Abstract
Distinct progenitor cell populations exist in cardiac mesoderm important for patterning of the heart. During heart tube formation in mouse, Tbx5 is expressed in progenitors located more laterally, whereas Isl1 and Fgf8 are expressed in progenitors located more medially. Signals that drive mesodermal progenitors into various cardiac lineages include Fgf8 which functions to induce Isl1. Studies in chick and zebrafish have shown that retinoic acid restricts the number of cardiac progenitors, but its role in mammalian cardiac development is unclear. Here, we demonstrate that Raldh2−/− mouse embryos lacking retinoic acid signaling exhibit a posterior expansion of the cardiac Fgf8 expression domain as well as an expansion of Isl1 expression into mesoderm lying posterior to the cardiac field. We provide evidence that retinoic acid acts specifically in the posterior-medial region of the cardiac field to establish the heart posterior boundary potentially by reducing Fgf8 expression which restricts the Isl1 domain.
Keywords: heart field, cardiac splanchnic mesoderm, retinoic acid signaling, FGF signaling, Isl1, Raldh2, Fgf8, Tbx5, mouse
INTRODUCTION
During early heart organogenesis, studies in vertebrate embryos have identified distinct progenitor cell populations in the splanchnic lateral plate mesoderm of the cardiac field that express unique combinations of transcription factors essential for heart development (Mjaatvedt et al., 2001; Waldo et al., 2001; Buckingham et al., 2005; Brade et al., 2007). Prior to heart tube formation in mouse it has been reported that Isl1 is expressed widely in cardiac progenitors (Prall et al., 2007), but later during heart tube formation Isl1 is expressed more medially (Cai et al., 2003) whereas Tbx5 is expressed more laterally in the cardiac crescent (Hochgreb et al., 2003; Ryckebusch et al., 2008). Genetic studies in mouse embryos indicate that a loss of Isl1 expression results in defects in the outflow tract, right ventricle, atria, and inflow tract (Cai et al., 2003), whereas a loss of Tbx5 expression results in sinoatrial defects and hypoplastic left ventricle (Bruneau et al., 2001). Thus, Tbx5 is important primarily for posterior heart development, whereas Isl1 seems to function all along the heart anteroposterior axis with the possible exception of the left ventricle. As mutations in cardiac transcription factors are associated with human congenital heart disease (Olson, 2006; Srivastava, 2006; Bruneau, 2008), further knowledge of how these genes are regulated during cardiac development may be important therapeutically. Also, multipotent progenitors expressing Isl1 have been observed in postnatal as well as embryonic heart, suggesting that Isl1+ cells may be useful in regenerative stem cell therapies for heart disease (Moretti et al., 2006; Laugwitz et al., 2008).
Early in the process of heart development, decisions are made that drive cardiac mesodermal progenitors down distinct pathways. Various overlapping heart fields (first, second, anterior) have recently been described marked by expression of various transcription factors such as Tbx5 (first heart field) or Isl1 and Fgf8 (second heart field); to avoid confusion it has been recommended that one refer to heart regions by the marker gene analyzed such as Tbx5+ or Isl1+ domains (Abu-Issa and Kirby, 2007). Factors that drive mesoderm to a cardiac fate are not well defined. However, recent studies on mouse embryos indicate that Fgf8 is required to drive splanchnic mesoderm and ventral pharyngeal mesodermal progenitors toward an Isl1+ fate, placing Fgf8 near the top of a cardiac signaling hierarchy (Ilagan et al., 2006; Park et al., 2006). As the vitamin A derivative retinoic acid (RA) has been found to repress Fgf8 expression (Del Corral et al., 2003; Sirbu and Duester, 2006) this suggested to us that RA may play a role in limiting the number of mesodermal progenitors achieving an Isl1+ cardiac fate via Fgf8 repression. Studies in zebrafish have demonstrated that RA limits the overall number of cardiac progenitor cells (Keegan et al., 2005) and chick studies demonstrate that RA limits the location of ventricular progenitors (Hochgreb et al., 2003), but the mechanism of RA action is unclear. RA directly controls gene expression by acting as a ligand for nuclear retinoic acid receptors that bind DNA regulatory elements (Mic et al., 2003). Support for Fgf8 as an RA target gene has come from the identification of a functional retinoic acid response element upstream of the Fgf8 promoter (Brondani et al., 2002), plus this regulatory element is conserved in the mouse, rat, and human Fgf8 genes (Balmer and Blomhoff, 2005).
An RA requirement for anteroposterior patterning of the heart tube has been demonstrated in vitamin A deficient quail embryos (Heine et al., 1985; Dersch and Zile, 1993) as well as mouse embryos unable to convert vitamin A to RA (Niederreither et al., 2001). Loss of RA synthesis in mouse embryos leads to a reduction posteriorly in the atria/inflow tract domain, plus the outflow tract/ventricular domain located more anteriorly forms an abnormal medial distended cavity rather than undergoing rightward looping and septation into right and left ventricles (Niederreither et al., 2001). During early organogenesis the first step of RA synthesis (oxidation of retinol to retinaldehyde) is catalyzed throughout the embryo by overlapping alcohol/retinol dehydrogenases encoded by Adh3, Adh4, and Rdh10 (Molotkov et al., 2002; Sandell et al., 2007), whereas the second step of RA synthesis (oxidation of retinaldehyde to RA) is catalyzed exclusively in trunk mesoderm by the tissue-specific retinaldehyde dehydrogenase encoded by Raldh2 (Aldh1a2) (Sirbu et al., 2005; Sirbu and Duester, 2006). In both mouse and chick embryos, Raldh2 is first expressed in the presomitic mesoderm, and then during the early somite stages a caudorostral wave of Raldh2 expression occurs in the lateral plate mesoderm up to a location just posterior to the cardiac field (Hochgreb et al., 2003). Although Raldh2 is likely to function in regulation of cardiac gene expression, the mechanism for how RA acts during early mouse heart development remains unclear as no target genes have been identified. In the studies reported here, we demonstrate that RA is required to limit the posterior extent of Isl1 expression in the cardiac field, thus helping to establish the posterior boundary of the heart. We provide evidence that RA performs this function through RA repression of Fgf8 in the posterior region of the cardiac field, thus limiting the action a potent inducer of Isl1.
RESULTS
RA Functions Specifically in the Posterior-Medial Region of the Heart Field
The early expression pattern of Raldh2 is consistent with secreted RA reaching only the posterior heart domain (atria/inflow tract) (Moss et al., 1998; Xavier-Neto et al., 2000; Hochgreb et al., 2003). Here, we confirm that Raldh2 expression in a wild-type mouse embryo (E8.25, 6-somite stage) is limited to somitic and lateral plate mesoderm lying just posterior to the cardiac crescent (Fig. 3F). Analysis of wild-type and Raldh2−/− mouse embryos carrying the RARE-lacZ RA-reporter transgene has shown that Raldh2 is responsible for all RA signaling in the heart at E8.75 (15-somite stage) (Mic et al., 2002), but analysis of RA signaling in wild-type embryos at earlier stages is necessary to determine where RA first acts during cardiac development. Here, we demonstrate that RA signaling at the cardiac crescent stage (6-somite stage) is limited to the posterior portion of the cardiac field as shown by RARE-lacZ expression in a wild-type embryo (Fig. 1C). These findings demonstrate that early cardiac RA activity is normally limited posteriorly to the atria/inflow tract region (thus absent anteriorly in the outflow tract/ventricular region), and that a loss of Raldh2 eliminates this RA activity.
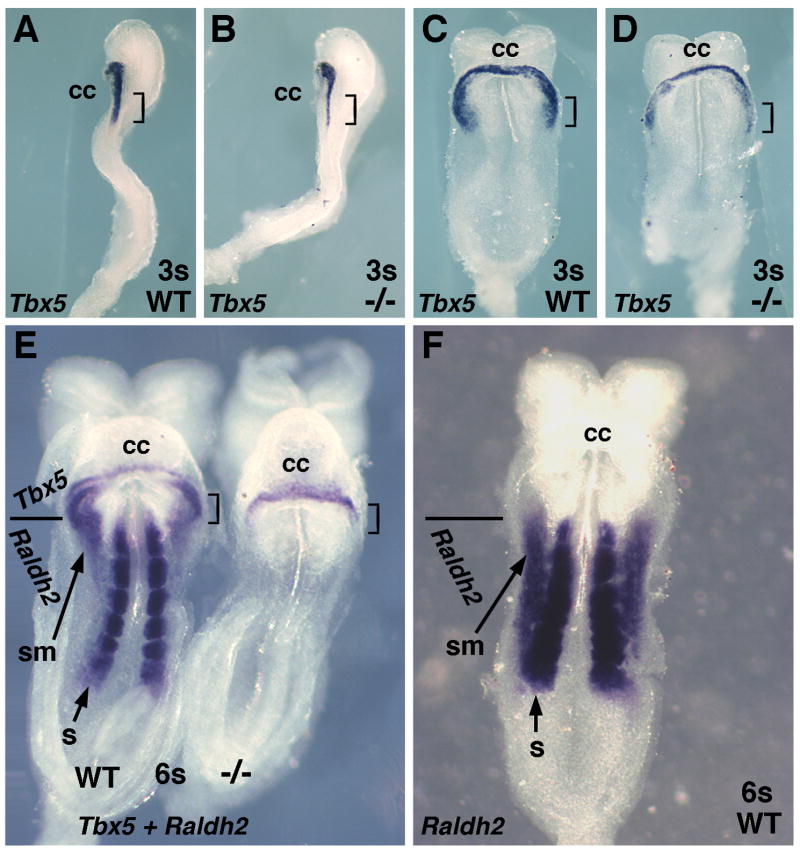
Fig. 3.
Loss of RA reduces the size of the Tbx5+ cardiac domain. (A–B) Tbx5 mRNA in 3-somite wild-type and Raldh2−/− embryos (lateral view); brackets point out reduced Tbx5 mRNA in posterior region of cardiac crescent in mutant. (C–D) Ventral view of 3-somite embryos shown in panels A and B. (E) Double-labeling of Tbx5 mRNA (anterior) and Raldh2 mRNA (posterior) in 6-somite wild-type and Raldh2−/− embryos (ventral view); mutant lacks Raldh2 mRNA; brackets identify reduction in posterior-lateral Tbx5 domain in mutant. (F) Detection of Raldh2 mRNA in a 6-somite wild-type embryo (compare to panel E). cc, cardiac crescent; s, somite; sm, splanchnic mesoderm.
Fig. 1.
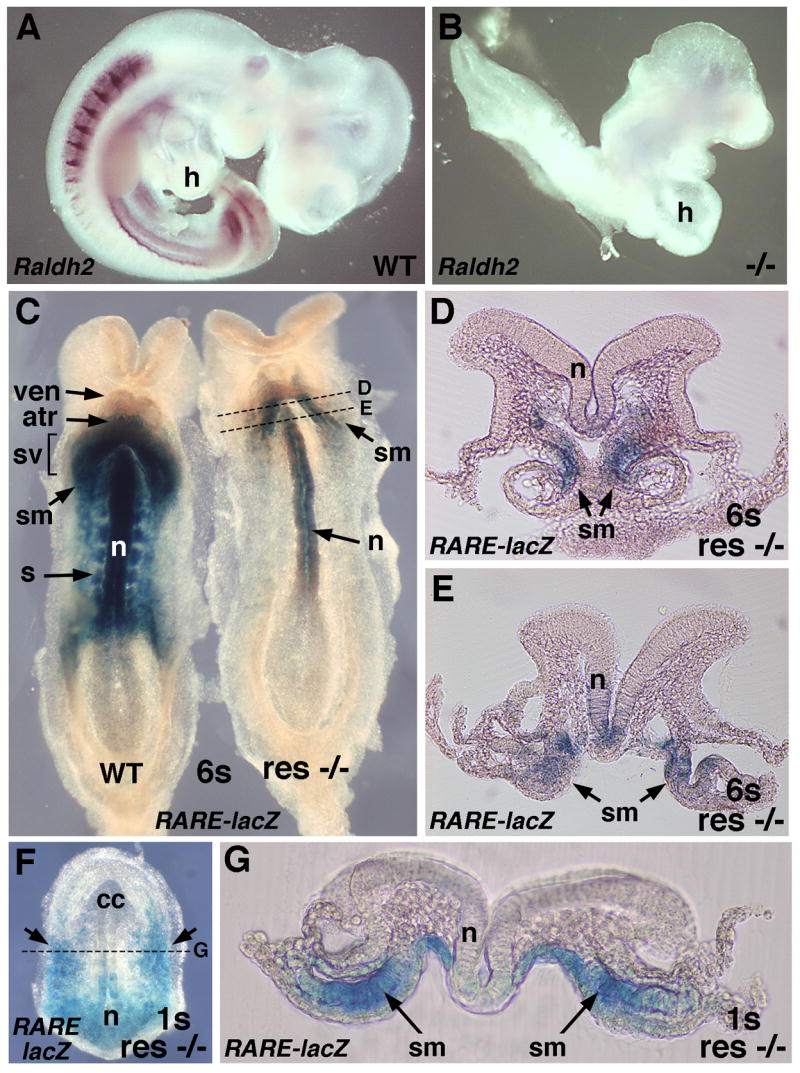
RA rescue targets the posterior-medial region of the heart field during heart tube formation. (A–B) Detection of Raldh2 mRNA in E9.5 wild-type (WT) and Raldh2−/− embryos; mutant lacks Raldh2 mRNA and exhibits a medial distended cardiac cavity. (C) RA activity in 6-somite wild-type and Raldh2−/− embryos carrying RARE-lacZ RA-reporter transgene following rescue by maternal dietary RA administration (ventral view); mutant exhibits RA activity only in the neuroectoderm and posterior-medial region of the heart field. (D–E) Serial transverse sections through the RA-rescued Raldh2−/− embryo indicated in panel C. (F) RA activity in 1-somite Raldh2−/− embryo rescued by maternal dietary RA administration (ventral view); RA activity is observed in the posterior region of the heart field and in the posterior neuroectoderm. (G) Transverse section through the RA-rescued Raldh2−/− embryo indicated in panel F. atr, atrial progenitor; cc, cardiac crescent; h, heart; n, neuroectoderm; s, somite; sm, splanchnic mesoderm (component of lateral plate mesoderm); sv, sinus venosa (inflow tract); ven, ventricular progenitor.
A comparison of E9.5 wild-type and Raldh2−/− embryos stained for Raldh2 mRNA demonstrates a complete absence of Raldh2 expression in the mutant which exhibits a heart tube with an abnormal medial distended cavity (Fig. 1A–B); this abnormal Raldh2−/− heart cavity has previously been demonstrated to exhibit a significantly reduced atria/inflow tract domain plus an enlarged outflow tract/ventricular domain that fails to undergo rightward looping and fails to septate into right and left ventricles (Niederreither et al., 2001). Maternal administration of RA at a low dose is known to rescue these Raldh2−/− cardiac patterning defects (Niederreither et al., 2001; Mic et al., 2002); this treatment provides less RA activity than that normally observed in wild-type embryos (Mic et al., 2003). We previously reported that low-dose maternal dietary RA administration from E6.75–E8.25 is sufficient to rescue early somite and cardiac defects observed in Raldh2−/− embryos, plus analysis of rescued mutants carrying RARE-lacZ can be used to identify where RA signaling acts during embryonic rescue (Sirbu and Duester, 2006). In those studies, we found that RA activity in rescued Raldh2−/− embryos at the 1–6 somite stages occurs in the posterior neuroectoderm, node ectoderm, and anterior mesoderm (near the cardiac field), but not in the somitic or presomitic mesoderm where Raldh2 is normally expressed indicating that some tissues respond to dietary RA whereas others do not (we provided evidence for RA-binding proteins that sequester RA at critical target sites). We have now further characterized RA activity in anterior mesoderm and report that RA-rescued Raldh2−/− embryos at the 1–6 somite stages exhibit RARE-lacZ expression in the posterior region of the cardiac crescent but limited to a medial domain (Fig. 1C; n = 6/6). Thus, RA activity in rescued Raldh2−/− embryos was not observed throughout the lateral-medial extent of the posterior cardiac crescent (as seen in a 6-somite wild-type embryo; Fig. 1C), but was instead focused on the medial region where the previously described Isl1+ domain exists (Cai et al., 2003). Transverse sections of a rescued 6-somite embryo confirmed that cardiac RA activity was confined to the medial splanchnic mesoderm (Fig. 1D–E). Wild-type embryos as early as the 1-somite stage have previously been shown to exhibit an anterior boundary of RA activity (RARE-lacZ expression) at the level of the posterior cardiac field (Sirbu et al., 2005). RA activity in a 1-somite stage RA-rescued Raldh2−/− embryo was also observed in the posterior heart field, and transverse sections revealed that RA was limited to the medial splanchnic mesoderm (Fig. 1F–G). The RA activity we detect in RA-rescued Raldh2−/− embryos at 1–6 somites has very little if any overlap with the lateral cardiac domain where Tbx5 is expressed (Ryckebusch et al., 2008). Our mutant rescue studies indicate that RA signaling activity in posterior-medial cardiac mesoderm is sufficient for heart tube patterning.
Loss of RA Signaling Expands the Isl1+ Cardiac Domain Posteriorly
As our RA rescue studies indicate that cardiac RA signaling is required medially where Isl1+ progenitors exist, we examined Raldh2−/− embryos to determine whether the absence of RA affected Isl1 expression in the cardiac crescent. Raldh2−/− embryos exhibit a marked increase in Isl1 expression in the cardiac field which begins to be detectable at the 3-somite stage and becomes increasingly evident by the 5-somite stage (Fig. 2A–D; n = 5/5). The observed expansion in Raldh2−/−cardiac Isl1 expression occurs in the posterior region of the developing heart tube, and extends posteriorly into splanchnic mesoderm not normally marked by Isl1 expression. Examination of Isl1 expression in Raldh2−/− embryos at 6-somites revealed further posterior expansion of Isl1 expression into non-cardiac mesoderm that does not express Isl1 in wild-type embryos (Fig. 2G–H; n = 2/2); also see transverse sections (Fig. 2K–N). These findings indicate that RA signaling is required to limit expansion of the cardiac Isl1+ domain along the anteroposterior axis.
Fig. 2.
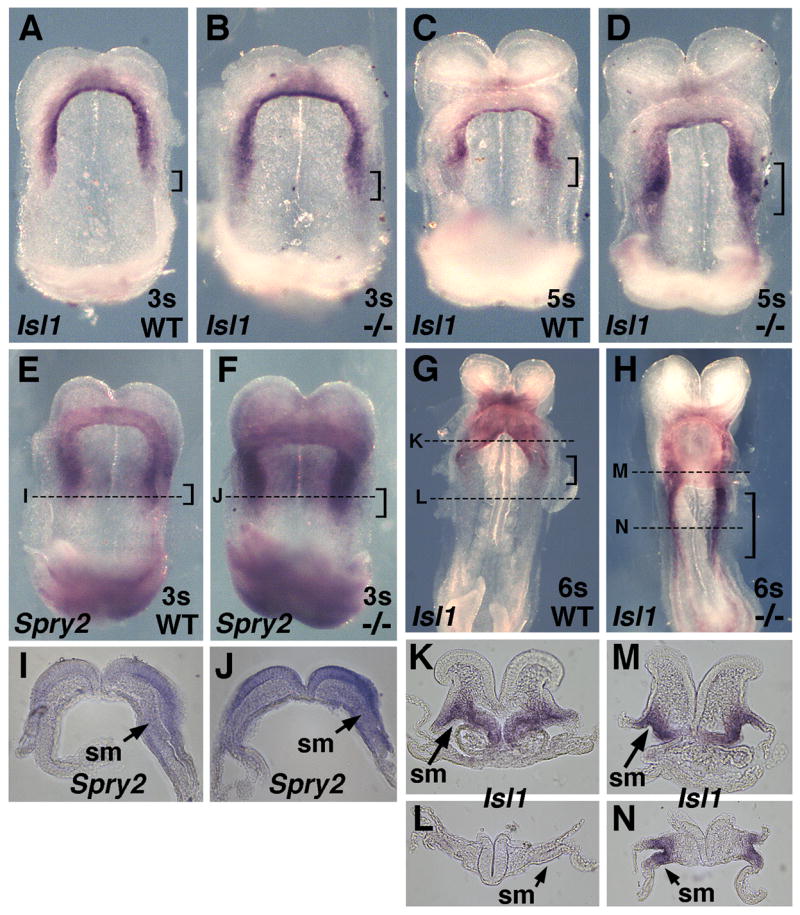
Isl1+ cardiac domain expands posteriorly after loss of RA. Isl1 mRNA in wild-type and Raldh2−/− embryos at (A–B) 3-somites, ventral view. and at (C–D) 5-somites, ventral view. Brackets indicate a marked increase in Isl1 mRNA in the posterior cardiac field of mutants. (E–F) Spry2 mRNA in 3-somite wild-type and Raldh2−/− embryos (ventral view) demonstrating a significant increase in posterior cardiac expression in the mutant (brackets); transverse sections shown in panels I and J. (G–H) Isl1 mRNA in wild-type and Raldh2−/− embryos at 6-somites (ventral view) with brackets pointing out posterior expansion of Isl1 mRNA in mutant; transverse sections shown in panels K–N. sm, splanchnic mesoderm.
Tbx5+ Cardiac Domain is Reduced in the Absence of RA Signaling
Previous molecular examination of mouse Raldh2−/− cardiac development (performed prior to reports of early cardiac Isl1 expression) revealed that expression of Nkx2.5, Mef2c, Hand2, and Gata4 are not significantly altered, but that a reduction in Tbx5 expression was noticed at E8.5–E9.0 (Niederreither et al., 2001). As our results suggest that RA signaling acts earlier than E8.5 (10 somites), we examined E8.0 embryos for the effect of a loss of RA on the Tbx5+ domain which is normally limited to a lateral location during heart tube formation (Hochgreb et al., 2003; Ryckebusch et al., 2008). Tbx5 expression in Raldh2−/− embryos ranging from 3–6 somites (E8.0–E8.25) was observed to be greatly reduced in the posterior-lateral region of the cardiac crescent (Fig. 3A–D; n = 4/4). Double-staining for Tbx5 and Raldh2 mRNAs at the 6-somite stage demonstrated a complete loss of Raldh2 mRNA in Raldh2−/− embryos along with a reduction in Tbx5 mRNA in the posterior-lateral region of the cardiac crescent (Fig. 3E). Comparison of a double-stained wild-type embryo (Fig. 3E) with an embryo stained for only Raldh2 mRNA (Fig. 3F), demonstrates that expression of Tbx5 and Raldh2 in the splanchnic mesoderm overlaps only slightly. A similar slight overlap of Tbx5 and Raldh2 expression was previously reported in sections of double-stained wild-type embryos which demonstrated that these two expression domains are largely distinct (Hochgreb et al., 2003; Ryckebusch et al., 2008). Also, we find that Raldh2 mRNA in paraxial mesoderm reaches a location no further anterior than the first somite which is located posterior to the cardiac crescent (Fig. 3E–F). These findings provide evidence that RA synthesized by RALDH2 in the splanchnic and somitic mesoderm located posterior to the heart field diffuses into the posterior heart field where it is needed to maintain high levels of Tbx5 expression. However, our RA rescue studies indicate that RA activity is not required in lateral cardiac mesoderm where Tbx5 is expressed, but only in medial cardiac mesoderm (Fig. 1C). Also, since Tbx5 is still expressed in the anterior portion of the Raldh2−/− cardiac crescent, it is unlikely that RA is needed instructively to induce Tbx5 expression. Thus, we suggest that RA is functioning in posterior-medial mesoderm as a permissive factor for posterior-lateral cardiac Tbx5 expression, potentially by regulating expression of another secreted factor.
RA is Required to Limit Fgf8 Expression and FGF Signaling in the Posterior Cardiac Field
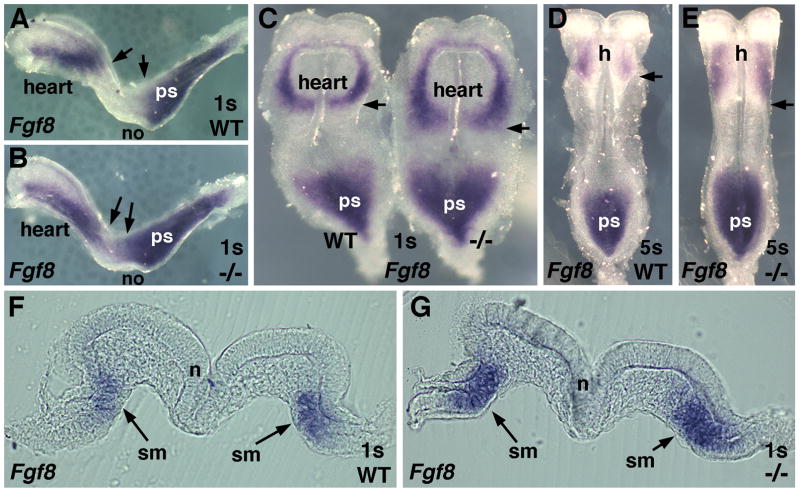
Recent studies indicate that Fgf8 is expressed at high levels in an early cardiac domain overlapping the Isl1+ domain, and that Fgf8 is required for induction of cardiac Isl1 expression (Ilagan et al., 2006; Park et al., 2006). We found that Raldh2−/− embryos exhibited a marked posterior expansion of Fgf8 expression in the developing heart at the 1-somite stage (Fig. 4A–B; n = 4/4); this is in addition to an anterior expansion of Fgf8 expression in the primitive streak region that we previously reported (Sirbu and Duester, 2006). A ventral view of 1-somite wild-type and Raldh2−/− embryos demonstrated that Fgf8 is normally expressed most highly in posterior-medial cardiac mesoderm (with decreasing expression as one proceeds anteriorly), and that the expanded cardiac Fgf8 expression domain in the mutant is a posterior extension of its normal domain in the cardiac crescent (Fig. 4C). At the 3–5 somite stages, the cardiac Fgf8 expression domain was still expanded posteriorly, although the overall level of expression was now much lower (Fig. 4D–E; n = 5/5). Transverse sections of 1-somite embryos through the posterior region of the heart demonstrated that Fgf8 mRNA in the heart field is normally localized to the medial splanchnic mesoderm and that Raldh2−/− embryos exhibit expanded Fgf8 mRNA in the splanchnic mesoderm relative to wild-type embryos at the same axial level (Fig. 4F–G). Thus, the expanded domain of Fgf8 expression observed in the absence of RA is located within the same posterior splanchnic mesodermal domain where RA was found to act during rescue of Raldh2−/− embryos (compare Fig. 1G and 4G).
Fig. 4.
Posterior expansion of cardiac Fgf8 expression following loss of RA signaling. (A–B) Detection of Fgf8 mRNA in 1-somite wild-type and Raldh2−/− embryos (lateral view); arrows point out posterior expansion of the cardiac domain and anterior expansion of the primitive streak domain in the mutant. (C) Ventral view of 1-somite embryos in panels A and B; note posterior expansion of Fgf8 mRNA in the heart field. (D–E) Detection of Fgf8 mRNA in 5-somite wild-type and Raldh2−/−embryos; arrows show posterior expansion in heart field. (F–G) Transverse sections of 1-somite wild-type and Raldh2−/− embryos through posterior region of Fgf8-positive cardiac domain (just below the label ‘heart’ in embryos shown in panel C). h, heart; n, neural tube; no, node; ps, primitive streak; s, somite; sm, splanchnic mesoderm.
As Fgf8 overexpression expands the cardiac field (Reifers et al., 2000; Alsan and Schultheiss, 2002) and Fgf8 loss-of-function reduces the mouse cardiac field and reduces Isl1 expression (Ilagan et al., 2006; Park et al., 2006), our studies suggest that RA may limit the size of the Isl1+ domain by limiting the amount of FGF8 signaling. To determine whether our observed increase in Fgf8 mRNA in the posterior region of the heart results in excessive FGF signaling, we examined expression of Sprouty2 (Spry2) which is induced in an Erk-dependent fashion by FGF signaling and functions to limit transmission of the signal resulting in phosphorylation of Erk1/2 (Minowada et al., 1999). Raldh2−/− embryos at the 3–5 somite stages exhibited a marked increase in Spry2 expression in the posterior heart field, overlapping the region where expanded expression of Fgf8 and Isl1 is observed (Fig. 2E–F; n = 3/3); transverse sections show increased Spry2 expression in the splanchnic mesoderm and neuroectoderm (Fig. 2I–J). Spry2 mRNA was also upregulated at the posterior end (primitive streak region) of Raldh2−/− embryos (Fig. 2E–F), consistent with the increase in Fgf8 expression in that region noted here (Fig. 4A–B) and reported previously (Sirbu and Duester, 2006). These results indicate that a loss of RA signaling leads to an increase in cardiac FGF8 signaling through the MAPK-Erk1/2 pathway, thus supporting a model in which expansion of the Isl1+ domain in Raldh2−/− embryos is due to increased FGF8 signaling.
RA Treatment Rescues Aberrant Expression of Fgf8 and Isl1 in Raldh2−/− Embryos
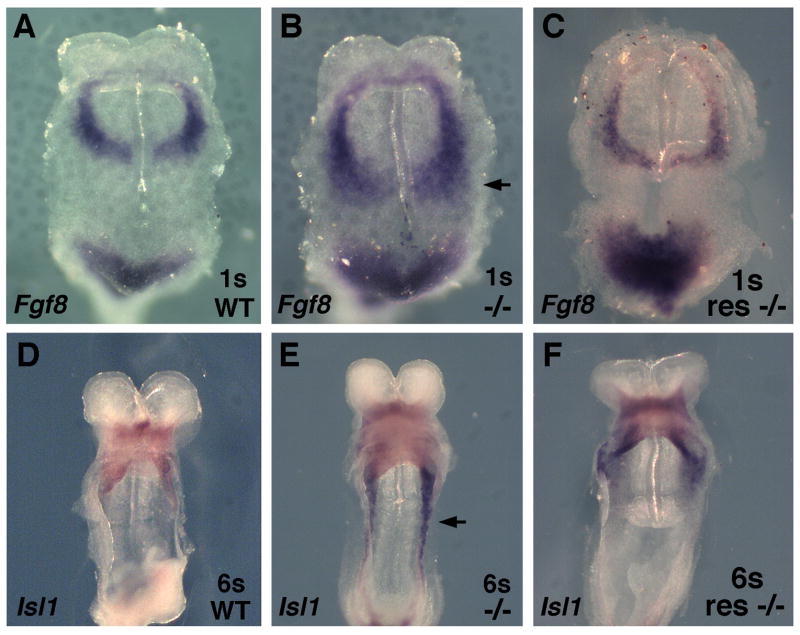
The studies detailed above suggest that RA treatment of Raldh2−/− embryos should repress Fgf8 and Isl1 expression. Raldh2−/− and wild-type embryos were collected from pregnant mice treated with a low dose dietary RA supplement from E6.75–E7.75 or E6.75–E8.25 or no RA as a control. At the 1-somite stage, untreated Raldh2−/− embryos exhibited a posterior expansion of Fgf8 mRNA compared to wild-type, whereas this posterior expansion was prevented in RA-treated Raldh2−/−embryos (Fig. 5A–C). Likewise, RA treatment of Raldh2−/− embryos at the 6-somite stage prevented the posterior expansion of Isl1 mRNA observed in untreated Raldh2−/− embryos (Fig. 5D–F).
Fig. 5.
RA treatment represses Fgf8 and Isl1 expression. (A–C) Fgf8 mRNA in 1-somite embryos, and (D–F) Isl1 mRNA in 6-somite embryos; all embryos are displayed from a ventral view to observe the heart field. The arrows indicate posterior expansion of Fgf8 or Isl1 expression in untreated Raldh2−/− embryos relative to wild-type, but this posterior expansion is prevented in Raldh2−/−embryos treated with maternal dietary RA supplementation (res −/−).
DISCUSSION
The findings reported here demonstrate that a lack of RA in mouse embryos changes the fate of mesodermal progenitors such that cardiac Isl1+ progenitors are expanded posteriorly by the 3-somite stage. Expansion of Isl1 expression in Raldh2−/− embryos is accompanied by an even earlier increase in cardiac Fgf8 expression by the 1-somite stage. Repression of Fgf8 is possibly the earliest role of RA signaling in cardiac development as it occurs by E7.75 (1-somite stage) just 6 hours after first onset of Raldh2 expression and RA signaling which occurs at E7.5 (Sirbu et al., 2005). Previous studies demonstrated that RA deficiency results in a decrease in posterior cardiac Tbx5+ progenitors observed at the 10-somite stage and later (Niederreither et al., 2001) and we demonstrate here that loss of posterior Tbx5 expression occurs by the 3-somite stage. Gene disruption studies in mouse have demonstrated crucial roles for Isl1 (Cai et al., 2003) and Tbx5 (Bruneau et al., 2001) in heart tube morphogenesis along the anteroposterior axis. Thus, the early changes in Isl1 and Tbx5 expression we observe in Raldh2−/− embryos could lead to significant alterations in anteroposterior patterning of the heart tube. Such changes in Raldh2−/− embryos may result in the previously observed cardiac malformations which include a smaller sinoatrial domain posteriorly (likely due to decreased Tbx5 expression) and an aberrant medial distended cavity located anteriorly where the ventricular/outflow tract domain normally resides (Niederreither et al., 2001). We suggest that the medial distended outflow tract/ventricular domain observed in Raldh2−/−embryos may be due to aberrant cardiac morphogenesis in response to an expanded population of Isl1+ progenitors following increased FGF8 signaling. After submission of this paper another article reported that Raldh2−/− embryos exhibit altered second heart field formation resulting in expanded expression of Isl1 and other second heart field markers, but that this expanded Isl1+ progenitor population was not able to differentiate into cardiomyocytes and likely contributed to the abnormal heart morphology observed (Ryckebusch et al., 2008).
Our studies have provided evidence that RA action during early heart patterning is normally limited to the posterior cardiac region, and we have demonstrated that a posterior-medial cardiac mesodermal cell population overlapping the Fgf8+ and Isl1+ domains (but not the lateral Tbx5+ domain) is the only mesoderm exhibiting RA activity during our low-dose RA rescue of Raldh2−/−cardiac patterning. These findings suggest that this posterior-medial domain represents the RA target tissue during early cardiac development and that it is required for proper anteroposterior patterning of the heart tube. Also, we provide evidence that Fgf8 expression in the posterior-medial cardiac field is an early target of RA signaling during heart development, and that excessive FGF8 signaling following a loss of RA signaling is associated with posterior expansion of the Isl1+ cardiac domain. Cardiac Fgf8 function is conserved as it is required for induction and expansion of the zebrafish heart (Reifers et al., 2000), and mouse genetic studies have uncovered an essential role for Fgf8 in upregulating Isl1 expression (Ilagan et al., 2006; Park et al., 2006). Previous studies in RA-deficient zebrafish embryos demonstrated that a loss of RA transforms non-cardiac mesoderm into cardiac mesoderm (Keegan et al., 2005). We propose that the general principle of RA limiting the allocation of mesoderm to a cardiac cell fate has been conserved from fish to mammal, with mammalian heart development employing RA signaling to limit the posterior extent of Isl1+ progenitors in lateral plate mesoderm. Our findings thus suggest that RA limits the number of cells achieving an Isl1+ fate by restricting expression of Fgf8, thus placing RA near the top of an important cardiac signaling hierarchy: RA –| FGF8 → Erk1/2 → Isl1. As our findings suggest that RA signaling does not function directly in the posterior-lateral cells expressing Tbx5, the ability of RA to stimulate Tbx5 expression may function permissively through RA downregulation of Fgf8 in posterior-medial cardiac mesoderm which may affect posterior-lateral cardiac mesoderm in a non-cell autonomous fashion. Further experiments are needed to determine if Tbx5 expression is repressed by FGF8 signaling.
There is no evidence that RA is required to induce expression of genes involved in early cardiac development (Niederreither et al., 2001). Instead, our studies indicate that RA plays a repressive role during cardiac development, by limiting the amount of FGF8 signaling posteriorly. The primary action of RA signaling during early heart tube formation may thus be downregulation of Fgf8 leading to reduced Isl1 expression needed to define the posterior limit of the cardiac field. This conclusion is supported by studies demonstrating the existence of a functional retinoic acid response element upstream of the Fgf8 promoter (Brondani et al., 2002). Also, other studies have demonstrated the existence of a mutual RA-FGF8 antagonism during posterior body axis extension (Del Corral et al., 2003), with RA generated by Raldh2 repressing Fgf8 in the epiblast (Sirbu and Duester, 2006) and FGF8 repressing Raldh2 expression in the presomitic mesoderm (Del Corral et al., 2003). Additionally, a reduction in FGF8 signaling through mutation of Tbx1 and Crkl leads to aortic arch and thymic defects in a mouse model of human del22q11 (DiGeorge) syndrome, and this loss results in higher Raldh2 expression and increased RA signaling; these defects can be rescued by a partial loss of Raldh2 function (Raldh2−/+) suggesting that Fgf8 normally functions to repress Raldh2 (Guris et al., 2006; Moon et al., 2006). As our findings indicate that Raldh2 represses Fgf8 in the posterior cardiac field, it appears that a mutual antagonism may exist between RA and FGF8 signaling during various stages of cardiac development. A disruption in RA-FGF8 antagonism during heart tube formation may thus represent the underlying molecular defect in some types of congenital heart disease.
EXPERIMENTAL PROCEDURES
Generation of Raldh2−/− Embryos and Conditional RA Rescue
A line of Raldh2+/− adult mice was described previously (Mic et al., 2002). Embryos derived from timed matings of Raldh2+/− mice were genotyped by PCR analysis of yolk sac DNA to identify Raldh2−/− embryos. Following mating, noon on the day of vaginal plug detection was considered embryonic day 0.5 (E0.5). Embryos were staged according to somite number. Rescue of Raldh2−/−embryos by maternal dietary RA supplementation was performed as previously described (Sirbu and Duester, 2006); the RA dose employed has been demonstrated to be in the normal physiological range (Mic et al., 2003). Briefly, all-trans-RA (Sigma Chemical Co.) was dissolved in corn oil and mixed with powdered mouse chow to provide a final concentration of 0.1 mg/g for treatment from E6.75–E8.25. Such food was prepared fresh each morning and evening and provided ad libitum. Embryos were analyzed at E8.0 and E8.25 when the mother was still on the RA-supplemented diet. All mouse studies conformed to the regulatory standards adopted by the Animal Research Committee at the Burnham Institute for Medical Research.
In situ Hybridization and Retinoic Acid Detection
Whole-mount in situ hybridization was performed as described previously to detect mRNA (Mic et al., 2002); wild-type and Raldh2−/− embryos were treated under identical hybridization conditions and stained for the same length of time. For double-labeling, two antisense RNA probes were used and detection was performed simultaneously. RA activity was detected by examining embryos carrying the RARE-lacZ RA-reporter transgene which places lacZ (encoding β-galactosidase) under the transcriptional control of a retinoic acid response element (RARE) (Rossant et al., 1991). β-galactosidase activity was detected using staining with X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) as previously described (Sirbu and Duester, 2006). Stained embryos were embedded in 3% agarose and sectioned at 30 mm with a vibratome.
Acknowledgments
We thank Pilar Ruiz-Lozano and Thomas Brade for helpful discussions. We are grateful to the following individuals for cDNAs used to prepare in situ hybridization probes: G. Martin (Fgf8, Spry2), V. Papaioannou (Tbx5), and P. Ruiz-Lozano (Isl1). We thank J. Rossant for providing RARE-lacZ mice. This work was funded by Deutsche Forschungsgemeinschaft grant Si1381/1-1 (I.O.S.) and National Institutes of Health grant GM062848 (G.D.).
Grant sponsor: The National Institutes of Health; Grant number: GM062848
References
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347–354. doi: 10.1016/j.jsbmb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Brade T, Gessert SMKh, Pandur P. The amphibian second heart field: Xenopus islet-1 is required for cardiovascular development. Dev Biol. 2007;311:297–310. doi: 10.1016/j.ydbio.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Brondani V, Klimkait T, Egly JM, Hamy F. Promoter of FGF8 reveals a unique regulation by unliganded RARa. J Mol Biol. 2002;319:715–728. doi: 10.1016/S0022-2836(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dersch H, Zile MH. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev Biol. 1993;160:424–433. doi: 10.1006/dbio.1993.1318. [DOI] [PubMed] [Google Scholar]
- Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006;10:81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Heine UI, Roberts AB, Munoz EF, Roche NS, Sporn MB. Effects of retinoid deficiency on the development of the heart and vascular system of the quail embryo. Virchows Arch B Cell Path. 1985;50:135–152. doi: 10.1007/BF02889897. [DOI] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CYI, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farrés J, Parés X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously-expressed alcohol dehydrogenase Adh3. Proc Natl Acad Sci USA. 2002;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo JH, Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Dräger UC, Rosenthal N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199:55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An nkx2–5/bmp2/smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers F, Walsh EC, Leger S, Stainier DY, Brand M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar) Development. 2000;127:225–235. doi: 10.1242/dev.127.2.225. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin S-C, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J, Shapiro MD, Houghton L, Rosenthal N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev Biol. 2000;219:129–141. doi: 10.1006/dbio.1999.9588. [DOI] [PubMed] [Google Scholar]