Abstract
BACKGROUND
The purpose was to evaluate the incidence and risk factors of thromboembolism associated with lenalidomide therapy in newly diagnosed myeloma.
METHODS
A pooled analysis was performed of patients with previously untreated multiple myeloma enrolled in clinical trials of lenalidomide-based therapy at the Mayo Clinic, Rochester, Minnesota, and the Italian Myeloma Network, Italy. The incidence of thrombosis, the effect of risk factors such as steroid dose and erythropoietin supplementation, and the effect of prophylaxis were examined.
RESULTS
In all, 125 patients enrolled in 3 clinical trials were identified. Patients were stratified based on the concomitant corticosteroid dose. Fifty-two patients were in the high-dose group (dexamethasone 40 mg, 12 days a month); 73 patients were in the low-dose group (prednisone at any dose; or dexamethasone 40 mg, 4 days a month). A total of 110 patients were initiated on thromboprophylaxis; of these, 104 patients (95%) received aspirin. Ten patients (8%) developed deep vein thrombosis, including 4 who were not receiving any thromboprophylaxis at the time of the event. The rate of thromboembolic events was not different between patients who received concomitant erythropoietin therapy and those who did not, 4.8% and 8.6%, respectively (P = .54). A higher number of venous thrombotic episodes occurred in the high-dose corticosteroid group compared with the low-dose corticosteroid therapy group (12% vs 6%), but the difference was not statistically significant (P = .3).
CONCLUSIONS
The incidence of deep vein thrombosis is lower than previously reported in the literature. There was a trend to a higher incidence of thrombosis in patients receiving high-dose corticosteroid therapy.
Keywords: multiple myeloma, lenalidomide, thrombosis, anticoagulation
Thromboembolic complications have been recognized for many years in patients with multiple myeloma (MM).1 Various mechanisms have been proposed to explain the increased risk, including defective fibrin structure and fibrinolysis due to increased immunoglobulin levels, the presence of autoantibodies like lupus anticoagulant, increased rates of acquired activated protein C resistance (aAPCR), and increased synthesis of inflammatory markers like interleukin (IL)-6.2,3 However, despite these procoagulant effects the incidence of thrombosis in myeloma was historically lower when compared with adenocarcinoma of the pancreas or lung. It was only after thalidomide was introduced in the late 1990s that the venous thromboembolic complications in MM gained prominence.4,5
Thalidomide monotherapy is associated with low rates of thrombosis, but the risk rises dramatically when the drug is combined with corticosteroids. In newly diagnosed myeloma the combination of thalidomide plus dexamethasone (Thal/Dex) has a high response rate of approximately 65% to 70% but is associated with a thrombosis risk of 17% to 26% in the absence of routine thromboprophylaxis.6,7 In patients with recurring myeloma the incidence of deep vein thrombosis with Thal/Dex is elevated, but lower (8%) compared with patients with newly diagnosed disease.8 The thrombotic episodes associated with thalidomide tend to occur in the first few months of therapy and are not correlated with age or response to therapy.4,5 The high rates of thrombosis remain unexplained and endothelial damage may play a role.
Lenalidomide is a more potent immunomodulatory derivative of thalidomide with a favorable side-effect profile.9–11 However, it appears that thrombotic risk is significant with lenalidomide-based regimens as well. Similar to thalidomide, thrombotic complications seem to be increased primarily when the agent is combined with corticosteroids or other chemotherapeutic agents.12 Another potential risk factor is the use of erythropoietic agents.13 Several different thromboprophylaxis strategies have been effective in lowering the risk of thrombosis: aspirin (81–325 mg/day), full-intensity warfarin (INR 2–3), and prophylactic enoxaparin (40 mg subcutaneous daily). However, none of these preventive strategies have been tested prospectively, so the choice often reflects physician and/or patient preferences.12,14–16
Although lenalidomide was approved by the Food and Drug administration (FDA) in July 2006, the incidence of deep vein thrombosis with this agent and the associated risk factors have not been well studied. The purpose of this article is to describe the incidence of deep vein thrombosis with lenalidomide therapy and assess the associated risk factors and efficacy of aspirin thromboprophylaxis.
MATERIALS AND METHODS
After approval from the respective Institutional Review Boards, a pooled analysis of newly diagnosed MM patients enrolled in lenalidomide clinical trials was performed jointly by the investigators in Italy and at the Mayo Clinic in Rochester, Minnesota. Data regarding the rate of venous and arterial thrombotic events were obtained by review of medical records and existing databases. Serious thromboembolic events, specifically deep vein thrombosis, arterial thrombosis, and pulmonary embolism, were reviewed and recorded. All clinical events were symptomatic and objectively diagnosed and confirmed by radiologic studies like ultrasound and contrast-enhanced computed tomographic (CT) scan during the treatment with lenalidomide or within 1 month of discontinuing the drug. In addition, various patient characteristics such as age, sex, performance status, previous history of deep vein thrombosis, pulmonary embolism, myocardial infarction, stroke, and disease status were assessed. We also examined whether aspirin prophylaxis and erythropoietin use influenced the thrombosis risk. The primary objective was to ascertain the rate of thrombotic episodes with lenalidomide-based therapy for newly diagnosed myeloma and to evaluate whether there is a higher risk of thrombosis in the high-dose steroid group.
Statistical analysis was done with Stat-View v. 5.01 (Cary, NC). All analyses were on an intent-to-treat principle. The chi-square and Fisher exact test were used to compare differences in nominal variables; the rank sum test was used for continuous variables.
RESULTS
Trial and Patient Characteristics
Three eligible trials were identified (Table 1). A total of 125 patients were analyzed (70 males and 55 females). The median age was 67.3 years (range, 32.7–72.3 years). In all, 110 patients (88%) received deep vein thrombosis prophylaxis (Table 2). Aspirin was the most common form of prophylaxis and was instituted in 104 patients. Five patients received coumadin and 1 was on low molecular weight heparin. Twenty-one patients (17%) were on erythropoietin supplementation.
TABLE 1.
Trial Characteristics
| Regimen | No. of patients. N = 125 | Lenalidomide dose | Corticosteroid dose |
|---|---|---|---|
| Lenalidomide plus dexamethasone in newly diagnosed multiple myeloma | 34 | 25 mg/d Days 1–21 every 28 d | Dex 40 mg Days 1–4, 9–12, 17–20 every 28 d |
| Lenalidomide plus dexamethasone in newly diagnosed multiple myeloma | 38 | 25 mg/d, Days 1–21 every 28 d | Dexamethasone 40 mg Days 1–4, 9–12, 17–20 every 28 d (high dose) or 40 mg Days 1, 8, 15, 22 every 28 d (low dose) |
| Melphalan, prednisone, lenalidomide in newly diagnosed multiple myeloma in patients > 65 y of age | 53 | 5–10 mg/d, Days 1–21 every 4–6 wk | Prednisone 2 mg/kg for 4 d every 4–6 wk |
TABLE 2.
Frequency Distribution of Deep Vein Thrombosis
| Risk factor | No. of patients | Rate of deep vein thrombosis, % | Hazard ratio (95% CI) | P |
|---|---|---|---|---|
| Prophylaxis | ||||
| Yes | 110 | 7.3 | 0.5 (0.1–2.7) | 0.42 |
| No | 15 | 13.3 | ||
| Steroid Dose | ||||
| Low-dose (Prednisone 2 mg/kg Days 1–4 or dexamethasone 40 mg Days 1, 8, 15, 22 administered every 28 d) | 73 | 6 | 0.4 (0.12–1.7) | 0.2 |
| High-dose (Dexamethasone 40 mg Days 1–4, 9–12, 17–20 every 28 d) | 52 | 12 | ||
| Regimen | ||||
| RD | 52 | 12 | ||
| Rd | 20 | 5 | 0.4 (0.1–3.6) | 0.4 |
| MPR | 53 | 5.6 | 0.5 (0.1–1.9) | 0.3 |
| Erythropoietin use | ||||
| Yes | 21 | 4.8 | 0.5 (0.1–4.4) | 0.55 |
| No | 104 | 8.6 | ||
| Sex | ||||
| Women | 55 | 5.5 | ||
| Men | 70 | 10 | 1.9 (0.5–7.8) | 0.4 |
| Age, y | ||||
| <60 | 27 | 0 | 0.08 | |
| ≥60 | 98 | 10.2 | ||
RD indicates lenalidomide plus high dose dexamethasone; RLD, lenalidomide plus low dose dexamethasone; MPR, lenalidomide + melphalan + prednisone.
Patients were stratified based on the concomitant corticosteroid dose to a high-dose and low-dose group. There were 52 patients in the high-dose group, receiving dexamethasone 40 mg daily for 4 days on Days 1–4, 9–12, and 17–20 of a 28-day cycle. The low-dose steroid group had 73 patients: 53 were on prednisone as per the melphalan, prednisone, and lenalidomide (MPR) regimen; 20 were on dexamethasone 40 mg on Days 1, 8, 15, and 22 of a 28-day cycle (Table 1).
Incidence and Risk Factors for Venous Thrombotic Events
Ten patients developed venous thrombotic events for an overall rate of 8%. The median age of patients who developed deep vein thrombosis was 69.3 years (range, 63.6–78.2 years). In patients who did not have a thromboembolic event the median age was 67.3 years (range, 32.7–78.3 years). The characteristics of patients who developed deep vein thrombosis are listed in Table 3. Six patients developed an extremity deep vein thrombosis and 4 patients had pulmonary embolism. There were no fatalities from these thrombotic events.
TABLE 3.
Characteristics of Patients With Deep Vein Thrombosis
| Patient | Age, y | Sex | Event | Regimen | Time of event | Prophylaxis at time of DVT | Steroid dose at time of DVT | Lenalidomide dose at time of DVT | Contributing factors |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | PE | RD | 4th cycle | None | 40 mg Days 1–4, 9–12, 17–20 every 28 d | 25 mg Days 1–21, every 28 d | None |
| 2 | 67 | M | DVT | RD | 3rd cycle | Aspirin 325 mg/d | 40 mg Days 1–4, 9–12, 17–20 every 28 d | 25 mg Days 1–21, every 28 d | None |
| 3 | 79 | M | DVT | RLD | 6th cycle | Aspirin 325 mg/d | 40 mg Days 1, 8, 15, 22 every 28 d | 15 mg Days 1–21, every 28 d | None |
| 4 | 67 | M | DVT | RD | 5th cycle | Aspirin 325 mg/d | 40 mg Days 1–4, 9–12, 17–20 every 28 d | 25 mg Days 1–21, every 28 d | None |
| 5 | 72 | M | DVT | RD | 4th cycle | Aspirin 325 mg/d | 40 mg Days 1–4, 9–12, 17–20 every 28 d | 15 mg Days 1–21, every 28 d | None |
| 6 | 71 | W | PE | RD | 4th cycle | None | 40 mg Days 1–3, 9–11, 17–19 every 28 d; plus tapering doses on Days 4, 5, 12, 13, 20, 21 | Not available | None |
| 7 | 64 | M | PE | RD | 16th cycle | Aspirin 325 mg/d | None | 25 mg Days 1–21, every 28 d | On coumadin at baseline for PE diagnosed during w/u of myeloma. Developed PE within a mo of switching to ASA from coumadin |
| 8 | 71 | W | DVT | MPR | 7th cycle | None | Pred 2 mg/kg Days 1–4 every 28 d | 10 mg Days 1–21, every 28 d | Stopped ASA 3 mo before for thrombocytopenia |
| 9 | 72 | W | PE | MPR | 1st cycle | Aspirin 100 mg/d | Pred 2 mg/kg Days 1–4 every 28 d | 10 mg Days 1–21, every 28 d | None |
| 10 | 68 | M | DVT | MPR | 6th mo of maintenance phase | None | None | 10 mg Days 1–21, every 28 d | ASA discontinued for thrombocytopenia |
M indicates men; W, women; RD, lenalidomide plus high dose dexamethasone; RLD, Lenalidomide plus low dose dexamethasone; MPR, melphalan, prednisone, lenalidomide; DVT, deep vein thrombosis; PE, pulmonary embolism.
Eight of 110 patients (7.3%) who received thromboprophylaxis developed deep vein thrombosis, compared with 2 of 15 patients (13.3%) who did not receive any thromboprophylaxis (P = .04). However, not all patients who were initiated on thromboprophylaxis remained on such treatment at the time of deep vein thrombosis. On the basis of review of the records, 2 of the estimated 10 patients (20%) who had stopped thromboprophylaxis developed deep vein thrombosis; thus, 6 of the remaining 100 patients (6%) who received continuous uninterrupted thromboprophylaxis developed thrombosis.
There was a higher rate of deep vein thrombosis in the high-dose corticosteroid group compared with the low-dose corticosteroid group (12% vs 6%), but this difference was not statistically significant, P = .3 (Table 2). Twenty-one patients (17%) received concurrent erythropoietin therapy. There was no effect of erythropoietin use on the incidence of deep vein thrombosis in this study, 4.8% (with erythropoietin) versus 8.6% (no erythropoietin) (P = .5). Male sex was associated with a 2-fold increase in thrombosis risk, but the increase was not statistically significant. There were no venous thrombotic events in patients younger than 60 years. The occurrence of venous thrombotic events did not affect overall survival. The overall survival of all patients in this study is shown in Figure 1.
FIGURE 1.
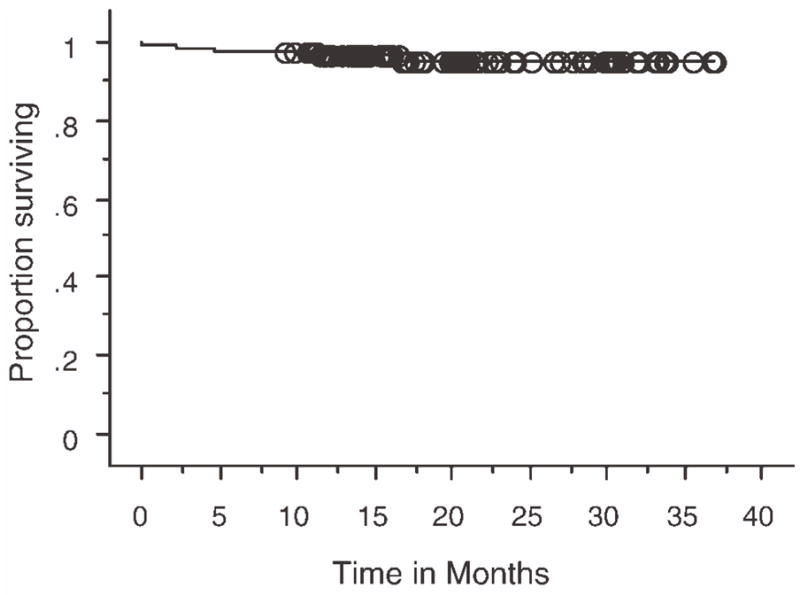
Kaplan-Meier plot showing overall survival of all 125 patients measured in months. At a median follow-up of 3 years overall survival was 95% at 3 years.
The timing of deep vein thrombosis with respect to lenalidomide treatment is shown in Table 3. In 6 patients (60%), thrombotic events occurred between the third and sixth cycle of treatment. Among the 4 patients whose thrombotic event did not occur within this time frame, 3 had possible contributing factors. In 2 patients in the MPR arm aspirin had been discontinued: 1 of them developed deep vein thrombosis in the seventh cycle of therapy and the other during maintenance. One patient in the high-dose steroid arm developed a pulmonary embolus in the sixteenth cycle of his treatment. He had recently switched from therapeutic coumadin to aspirin. One patient in the MPR arm developed a pulmonary embolus within the first cycle of treatment and had no known risk factors.
Arterial thrombosis was uncommon, with 3 reported myocardial infarctions and no episodes of stroke. Of this, only 1 event was thought to be related to the study drug. This patient developed myocardial infarction within a week of starting lenalidomide and high-dose dexamethasone. On coronary catheterization he was found to have extensive lesions in the right coronary artery, which was stented. There were underlying risk factors including hypertension, obesity, and previous smoking. Lenalidomide was discontinued.
DISCUSSION
Thrombotic complications of lenalidomide therapy have evoked a great deal of interest. The underlying mechanisms remain unclear. We performed a pooled analysis of newly diagnosed myeloma patients receiving lenalidomide as initial therapy and report a cumulative deep vein thrombosis rate of 8%. This rate is in the lower range of what has been previously reported in the literature in this setting (Table 4). The lower rate may be related to the high percentage of our patient population (88%) who received anticoagulant prophylaxis. In addition, 58% of patients studied were on low-dose steroids, namely, weekly dexamethasone, or prednisone. The thrombotic rate in patients receiving low-dose corticosteroids in this study was 6%. In contrast, the thrombotic rate in patients on high-dose corticosteroids was 12% (P = .4). The difference in incidence of thrombosis based on the dose of corticosteroids did not achieve statistical significance, probably due to the low event rate and the relatively small sample size of this study. However, these data are consistent with a prior report in which the thrombosis risk with lenalidomide was significantly associated with steroid dose.17
TABLE 4.
Incidence of Lenalidomide Associated Thrombosis in Recent Clinical Trials
| Study | Regimen | Disease status | No. of patients | Rate of deep vein thrombosis | Thromboprophylaxis |
|---|---|---|---|---|---|
| Weber, 200627 | Lenalidomide 25 mg Days 1–21 every 28 d plus dexamethasone 40 mg Days 1–4, 9–12, 17–20 every 28 d | Relapsed, refractory | 177 | 15% | None |
| Zonder, 200525 | Lenalidomide 25 mg Days 1–28 every 35 d plus dexamethasone 40 mg Days 1–4, 9–12, 17–20 every 35 d | Newly diagnosed | 38 | 75%; reduced to 19% after institution of aspirin prophylaxis | ASA in the second phase |
| Richardson, 200628 | Lenalidomide 30 mg daily (or15 mg twice a d) administered on Days 1–21 every 28 dd with or without dexamethasone Days 1–4, 15–18 every 28 d | Relapsed, refractory | 102 | 3% (rate is 4.4% among the 68 patients who received lenalidomide plus dexamethasone | None |
| Rajkumar, 200726 | Lenalidomide 25 mg Days 1–21 every 28 d plus dexamethasone 40 mg Days 1–4, 9–12, 17–20 every 28 d | Newly diagnosed | 223 | 22.1% | ASA (randomized between coumadin and ASA in the expansion phase) |
| Rajkumar, 200726 | Lenalidomide 25 mg Days 1–21 every 28 d plus dexamethasone 40 mg Days 1, 8, 15, 22 every 28 d | Relapsed, refractory | 222 | 6.1% | ASA |
ASA indicates aspirin; High dose dex, dexamethasone 40 mg (Days 1–4, 9–12 and 17–20 in a 28 d cycle).
Erythropoietin is thought to increase the risk of deep vein thrombosis with lenalidomide therapy. When 2 large randomized trials involving recurring refractory myeloma patients was compared, it was found that the thrombosis rate of 8.5% reported by Dimopoulos et al.18 in 176 patients in Europe, Israel, and Australia was lower than the 15% reported by Weber et al.27 in a similar group of patients in the US and Canada. Both groups had received the same dose of dexamethasone and lenalidomide, but more patients in the North American study received concomitant erythropoietin. On multivariate analysis, the use of erythropoietin was found to be a significant risk factor for thrombosis in the North American Study.13 In our study, only 17% of patients were on erythropoietin, and no association was seen between erythropoietin use and deep vein thrombosis. The risk of deep vein thrombosis was 4.8% in patients who received concomitant erythropoietin, and 8.6% in those who did not receive any erythropoietin. The high frequency of anticoagulant prophylaxis as well as the low dose steroids used in combination with lenalidomide may have reduced the thrombotic risk associated with erythropoietin usage.
It is well established that aspirin is effective in reducing the risk of myocardial infarction and stroke, but its role in reducing venous thrombosis is controversial.19 In a large study (the Pulmonary Embolism Prevention trial) involving more than 17,000 orthopedic patients, it was noted that aspirin significantly reduced the risk of venous thromboembolism compared with the placebo arm.20 There was some criticism regarding the study endpoints.21 Nevertheless, review of available evidence seems to suggest that platelets are involved in venous thrombus formation, and inhibiting the platelet function with aspirin may be of some benefit in reducing the risk of venous thrombosis.22 Currently, aspirin is not recommended for prophylaxis against venous thrombosis23 because we have more effective agents.
There are some data that aspirin has been beneficial in reducing the risk of deep vein thrombosis in the context of thalidomide or lenalidomide therapy. In 1 study, when aspirin was added to the regimen of DVd-T (liposomal doxorubicin, vincristine, dexamethasone, and thalidomide) there was a significant reduction in the risk of deep vein thrombosis.24 In a Southwest Oncology Group (SWOG) study of newly diagnosed myeloma,25 9 of the first 12 patients enrolled (75%) developed thromboembolic complications. Subsequently, after institution of aspirin prophylaxis the cumulative thrombotic rate in the study was reduced significantly, although the rate was still high, at 19%. In our cohort of patients the rate of thrombosis in patients on aspirin prophylaxis was approximately 7%, compared with 13% without prophylaxis.
Arterial thrombosis is uncommon with lenalidomide. The 1 patient who had a myocardial infarction attributed to lenalidomide had major risk factors for coronary disease. In more than half of the patients thrombosis occurred between the third and sixth month. This could have been affected by the finding that patients may have completed therapy within the first 6 months. In the 3 patients whose thrombotic event occurred after the sixth month, anticoagulant prophylaxis was either discontinued or modified.
The thrombosis rate with lenalidomide and high-dose corticosteroids was 12% in our study, and approximately 20% in other studies (Table 4). Given this rate, prophylaxis with low molecular weight heparin or therapeutic-dose coumadin rather than aspirin is required in such patients. Preliminary data suggest that overall survival with high-dose steroids is inferior to low-dose steroids in newly diagnosed patients receiving lenalidomide-based therapy.26 Hence, this regimen may be less widely used in the future. In contrast to lenalidomide plus high-dose corticosteroids, based on our study and a review of other trials, it appears that the rate of deep vein thrombosis with lenalidomide and low-dose steroid therapy is approximately 5% to 8% with aspirin prophylaxis. Regardless of whether there is a true protective effect or not, the low overall rate of thrombotic events strongly argues in favor of routine aspirin prophylaxis with lenalidomide and low-dose corticosteroid-containing regimens, and negates the need for routine full-dose anticoagulation in this setting.
Acknowledgments
Supported in part by the Università degli Studi di Torino; Fondazione Neoplasie Sangue Onlus, Associazione Italiana Leucemie, Compagnia di S Paolo, Fondazione Cassa di Risparmio di Torino, Ministero dell’Università e della Ricerca (MIUR), and Consiglio Nazionale delle Ricerche (CNR); Italy. Also supported by the National Institutes for Health under Grants CA 62242, CA107476, CA 100080 and CA 93842 (to S.V.R.).
References
- 1.Catovsky D, Ikoku NB, Pitney WR, Galton DA. Thromboembolic complications in myelomatosis. BMJ. 1970;3:438–439. doi: 10.1136/bmj.3.5720.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elice F, Fink L, Tricot G, Barlogie B, Zangari M. Acquired resistance to activated protein C (aAPCR) in multiple myeloma is a transitory abnormality associated with an increased risk of venous thromboembolism. Br J Haematol. 2006;134:399–405. doi: 10.1111/j.1365-2141.2006.06208.x. [DOI] [PubMed] [Google Scholar]
- 3.Zangari M, Saghafifar F, Mehta P, Barlogie B, Fink L, Tricot G. The blood coagulation mechanism in multiple myeloma. Semin Thromb Hemost. 2003;29:275–282. doi: 10.1055/s-2003-40965. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Thalidomide therapy and deep venous thrombosis in multiple myeloma. Mayo Clin Proc. 2005;80:1549–1551. doi: 10.4065/80.12.1549. [DOI] [PubMed] [Google Scholar]
- 5.Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001;344:1951–192. doi: 10.1056/NEJM200106213442516. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 7.Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100:2272–223. doi: 10.1182/blood-2002-06-1674. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostopoulos A, Weber D, Rankin K, Delasalle K, Alexanian R. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–771. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 9.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Lenalidomide in multiple myeloma. Expert Rev Anticancer Ther. 2006;6:1165–1173. doi: 10.1586/14737140.6.8.1165. [DOI] [PubMed] [Google Scholar]
- 12.Zonder JA. Thrombotic complications of myeloma therapy. Hematology. 2006:348–355. doi: 10.1182/asheducation-2006.1.348. [DOI] [PubMed] [Google Scholar]
- 13.Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–2080. doi: 10.1056/NEJMc053530. [DOI] [PubMed] [Google Scholar]
- 14.Durie BGM, Richardson P, Palumbo A, et al. Deep vein thrombosis in myeloma: estimate of prevelance and recommendations for therapy based upon a survey of members of the International Myeloma Working Group (IMWG) [abstract] ASH Annu Meet Abstr. 2006;108:3571. [Google Scholar]
- 15.Hirsh J. Risk of thrombosis with lenalidomide and its prevention with aspirin. Chest. 2007;131:275–277. doi: 10.1378/chest.06-2360. [DOI] [PubMed] [Google Scholar]
- 16.Bennett CL, Angelotta C, Yarnold PR, et al. Thalidomide-and lenalidomide-associated thromboembolism among patients with cancer. JAMA. 2006;296:2558–2560. doi: 10.1001/jama.296.21.2558-c. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Jacobus S, Callander N, Fonseca R, Vesole D, Greipp P. A randomized phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group [abstract] ASH Annu Meet Abstr. 2006;108:799. [Google Scholar]
- 18.Dimopoulos MA, Spencer A, Attal M, et al. Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of a phase 3 study (MM-010) [abstract] ASH Annu Meet Abstr. 2005;106:6. [Google Scholar]
- 19.Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ. 1994;309:1213–1215. doi: 10.1136/bmj.309.6963.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.No authors listed. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355:1295–1302. [PubMed] [Google Scholar]
- 21.Cohen A, Quinlan D. PEP trial. Lancet. 2000;356:247. doi: 10.1016/s0140-6736(00)02493-4. [DOI] [PubMed] [Google Scholar]
- 22.Hovens MMC, Snoep JD, Tamsma JT, Huisman MV. Aspirin in the prevention and treatment of venous thromboembolism. J Thromb Haemostasis. 2006;4:1470–1475. doi: 10.1111/j.1538-7836.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 23.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 24.Baz R, Li L, Kottke-Marchant K, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005;80:1568–1574. doi: 10.4065/80.12.1568. [DOI] [PubMed] [Google Scholar]
- 25.Zonder JA, Durie BGM, McCoy J, et al. High incidence of thrombotic events observed in patients receiving lenalidomide (L) + dexamethasone (D) (LD) as first-line therapy for multiple myeloma (MM) without aspirin (ASA) prophylaxis [abstract] ASH Annu Meet Abstr. 2005;106:3455. [Google Scholar]
- 26.Rajkumar SV, Jacobus S, Callander N, et al. Phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group [abstract] J Clin Oncol (Meet Abstr) 2007;25(18 suppl):LBA8025. [Google Scholar]
- 27.Weber D, Wang M, Chen C, et al. Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): results of 2 phase III studies (MM-009, MM-010) and subgroup analysis of patients with impaired renal function [abstract] ASH Annu Meet Abstr. 2006;108:3547. [Google Scholar]
- 28.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]


