower molecular weight form of the enzyme. Both of the genetically altered strains as well as the wild-type demonstrated an increase in gelatinase activity when comparing oxygen-treated animals to room-air raised animals. No MT1-MMP (MMP-14) levels were detected. These data are summarized in table 5.
Table 5.
Zymographic analysis of MMP-2 and -9 in knockout mice.
| MMP-2
|
MMP-9
|
|||
|---|---|---|---|---|
| Treatment | 72 kDa | 62 kDa | 92 kDa | 84 kDa |
| Wild Type | ||||
| Room Air Raised P12 | 4.10 ± 1.5 | ND | 6.95 ± 2.1 | ND |
| Room Air Raised P19 | 4.56 ± 1.8 | ND | 8.70 ± 1.3 | ND |
| Oxygen Treated P12 | 6.25 ± 2.5 | 1.38 ± 0.5 | 9.80 ± 0.3 | 0.66 ± 0.3 |
| Oxygen Treated P19 | 9.19 ± 1.6 | 4.66 ± 2.4 | 8.56 ± 0.7 | 0.74 ± 0.4 |
| MMP-2 −/− | ||||
| Room Air Raised P12 | ND | ND | 13.14 ± 1.8† | 0.39 ± 0.1† |
| Room Air Raised P19 | ND | ND | 15.62 ± 1.2† | 0.48 ± 0.1† |
| Oxygen Treated P12 | ND | ND | 10.89 ± 0.9 | 0.61 ± 0.1 |
| Oxygen Treated P19 | ND | ND | 9.02 ± 1.7 | 0.90 ± 0.2 |
| MMP-9 −/− | ||||
| Room Air Raised P12 | 7.30 ± 0.9† | 2.71 ± 1.0† | ND | ND |
| Room Air Raised P19 | 8.09 ± 1.7† | 2.43 ± 1.0† | ND | ND |
| Oxygen Treated P12 | 7.19 ± 0.5 | 3.67 ± 0.9† | ND | ND |
| Oxygen Treated P19 | 6.95 ± 1.3 | 4.15 ± 1.4 | ND | ND |
All values (N=4) expressed as pixels/microgram retinal protein.
ND Activity was below the level of detection
Sigificantly different from the same protein at the same time point in wild type retinas (P≤ 0.05).
Discussion
Multi-comparisons of the control and treatment groups shown in Tables 2–4 indicate no appreciable differences in average intraretinal vascular areas. These observations suggest that all of the compounds tested in this study, regardless of the MMP selectivity or specificity and route of administration, had no relevant effect on the normal retinal vascular development as seen by the measurements of vascular areas. Systemic administration of AG3340 or DPC-A37668 had no significant effect on weight gain when compared to the corresponding vehicle and untreated groups, indicating no toxicity within the dose range indicated (Tables 2–4).
Intravitreal injection of the Ro-31-9790 vehicle at day 14(2) and the DPC-A37668 vehicle at day 14(0), produced a significant reduction in retinal neovascular area when compared to eyes from VO rats that were not injected (p<0.01 and p<0.05, respectively). A consistent reduction in retinal neovascular area was observed for the rest of the intravitreal vehicle injection groups in this study; however, statistical significance was not achieved when compared to non-injected controls. Presumably, this anti-angiogenic effect is related to healing of the wound created by the needle puncture. Studies in our laboratory have shown that needle puncture of the globe upregulates several angiostatic proteins in the retina that may be responsible for attenuation of the vasoproliferative response.24
Intravitreal injection of the broad-spectrum inhibitor Ro-13-9790 at day 14(0) or day 14(2), produced a relatively small difference in percent inhibition of NV between the two injection times. These data suggest that MMP activity is critical to angiogenesis for at least 2 days after oxygen exposure. Intravitreal injection of the MMP-2- and -9 selective inhibitor AG3340 at day 14(0), resulted in 65% inhibition of NV. The inhibition constants (Ki values, Table 1) of Ro-31-9790 for MMP-2 and MMP- 9 are 5.2 nM and 10.4 nM compared to 0.05 nM and 0.26 nM for AG3340 respectively. 25, 26 The relative magnitudes of these Ki values suggest an approximate 100-fold and 40-fold greater inhibition of MMP-2 and MMP-9 by AG3340 in vitro. The relative magnitudes of the Ki values, and the modest decrease in percentage inhibition in retinal NV obtained for intravitreal injection of Ro-31-9790 from 78% to 65% for AG3340, suggests that the majority of pro-angiogenic MMP activity is contributed by MMP-2 and MMP-9. Although broad MMP inhibition may explain the increased inhibition observed following a single intravitreal injection of Ro-31-9790, it is also possible that the compound is acting through an MMP-independent mechanism and/or has increased bioavailability. Intravitreal injection of the MMP-2-selective inhibitor DPC-A37668 (150 μg) at day 14(0) (Ki = 0.48 nM)26 produced a 52% inhibition as compared to vehicle-injected controls. When viewed in light of the similar results with AG3340, this finding further suggests that MMP-2 plays a more dominant role than MMP-9 in retinal NV in this model. Another explanation for the reduced inhibition following intravitreal DPC-A37668 may be related to the 10-fold increase in Ki and/or limited bioavailability of DPC-A37668 as compared to AG3340.
Intraperitoneal injection of AG3340 or DPC-A37668 showed a dose-dependent increase in efficacy against retinal NV (Tables 2, 3). However, inhibition at the highest doses of i.p. AG3340 and DPC-A37668 (39% and 31%, respectively) were both substantially lower than that obtained following intravitreal injection of these compounds (65% and 52% respectively). These observations may be related to lower bioavailability to sites of retinal angiogenesis following intraperitoneal administration compared to intravitreal injection at these doses. The percent inhibition of NV at each dose of DPC-A37668 was lower than that for AG3340. As previously discussed in the context of intravitreal injection, these data may be accounted for in terms of MMP-2 selectivity vs. MMP-2 and MMP-9 selectivity, relative Ki values and differences in bioavailability between AG3340 and DPC-A37668 administered by intraperitoneal injection or a combination of these parameters.
Administration of AG3340 and DPC-A37668 by oral gavage produced a dose-dependent increase in the percent inhibition of NV compared to vehicle controls. The percent inhibition of NV at the highest dose of AG3340 was substantially lower than that obtained by intravitreal injection of this drug, again suggesting limited bioavailability by this route of administration at the doses tested. However, administration of the highest dose of DPC-A37668 gave a percent inhibition substantially higher than that obtained by intravitreal or intraperitoneal injection and comparable to that obtained by intravitreal injection of the broad-spectrum inhibitor Ro-31-9790. This suggests that high levels of bioavailable drug can be obtained at sites of retinal angiogenesis when this drug is repetitively dosed by oral gavage; moreover, bioavailability may be a dominant factor since the in vitro MMP-2 inhibition constant is ten-fold higher than that of AG3340. Since DPC-A37668 is MMP-2-selective, the relatively robust inhibition of NV suggests the important, perhaps exclusive, role of MMP-2 in the retinal angiogenic process in this disease model.
In a previous study, intravitreal injection of PAI-1 and vehicle into the eyes of VO rats resulted in a strong induction of the latent and active forms of MMP-9 as measured by analysis of zymograms,21 yet, these eyes showed the lowest degree of retinal NV relative to non-injected eyes with greater disease. Needle puncture of the globe may be responsible for this induction of MMP-9.21 Injection of PAI-1 demonstrated remarkable efficacy with respect to inhibition of retinal NV, and the activated form of MMP-2 in PAI-1 injected eyes was only one fourth of that measured in non-injected eyes of VO rats. These observations, as well as others suggest a more prominent role for MMP-2 in angiogenesis.30–34
Although there are well-documented differences in the pathologies associated with the rat ROP and the mouse OIR models, pre-retinal NV is a common component of the pathogenesis in both. When testing pharmacological inhibitors in animal models of disease, inherent ambiguities are associated with in vivo specific versus non-specific effects. To address this issue, we tested the susceptibilities of MMP-2−/− or -9−/− mice to the development of pre-retinal NV in a mouse model of OIR. MMP-2 deficiency produced a greater reduction in pre-retinal NV (75%) than MMP-9 deficiency (44%). A compensatory response of the non-deficient gelatinase in the genetically altered mice was found in the room-air raised animals. This compensation may have been absent or reduced in the oxygen-treated animals due to a maximal activity of these enzymes under the OIR conditions. However, even with a compensation of MMP-2 in the MMP-9 deficient mice at P12, this compensation did not overcome the removal of MMP-9, which resulted in a significant decrease in neovascularization compared wild type. This decrease was not as large as the decrease in the MMP-2 deficient mice further showing the importance of MMP-2 over that of MMP-9 in this model. Our rat experiments with pharmacological MMP inhibitors of variable selectivity/specificity also suggest that the dominant MMP activity in pre-retinal NV arises from MMP-2. Hence, our rat and mouse findings correlate well, and they point to the importance of MMP-2 activity in pre-retinal NV. However, a previous report indicated that an MMP-9−/− strain with a C57BJ/6L genetic background showed no difference in pre-retinal NV compared to the wild-type.35 The MMP-9−/− strain tested in our work is independent from the strain used by Ohno-Matsui et al., and the differences in susceptibility to OIR may be related to genetic variance resulting from differences in genome manipulation. Additionally, a report by Sarman et al. indicated no pathologic retinal angiogenic impairment in MMP-2-deficient mice.36 This direct discrepency is possibly the result of a mixed genetic background of their mice containing the 129 strain, which is known to have increased susceptibility to neovascularization.37 Also, their method of fluorescein-dextran infusion would not have detected much of the neovascular growth lacking patent, vascular lumena, which would have been shown by our ADPase staining.38 Without optimal assessment of neovascularization, much of the difference in wild-type and gelatinase-deficient mice could go unnoticed.
We did not measure any pharmacokinetic parameters for the drugs that were tested in this study. However, even with the uncertainties associated with bioavailability and the assumptions associated with correlating in vivo inhibition with in vitro Ki data,39,40 it is plausible the inhibition of MMP activity was directly responsible for the efficacy demonstrated in these experiments. Furthermore, the data presented in the current study suggest that specific inhibitors of MMP-2 may be sufficient to block a substantial proteolytic component of retinal NV in the rat. MMP-2-specific inhibition would be advantageous, as other MMP activities are likely to be required for the developing retina.21 The bioavailability of the drugs given as intravitreal injections should be nearly complete because of the drugs’ injection directly into the vitreous. The vitreous is not a barrier even to large proteins, meaning these molecules would have no barrier in their effects on the retina. The best test of this effect would be through zymography on the enzymes to see the inhibition of the gelatinase activity; however, the separation techniques of gel zymography isolate the drug from the enzymes preventing this inhibition. Therefore, an MMP gelatinase activity assay (Chemicon), specifically designed to measure the effect of MMP inhibitors on biological samples, was used showing a 35%, 40% and 58% reduction in gelatinase activity in comparison to vehicle-treated levels after treatment with DPC-AA3768, Ro-31-9790, or AG3340, respectively, as shown in figure 6. Additionally, despite the possibility of an MMP-independent pathway being inhibited in this process, both the genetic and pharmacologic manipulation of the matrix metalloproteinases rendered similar results, strongly supporting the bioavailability of the drugs and pharmacologic inhibition of the gelatinases in vivo.
FIGURE 6.
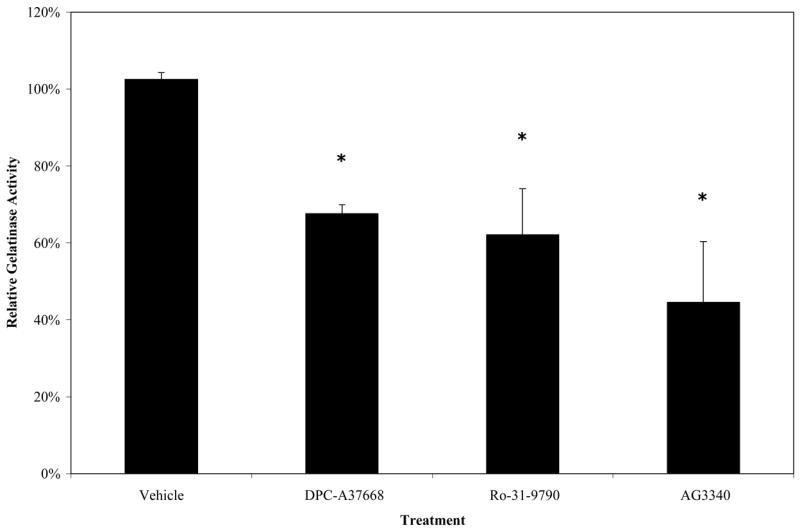
Effect of MMP inhibitor treatment on gelatinase activity levels one day after injection. *p<0.01.
VEGF is a potent endothelial cell mitogen, and it induces endothelial cell differentiation: both of these bioactivities are crucial to angiogenesis. As a result, VEGF, VEGF-receptors and downstream signaling intermediates have received considerable attention as chemotherapeutic targets.41–45 In this study, we have targeted MMP activity (extracellular matrix digestion) that is intrinsically linked to endothelial cell migration, an early event in the angiogenic process. It is likely that targeting only one component of the angiogenic process in the pathogenesis of ROP will not provide sufficient chemotherapeutic potential.21 Combination therapies targeting multiple components of tumor angiogenesis have proven successful,21 and a similar approach may be useful for ROP and other ocular conditions with an angiogenic component.
Acknowledgments
The authors would like to thank: Lynn Matrisian, Ph.D. for donating MMP-2−/− and MMP-9−/− mice; Rong Yang, M.D. and Xiang Q. Werdich, M.D., Ph.D. for performing retinal dissections.
Supported by NIH EY07533, NIH EY01826, Alcon Research, Ltd., a Challenge Grant from Research to Prevent Blindness, Inc., and a Research to Prevent Blindness Senior Scientific Investigator Award to JSP.
References
- 1.Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. [Review] [225 refs] Biochem Pharmacol. 2001;61(3):253–70. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 2.Berglin L, Sarman S, van der Ploeg I, Steen B, Ming Y, Itohara S, Seregard S, Kvanta A. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(1):403–8. doi: 10.1167/iovs.02-0180. [DOI] [PubMed] [Google Scholar]
- 3.Migatti P, Rifkin DB. Plasminogen activations and matrix metalloproteinase in angiogenesis. Enzyme Protein. 1996;49:117–37. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- 4.Loskutoff DJ, van Mourik JA, Erickson LA, Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natn Acad Sci. 1983;80(10):2956–60. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi F, Vassalli JD, Dano K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. [Review] [70 refs] J Cell Bio. 1987;104(4):801–4. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. [Review] [1000 refs] Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen P, Larsson LI, Nielsen LS, Grondahl-Hansen J, Andreasen PA, Dano K. Human endothelial cells contain one type of plasminogen activator. FEBS Letters. 1984;168(1):33–7. doi: 10.1016/0014-5793(84)80201-x. [DOI] [PubMed] [Google Scholar]
- 8.Jerdan JA, Kristensen P, Maglione A, Glaser BM. New blood vessel formation is associated with urokinase-type plasminogen activation (u-PA) Invest Opthalmol Vis Sci. 1988;29:109. [Google Scholar]
- 9.Saksela O. Plasminogen activation and regulation of pericellular proteolysis [Review] [373 refs] Biochimica et Biophysica Acta. 1985;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- 10.Soini Y, Hurskainen T, Hoyhtya M, Oikarinen A, Autio-Harmainen H. 72 KD and 92 KD type IV collagenase, type IV collagen, and laminin mRNAs in breast cancer: a study by in situ hybridization. J Histochem & Cytochem. 1994;42(7):945–51. doi: 10.1177/42.7.8014478. [DOI] [PubMed] [Google Scholar]
- 11.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58(5):1048–51. [PubMed] [Google Scholar]
- 12.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Bio. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24(2):82–7. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 14.Flynn JT, O’Grady GE, Herrera J, Kushner BJ, Cantolino S, Milam W. Retrolental fibroplasia: I. Clinical observations. Arch Ophthalmol. 1977;(2):217–23. doi: 10.1001/archopht.1977.04450020019002. [DOI] [PubMed] [Google Scholar]
- 15.Nelson I. Nelson Textbook of Pediatrics. Vol. 15. London: Saunders; 1996. Disorders of the Eye; p. 1790. [Google Scholar]
- 16.Foos RY. Chronic retinopathy of prematurity. Ophthalmol. 1985;92(4):563–74. doi: 10.1016/s0161-6420(85)34007-1. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. Multicenter trial of cryotherapy for retinopathy of prematurity. 3 1/2-year outcome--structure and function. Arch Ophthalmol. 1993;111(3):339–44. [PubMed] [Google Scholar]
- 18.Phelps DL. Retinopathy of prematurity [Review] [7 refs] Pediatr Rev. 1995;16(2):50–6. doi: 10.1542/pir.16-2-50. [DOI] [PubMed] [Google Scholar]
- 19.Penn JS, Rajaratnam VS, Collier RJ, Clark AF. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2001;42(1):283–90. [PubMed] [Google Scholar]
- 20.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36(6):724–31. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Penn JS, Rajaratnam VS. Inhibition of retinal neovascularization by intravitreal injection of human rPAI-1 in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44(12):5423–9. doi: 10.1167/iovs.02-0804. [DOI] [PubMed] [Google Scholar]
- 22.Blodi BA AG3340-Study-Group. Effects of prinomastat (AG3340), an angiogenesis inhibitor, in patients with subfoveal choroidal neovascularization associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42(4):S311. [Google Scholar]
- 23.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore P. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 24.Penn JS, McCollum GW, Barnett JM, Werdich XQ, Keopke KA, Rajaratnam VA. Angiostatic Effect of Penetrating Ocular Injury: Role of Pigment Epithelium-Derived Factor. Invest Ophthalmol Vis Sci. 2006;47(1):405–414. doi: 10.1167/iovs.05-0673. [DOI] [PubMed] [Google Scholar]
- 25.Personal communication; Keith Walker, Roche Diagnostics Corporation, Palo Alto, Ca.
- 26.Personal communication; David Bingaman, Alcon Research Laboratories, Ft. Worth, TX.
- 27.Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992;110(2):267–76. doi: 10.1001/archopht.1992.01080140123039. [DOI] [PubMed] [Google Scholar]
- 28.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34(3):576–85. [PubMed] [Google Scholar]
- 29.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36(10):2063–70. [PubMed] [Google Scholar]
- 30.Puyraimond A, Weitzman JB, Babiole E, Menashi S. Examining the relationship between the gelatinolytic balance and the invasive capacity of endothelial cells. J Cell Sci. 1999;112(Pt9):1283–90. doi: 10.1242/jcs.112.9.1283. [DOI] [PubMed] [Google Scholar]
- 31.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93(9):3064–73. [PubMed] [Google Scholar]
- 32.Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res. 2000;70(4):419–28. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- 33.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289(5482):1202–6. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 34.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natn Acad Sci. 1996;93(14):7069–74. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthamol Vis Sci. 2003;44(12):5370–5375. doi: 10.1167/iovs.03-0249. [DOI] [PubMed] [Google Scholar]
- 36.Sarman S, van der Ploeg I, Seregard S. Retinal Vascular Development and Pathologic Retinal Angiogenesis Are Not Impaired in Matrix Metalloproteinase-2 Deficient Mice. Curr Eye Res. 2005;30:259–267. doi: 10.1080/02713680590923212. [DOI] [PubMed] [Google Scholar]
- 37.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14(7):871–6. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 38.Penn JS, Henry MM. Assessing retinal neovascularization in an animal model of proliferative retinopathy. Microvasc Res. 1996;51:126–130. doi: 10.1006/mvre.1996.0014. [DOI] [PubMed] [Google Scholar]
- 39.Fayer JL, Zannikos PN, Stevens JC, Luo Y, Sidhu R, Kirkesseli S. Lack of correlation between in vitro inhibition of CYB3A-mediated metabolism by a PPAR-gamma agonist and its effect on he clinical pharmacokinetics of midazolam, and in vivo probe of CYP3A activity. J Clin Pharm. 2001;41:305–316. doi: 10.1177/00912700122010122. [DOI] [PubMed] [Google Scholar]
- 40.Maurer T, Fung HL. Comparison of methods for analyzing kinetic data from mechanism-based enzyme inactivation: application to nitric oxide synthase. Aaps Pharmsci. 2000;2(1):E8. doi: 10.1208/ps020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natn Acad Sci. 1995;92(23):10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natn Acad Sci. 1996;93(10):4851–6. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch of Ophthalmol. 1996;114(1):66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- 44.Danis RP, Bingaman DP, Jirousek M, Yang Y. Inhibition of intraocular neovascularization caused by retinal ischemia in pigs by PKC beta inhibition with LY333531. Invest Ophthalmol Vis Sci. 1998;39(1):171–9. [PubMed] [Google Scholar]
- 45.Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003;44(4):1722–31. doi: 10.1167/iovs.01-1193. [DOI] [PubMed] [Google Scholar]