Abstract
Background and Purpose: Infants with myelomeningocele (MMC) have difficulty with, and show delays in, acquiring functional skills, such as walking. This study examined whether infants with MMC will respond to treadmill practice by producing stepping patterns or at least motor activity during the first year after birth. This study also compared the stepping trajectories of infants with MMC across age with those of infants with typical development (TD) to analyze the characteristics of the development of stepping patterns in infants with MMC early in life.
Participants: Twelve infants with MMC (lumbar and sacral lesions) and 12 infants with TD were the participants in this study.
Methods: The infants were tested on a treadmill at ages 1, 3, 6, 9, and 12 months, with no treadmill practice between test sessions. Infants were supported on the treadmill for twelve 20-second trials. A digital camera and behavior coding were used to determine step rate, interlimb stepping patterns, step parameters, and motor activity level.
Results: Treadmill practice elicited steps in infants with MMC (14.4 steps/minute during the year) but less so than in infants with TD (40.8 steps/minute). Responsiveness was affected by lesion level but varied markedly among infants. Interlimb stepping was less readily alternating, but step parameters were similar to those produced by their peers with TD. Finally, holding infants with MMC on a moving treadmill resulted in greater motor activity (17% during the year) than holding infants on a nonmoving treadmill.
Discussion and Conclusion: Infants with MMC responded to the treadmill by stepping (but less so than infants with TD) and showing increased motor activity, but they demonstrated a different developmental trajectory. Future studies are needed to explore the impact of enhancing sensory input during treadmill practice to optimize responses in infants with MMC.
Myelomeningocele (MMC) is the most common neural tube defect in the United States, affecting 1,500 to 2,000 of the more than 4 million babies born in the country each year.1 In recent years, the incidence of MMC has decreased in many developed countries as a function of improved prepregnancy health care for women of parenting age and folic acid dietary supplementation. However, the incidence remains as high as 8 in 1,000 in India and 1.5 in 1,000 in Mexico.2–4 This disorder frequently results in numerous neuromusculoskeletal complications, including paraparesis, neurogenic bowel and bladder, hydrocephalus, and cognitive issues. In this study, we focus, in particular, on the issue of lower-limb function.
For children born with MMC, walking onset is delayed by an average of 2 years compared with that in their peers with typical development (TD). Walking in children born with MMC comes with a high energy cost, high motion variability (such as pelvic obliquity and hip rotation5), and often the need for braces, orthoses, or both to manage the impact of paresis.6–8 The likelihood that infants with MMC will walk is affected by the level at which the functional lesion occurs. Current data suggest a 20% chance of walking for those born with lesions at a high lumbar level, 80% for infants with lesions at a low lumbar level, and 90% for infants with lesions at the sacral level.8,9 Unfortunately, by late childhood to early adolescence, many children with MMC are unable to maintain upright locomotion for community mobility and transition to wheelchair use; this transition introduces or reinforces significant comorbidities, such as scoliosis and obesity.10,11
The possibilities for nonsurgical early therapeutic interventions that might help infants with MMC acquire stronger and better functional control of their lower limbs have largely been neglected. Medical studies that describe the spontaneous leg activity of infants with MMC have been done. Ultrasound examinations of 16- to 24-week-old fetuses with thoracic, lumbar, and sacral MMC lesions have indicated that these fetuses are as active as fetuses with TD, showing flexion and extension movements of their hips and knees.12–14 However, in the weeks after birth, rather than showing increased leg activity, infants with MMC exhibit decreased leg activity.15,16 Although this decreased activity after birth is not surprising, it is encouraging to note that 4- to 6-month-old infants with MMC will respond to contexts that encourage activity, such as a specially designed chair, by increasing their frequency of spontaneous leg movement.17
The fact that infants with MMC will show active neuromotor responses to environmental manipulations is encouraging. Current theory and empirical data indicate that for infants, generally, early exploration and spontaneous motor activity drive the development of motor control and the organization of underlying neural structures.18–21 Through their own activity, infants create recurrent and reciprocal cycles of sensorimotor input and output via the central and peripheral nervous systems. Through these perception-action cycles, the capacity of populations of neurons to be activated in a coordinated and functional manner is strengthened. Because MMC involves damage to the spinal cord, the number of intact motor units available to initiate and sustain activity and the number of sensory receptor pathways are reduced.22,23 The task of gaining strength (force-generating capacity) and control not only is metabolically more demanding but also may require more cycles of neurological activity over time to build a foundation of organized and functional neuromotor pathways.20,24
One potential way in which to assist the development of leg control in babies with MMC is to create an environment that encourages them to produce more cycles of leg activity, in particular, patterns of movement that relate to functional behavior, such as walking. In previous studies, Ulrich and colleagues25–27 showed that infants with TD respond to being supported upright on a small motorized treadmill by producing well-coordinated and adaptive stepping patterns. Subsequently, Ulrich and colleagues28–31 demonstrated that infants with Down syndrome also are able to step when supported on a treadmill, but with a delay in the age of onset of responsiveness compared with their peers with TD. Furthermore, it was shown that treadmill training significantly reduces the delay in walking onset for infants with Down syndrome and improves the quality of gait for toddlers.28–31
Therefore, in this study, we asked a much-needed question about whether the use of a treadmill might provide experience and input relevant to and usable by the neuromotor systems of infants with spinal sensorimotor lesions that are incomplete in nature. Specifically, our goal was to determine whether infants with MMC are able to increase their step rate or at least their motor activity level (moving in any manner) when supported on a motorized treadmill during the first year after birth. We also compared the quantity of stepping (step rate) and the quality of stepping (interlimb stepping patterns and step parameters) in infants with MMC across age with those in infants with TD to identify the characteristics of the development of stepping patterns in infants with MMC. In addition, for the group with MMC, we examined the relationship between neuromotor damage (on the basis of the level of the lesion) and stepping trajectories.
Method
Participants
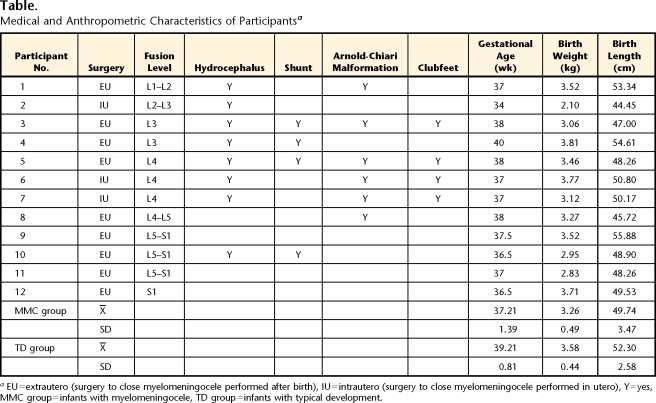
Twelve infants with MMC and 12 infants with TD were enrolled in our longitudinal study at 1 month of age (7 girls and 5 boys in each group). Infants with both MMC and chromosomal or central nervous system abnormalities not known to be associated with MMC were excluded. Lesion levels were limited to lumbar and sacral and were based on the fusion sites recorded by hospitals (Table). Infants with TD were without known cognitive, sensory, or motor impairments (on the basis of parental reports). One additional baby with MMC and 1 additional baby with TD were tested but were excluded from this study because they moved from the geographical area or their parents did not want to continue with the study.
Table.
Medical and Anthropometric Characteristics of Participantsa
EU=extrautero (surgery to close myelomeningocele performed after birth), IU=intrautero (surgery to close myelomeningocele performed in utero), Y=yes, MMC group=infants with myelomeningocele, TD group=infants with typical development.
Infants with MMC were referred by physicians at the University of Michigan and Toledo, Ohio, hospital systems. Parents gave written informed consent for their infants to participate in this study and completed a medical status and history form (including shunt status, level of lesion, and surgeries). Infants with TD were recruited through newspaper advertisements, fliers, and word-of-mouth communication in the Ann Arbor, Michigan, area.
Procedure
Infants visited our laboratory to be tested at 1, 3, 6, 9, and 12 months of age and at walking onset. For babies born more than 2 weeks before their due date, we used corrected ages for test sessions and analyses. During each session, we tested infants in 3 conditions: newborn stepping, treadmill stepping, and supine spontaneous movement. In this report, we address only the second and most extensive testing condition, treadmill stepping. To prepare infants for testing, we removed their clothing and diaper. We placed reflective markers (8-mm diameter at ages 1 and 3 months; 18-mm diameter at ages 6 months and on) bilaterally on the iliac crest, greater trochanter, knee joint, malleolus, and ventral surface of the third metatarsophalangeal joint. We placed preamplified bipolar electromyograph electrodes over the muscle bellies of the left gastrocnemius, tibialis anterior, rectus femoris, and biceps femoris muscles.
The treadmill stepping condition consisted of a custom-made motorized treadmill placed on a large table (73 cm high, 118 cm wide, and 190 cm long) surrounded by 6 motion capture cameras and a digital video camera. The treadmill had a frame measuring 18 cm high, 42 cm wide, and 82 cm long; a smooth belt surface (30 cm wide); and adjustable speed control. We placed 3 Peak Motus* real-time cameras on each side of the table to capture the movements of each leg.
A digital video camera (60 Hz) was placed at the side of the table perpendicular to the treadmill motion and with the height adjusted to the baby's size to videotape stepping behavior. The data from this digital video camera, as well as the data from the motion capture system and the electromyograph, were synchronized and recorded with the Peak Motus cameras.
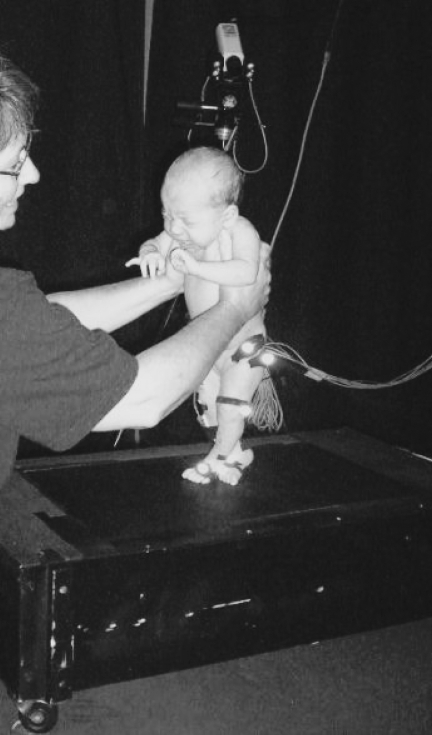
We held infants upright so that their feet rested on the belt of the treadmill in a partial body-weight-supported position for twelve 20-second trials, split into 2 sets of 6 trials (Fig. 1). Between sets, infants were given a break, and breaks were taken throughout testing as needed. During trials 1 and 12, the belt of the treadmill was not moving. For trials 2 through 6 and 7 through 11, the belt was moving and the speed was increased in increments of 0.038 m·s−1, from 0.068 m·s−1 to 0.22 m·s−1. Speed adaptations will be assessed in a separate article in preparation. For this article, we collapsed data across speed within each test session for each baby.
Figure 1.
Photograph of a 1-month-old infant being held on the small motorized treadmill.
After testing, we measured total body weight and length, and for each leg, we measured length (greater trochanter to lateral malleolus), thigh, and shank circumference. Because the neuromotor outcomes of spinal lesions in babies with MMC often are asymmetrical, we classified each leg as more or less affected on the basis of the infants’ medical records. When no differences were reported, we assigned the right leg as the less-affected leg.
Data Reduction
For the purposes of the present investigation, only data collected with the digital video camera during the treadmill testing are presented here to focus on the quantity and quality of the stepping of the babies when supported on the treadmill during the first year of life.
For determination of the occurrence and temporal aspects of steps, as well as the baby's general level of motor activity, the digital video data were behaviorally coded using frame-by-frame (60-Hz) analysis of each trial with Peak Motus computer software. Before coders (2 of the study authors and 4 student assistants) could begin working on data for this study, they had to obtain a coefficient of agreement of .85 (interobserver reliability coefficient, kappa) through comparison of their work with that of previously validated coders for the same set of trials by using training tapes.
Step rate.
First, coders counted the number of steps produced in each trial. Because portions of some trials could not be completed as a result of infants’ fussiness or other reasons, we calculated the rate of steps to allow comparisons among infants and among ages. The rate of steps was defined as the total number of steps taken per session for each baby divided by the total number of seconds spent on the treadmill during moving-belt trials. Two infants with MMC missed one visit; we interpolated the points surrounding this event to create a surrogate value for this visit for longitudinal analysis purposes. Two infants with TD missed the treadmill data collection session at 12 months. Because they stepped continuously at 9 months and it was not reasonable to expect further improvement, we used their performance values for 9 months at 12 months.
Interlimb stepping patterns.
Coders were trained to recognize 4 interlimb stepping patterns: alternating (a step of one leg overlaps a step of the opposite leg), single (a step of one leg does not overlap a step of the other leg), parallel (both legs swing forward simultaneously), and double (a “stutter step” within a series of alternating steps). For these patterns, we calculated the 4 types of steps produced at each visit for each baby as a percentage of the total steps.
Step parameters.
Coders identified the time (frame) when events occurred: toe-off, touch-down, and end of stance for alternating steps only. To account for developmental differences in body size, we normalized stride cycle and swing- and stance-phase durations by leg length and transformed the data to dimensionless variables with the following formula: normalized cycle duration = cycle duration/(gravity/leg length)2.32 For alternating steps, the part of the infant's foot that made contact at touch-down and during mid-stance was coded as toe, flat, heel, lateral, or medial. Next, the percentage of each foot posture that was used for touch-down and mid-stance was calculated.
Motor activity level.
Motor activity level was coded for all nonmoving-belt and moving-belt trials to describe infants’ movement levels even if they did not respond to the treadmill with steps. We used 2 categories of activity: (1) overall activity, which reflected movement of any limbs, trunk, or head, and (2) leg activity, which reflected only leg behavior. Overall activity was scored every 5 seconds with 1 of 3 values: 0=no movement, 0.5=small movement, and 1=clear movement of arms, legs, head, or any of these parts. Leg activity was scored every 5 seconds with dichotomous values: 0=no leg movement and 1=clear leg movement. Next, we transformed the scores obtained at each session to a percentage for each infant on the basis of the maximal score possible for baseline trials and moving-belt trials.
Data Analysis
We used SPSS version 14.0 statistical software† for statistical analyses. The general linear model procedure was used to conduct multivariate analyses of variance (MANOVAs) and analyses of variance (ANOVAs) for repeated measures. The Huynh-Feldt epsilon correction for multiple comparisons (we report degrees of freedom rounded to the nearest whole number) was used to determine statistical significance, which was set at P<.05. The effect size for each ANOVA is reported as eta square (η2). Only statistics for results that were statistically significant are presented.
Results
Participant Characteristics
We used a 2 (group) × 5 (age) MANOVA for repeated measures on age to compare groups on overall body size. Dependent variables were height, weight, and the ponderal index: (3 /height ×100). Only a significant age effect emerged (Wilks lambda=0.12, F12,227=82.83, P<.001). Post hoc ANOVA results showed significant increases in body weight (F2,49=444.86, P<.001, η2=.95) and body length (F3,73=666.62, P<.001, η2=.97) and a significant decrease in the ponderal index (F2,64=38.24, P<.001, η2=.26) with age.
/height ×100). Only a significant age effect emerged (Wilks lambda=0.12, F12,227=82.83, P<.001). Post hoc ANOVA results showed significant increases in body weight (F2,49=444.86, P<.001, η2=.95) and body length (F3,73=666.62, P<.001, η2=.97) and a significant decrease in the ponderal index (F2,64=38.24, P<.001, η2=.26) with age.
We used a 2 (group) × 2 (leg) × 5 (age) MANOVA for repeated measures on age to compare groups on leg measures. Dependent variables were leg length, thigh circumference, and shank circumference. Both main effects were significant: age (Wilks lambda=0.03, F12,439=100.92, P<.001) and group (Wilks lambda=0.66, F3,40=6.79, P<.001). The age × group interaction also was significant (Wilks lambda=0.88, F12,439=1.84, P=.04). None of the post-hoc ANOVAs for individual dependent variables resulted in a significant interaction effect. We obtained a main effect of age for leg length (F3,113=519.7, P<.001, η2=.92), thigh circumference (F3,132=238.6, P<.001; η2=.85), and shank circumference (F3,108=378.9, P<.001, η2=.90). The main effect of group was significant for both leg length (F1,42=4.85, P=.03, η2=.10) and shank circumference (F1,42=14.74, P<.001, η2=.26). For leg length and shank circumference, values were higher for infants with TD than for infants with MMC. Inspection of the means suggests that each group showed very similar values at month 1 (19.4 and 19.6 cm for leg length for the infants with MMC and the infants with TD, respectively; 12.5 and 13.0 cm for shank circumference for the infants with MMC and the infants with TD, respectively). With age, the difference was greater (27.9 and 28.6 cm for leg length for the infants with MMC and the infants with TD, respectively; 17.6 and 19.3 cm for shank circumference for the infants with MMC and the infants with TD, respectively, at 12 months). Although not reaching the level of a significant univariate interaction, those values, in combination, may have resulted in the multivariate interaction effect reported above.
Step Rate
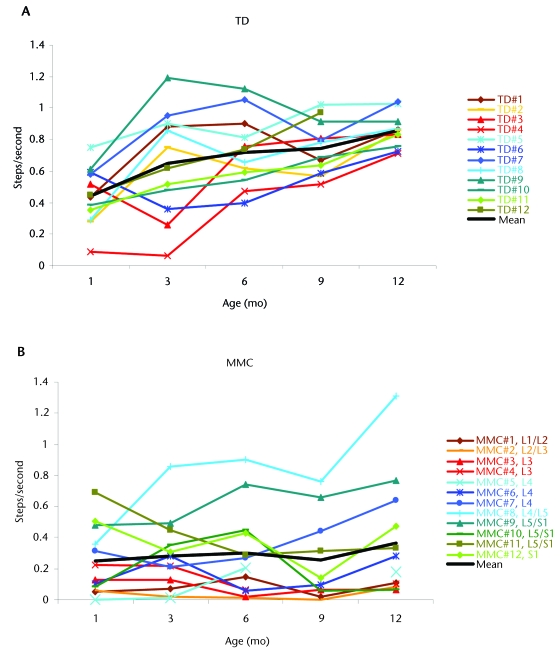
For our primary topic of interest, the infants’ step rate, we used a 2 (group) × 5 (age) ANOVA for repeated measures on age with step rate as the dependent variable. We found a significant age effect (F3,80=10.45, P<.001, η2=.32), a significant group effect (F1,22=21.3, P<.001, η2=.49), and an age × group interaction (F3,80=4.58, P=.003, η2=.17). Figures 2A and 2B display step rates across months for each infant as well as group means for infants with TD and infants with MMC, respectively. Infants with TD clearly showed an increase in step rate over time, shifting from high variability across individuals at month 1 to highly similar step rates by months 9 and 12. Infants in the MMC group did not show a change in the step rate over time and maintained high interindividual variability throughout the year.
Figure 2.
Number of steps taken per second across age, by groups and individuals: typical development (TD) (A), myelomeningocele (MMC) (B), and MMC with lesions at high (C), middle (D), and low (E) levels.
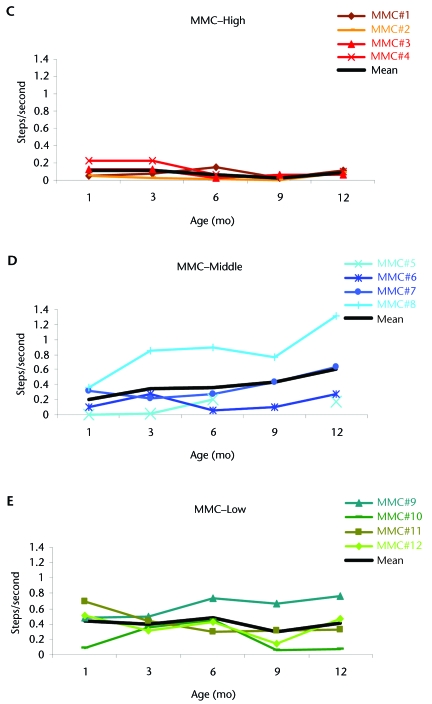
To better understand the variability in the MMC group, we divided infants into 3 subgroups on the basis of lesion level and current published research suggesting the inherent likelihood of these babies remaining community walkers into adulthood. Longitudinal studies of cohorts observed into adulthood33,34 suggested that for subjects with lesions above L3, the prognosis was not likely to be walking in adulthood. For subjects with L4 lesions, there seemed to be a 50% rate of community walking; this rate was as high as 90% for subjects with L5 and sacral lesions. On the basis of these published data, we classified babies with L1, L2, and L3 lesions as having high-level lesions; infants with L4 lesions as having middle-level lesions; and infants with L5 or sacral lesions as having low-level lesions. Figures 2C, 2D, and 2E show that the step rate tended to increase as the level of the lesion decreased. Infants with high-level lesions clearly produced the fewest steps, with little change with age and low variability. Infants with middle- and low-level lesions showed more improvement but also higher variability.
Interlimb Stepping Patterns
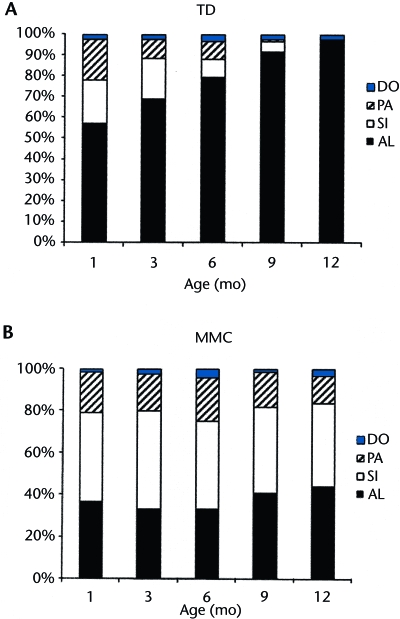
To examine differences in interlimb stepping patterns, we ran a 2 (group) × 5 (age) MANOVA for repeated measure on age and the following dependent variables: percentages of alternating, single, parallel, and double steps. We obtained significant group (Wilks lambda=0.44, F4,19=5.95, P=.003) and age (Wilks lambda=0.64, F16,260=2.59, P=.001) effects. Post-hoc ANOVAs were conducted for individual dependent variables. Means for each stepping pattern by group over time are shown in Figure 3. For alternating steps, we found significant group (F1,22=24.14, P<.001, η2=.52), age (F4,88=8.02, P<.001, η2=.27), and interaction (F4,88=4.27, P<.001, η2=.16) effects. Infants with TD produced more alternating steps than their peers with MMC. Both groups showed an increase in alternating steps with age, but infants with TD showed a considerably greater increase over time than infants with MMC. For single steps, one significant effect emerged: the main effect of group (F1,22=18.59, P<.001, η2=.46). Infants with MMC generated more single steps than their peers with TD. The main effects of group and age were significant for parallel steps (F1,22=5.43, P=.029, η2=.20 and F4,88=3.80, P=.007, η2=.15, respectively). Infants with MMC produced more parallel steps than their peers with TD, but both groups showed a decrease over time in the proportion of all steps that were parallel steps.
Figure 3.
Distribution of stepping patterns produced by infants with typical development (TD) (A) and infants with myelomeningocele (MMC) (B) across age: alternating (AL), double (DO), parallel (PA), and single (SI).
Step Parameters
We examined the parameters of infants’ alternating steps, the ones most similar to the interlimb stepping pattern used in walking. We included infants’ data only if they produced more than 4 alternating steps per session. Thus, for infants with MMC, sample sizes were 6 for month 9, 7 for months 6 and 12, and 8 for months 1 and 3. For infants with TD, the sample size was 12 throughout. Missing values for infants with MMC precluded calculating statistics.
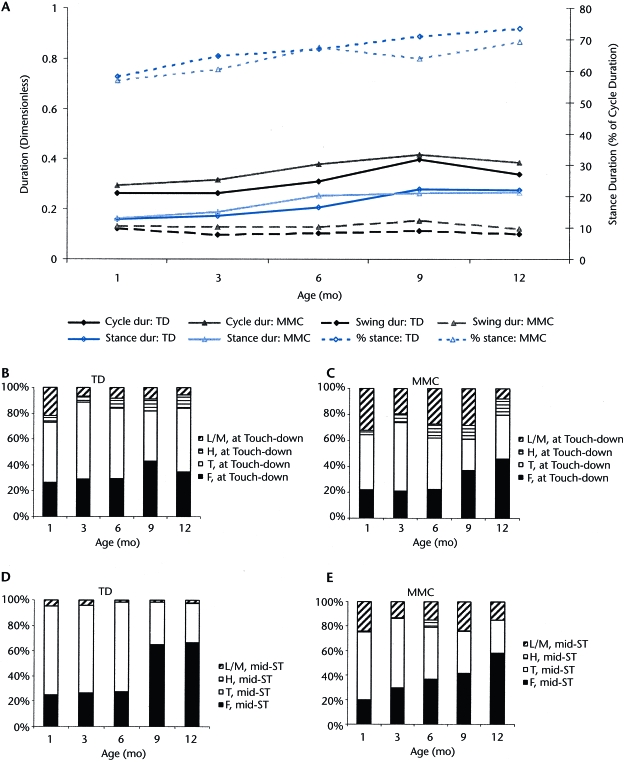
Figure 4A shows the values for step parameters across age: dimensionless cycle, swing phase and stance phase durations, and percent stance phase per cycle. The data suggested that the temporal parameters of alternating steps did not differ between groups. Both groups tended to show small increases in cycle duration and percent stance phase with increasing age. Figures 4B and 4C show foot posture at touch-down for infants with TD and MMC, respectively. At the end of the swing phase, the first contact for 60% of both groups was made with the toes or the flat part of the feet (soles). During the stance phase (Figs. 4D and 4E), both groups settled onto the flat part of the feet with greater frequency over time. Infants with MMC were more likely than their peers with TD to make initial contact with the lateral part of the feet at touch-down (20%) and during the stance phase (19%).
Figure 4.
Step parameters by group across age. (A) Normalized cycle duration, swing duration, stance duration, and percent stance. (B and C) Mean percentages of lateral or medial (L/M), heel (H), toe (T), or flat (F) part of foot making contact at touch-down for infants with typical development (TD) (B) and infants with myelomeningocele (MMC) (C). (D and E) Mean percentages of lateral or medial (L/M), heel (H), toe (T), or flat (F) part of foot in contact with belt during mid-stance (mid-ST) for infants with TD (D) and infants with MMC (E).
Motor Activity Level
To compare the motor activity levels of infants during moving-belt and nonmoving-belt conditions, we ran a 2 (group) × 5 (age) × 2 (condition) MANOVA for repeated measures on age. Dependent variables were overall activity and leg activity. We found significant main effects of group (Wilks lambda=0.80, F2,43=5.39, P=.008) and condition (Wilks lambda=0.50, F2,43=21.88, P<.001) and a significant age × group interaction (Wilks lambda=0.92, F8,350=1.20, P=.046). These results suggested that the groups differed in motor activity levels, with infants with TD moving more than their peers with MMC; however, the difference changed as infants grew older. Furthermore, both groups engaged in more motor activity when the belt was moving than when the support surface was stationary.
The post-hoc ANOVA for leg activity resulted in a significant condition effect (F1,44=21.72, P<.001, η2=.33) and an age × group interaction effect (F3,283=2.92, P=.023, η2=.06). For overall activity, the post-hoc ANOVA resulted only in a significant condition effect (F1,44=40.05, P<.001, η2=.48). Examination of means showed that leg activity for infants with TD remained stable over time, whereas infants with MMC showed a period of decreased leg activity—about 10% in each condition—beyond ages 6 and 9 months. The condition effect for both motor activity level variables revealed an increase during moving-belt trials compared with nonmoving-belt trials. On average, across months, infants with TD showed a 26% increase in overall activity when supported on the moving belt compared with the nonmoving belt. For infants with MMC, the average increase in overall activity was 18%. Leg activity increased by 22% in infants with TD and 16% in infants with MMC.
Discussion
Overall, our data showed that during the first year of life, supporting infants with MMC upright on the moving belt of a motorized treadmill elicits stepping patterns in addition to increased overall motor activity. In previous studies, researchers reported less leg activity in infants with MMC than in their peers with TD.15,17,35 Our current data parallel these earlier reports and extend them by demonstrating that although treadmill practice elicited stepping patterns in infants with MMC, their step rate, like their motor activity level, was lower than that of their peers with TD and the distribution of their interlimb stepping patterns was different from that of their peers.
Early signs of the impact of reduced motor activity in the legs may be evident in our finding of emergent group differences in leg size, which were the only significant anthropometric differences that we observed. At 1 month, leg length and shank circumference were similar between groups, but differences increased over time, such that the legs of infants with MMC were shorter and smaller in mass by the end of the first year. Although such growth impairment is typically attributed to impaired innervation, it may also be attributable, in part, to the less-frequent exposure of bones to the compression forces generated by repeated muscle contractions, which are known to facilitate bone growth.
As a group, infants with MMC had a step rate lower than that of infants with TD, clearly exposing their muscles to less contractile force over time than that seen in infants with TD. Still, they averaged approximately 48 steps per session (or 14.4 steps per minute), which is not a trivial number. However, their group mean step rate seemed quite flat across the year, whereas all infants with TD showed an increase in the step rate over time. Lesion level clearly affected both the step rate and the developmental trajectory across individual babies. Infants with the highest-level lesions (L1–L3), in particular, showed a very low step rate over time. We propose that the lack of improvement for infants in this subgroup over time represents a marked delay in the development of muscle strength and limb control compared with development in their peers, rather than an innate lack of a capacity for development. From the follow-up data that we collected for our infants with MMC after 12 months of age, we know that 3 of the 4 infants in this subgroup attained the ability to walk by using walkers to provide postural support (at 24 months, 29 months, and 44 months). Their high-level lesions likely caused the most depressed rate of development because of the increased degree of loss of sensorimotor units.36 Ulrich et al37 observed a similar delay in response to body-weight-supported treadmill practice in a study involving infants with Down syndrome. When given sufficient time, the infants showed improved stepping, particularly when provided with treadmill practice.28,29 We predict that at least 3 of our infants with high-level MMC would have responded with more steps on the treadmill had we continued to monitor their behavior beyond the first 12 months after birth.
Infants with MMC also demonstrated higher variability in their step rate than infants with TD, as individuals and as a group, and they showed several unique developmental trajectories during the first year of life. These findings are important for several reasons. First, the variability in stepping trajectories reinforced the notion that lesion level, while relevant, is a poor predictor, in isolation, of sensorimotor responsiveness at any point in time for individual babies with MMC.38 Other factors (such as shunt revisions, joint and ligament structures, specific medications, and overall family support resources) interact to make such predictions complex and difficult. Second, variability in infants with TD, but not in those with MMC, resolved markedly by the second half of the first year. This resolution, according to neuromotor development theory and data, occurs because infants’ spontaneous activity drives the organization of sensorimotor control and, thus, stable patterns of movement.20,21,39 Infants with TD are spontaneously quite active, whereas infants with MMC have decreased leg movements. Thus, infants with MMC seem to be caught in a cycle of inherently less spontaneous activity, which slows their rate of improvement in neuromotor control, which contributes to delays in acquiring functional motor skills and even nonfunctional behavioral responses, such as supported stepping. In addition to the inherent neural and physiological problems in babies with MMC, the medical procedures designed ultimately to improve outcomes, such as castings, joint surgeries, or even shunt surgeries, often interrupt progress in the short term.
Last, but perhaps most germane to motor development, the effects of congenital disruption of the spinal cord on motor and sensory communications between the periphery and the brain vary from mild to severe across individuals. When sensory feedback is decreased and motor unit input to muscles is reduced, the effects on establishing a repertoire of movement patterns and control may be inconsistent and varied. The processes of perceiving and acting may require even more repetitions than usual for babies with MMC to learn to control behavior. Such babies must “work harder” to build control and strength, an effort that may tax the motivation that is normally reinforced when active infants who are healthy perceive the impact of their actions on the environment.40–43
Although infants with MMC responded to treadmill practice by stepping, they also showed a developmental trajectory of interlimb stepping patterns that was different from that of their peers over time. Overall, infants with MMC were less likely to produce alternating steps at all ages than infants with TD; this finding was true even for those with lesions at the lowest levels (L5 and sacral). Throughout the 12-month study period, infants with MMC continued to produce a large proportion of parallel steps, with both legs moving in synchrony, or single steps, in which one leg stepped but the other did not. The ability to initiate and control alternating movements of the legs is critical to developing functional skills such as creeping and walking. We argue that the difficulty in developing rhythmic coupled oscillation is derived not only from innervation asymmetries but also from the infants’ diminished spontaneous efforts to explore with their legs.35 Asymmetrical movements are typical early in life and in the treadmill context.26 By 6 to 7 months of age, infants with TD increase strength and control. Then their capacity to shift control from one interlimb organization to another and to respond to the dynamics of the supported treadmill context becomes strongly dominated by an alternating organization.
In contrast to interlimb stepping patterns, the step parameters of infants with MMC seemed quite similar to those of their peers with TD. Perhaps this is because within the step motion, the legs tend to conform to their pendular qualities, with movement being influenced by gravity and motion-dependent torque as well as the dynamic motion of the treadmill belt under the feet.44,45 It seems that when a step cycle is elicited in infants with MMC in this situation, the outcome is a pattern that has many of the parameters that are desirable in steps that produce locomotion. Step cycle duration as well as the proportions of the cycle represented by swing and stance were similar between the groups. Where individual steps differ more between groups is in the part of the foot that makes contact with the surface at touch-down. It is clear that anomalies of joints, in particular, club foot, heighten the tendency to make first contact with the lateral part of the foot. However, although lateral contact occurred more often, both groups showed significant postural variability and a great deal of toe contact at initial touch-down as well as contact with the flat part of the foot. During stance, infants with MMC, like their peers with TD, tended to show decreased lateral contact and an increase in the likelihood that the flat part of the foot supported body weight.
Finally, our results showed that at each age infants in both groups produced more motor activity (either with their entire body or with their legs only) when the treadmill belt was moving than when the belt was not moving. This result indicates that treadmill practice seems to be able to increase the level of activity of the population with MMC, both for strong steppers and for weak steppers. This is an important discovery for a population known to have decreased levels of motor activity.23,35 This result, added to the capacity of this population to respond to the treadmill with stepping during the first year of life, suggests the possibility of using treadmill practice as an additional therapy for infants with MMC. In the present study, babies were in contact with the moving belt for a total of only 3.3 minutes per test session. Increasing the duration of each session and repeating the exposure on multiple days per week may accelerate the infants’ developmental processes. That is, providing babies with more opportunities to accumulate experience in an upright, partial body-weight-bearing movement situation may facilitate the development of bone and joint tissue and help the babies develop neuromotor control of their legs.
Study Limitations
The present study had several limitations. Infants with MMC typically came to our laboratory before or after a checkup at the university hospital. Some parents drove a distance to the university; thus, whether their laboratory visit occurred before or after the hospital checkup, infants may not have been tested at an optimal point for energy and arousal levels, compared with infants with TD. These factors could have added to the decreased levels of activity in the infants with MMC. We were able to obtain only lesion level, which may not be as meaningful for behavior as true neurological level. We also would like to have had a larger sample to allow regression analysis to examine relationships between factors such as intrautero versus extrautero surgery, shunts, and revisions and the infants’ early motor performance and subsequent locomotor outcomes. Two infants started but did not complete the study; one family moved to a different state (infant in the TD group), and one family indicated that they had schedule conflicts (infant in the MMC group).
Conclusion
Our results showed that partial body-weight-bearing treadmill practice can increase leg activity and, specifically, elicit stepping patterns in infants with MMC at an average rate of 14.4 steps per minute. Responsiveness varied among infants and was affected by lesion level but was not uniquely predicted by it. Interlimb stepping was less readily alternating, but the within-limb step parameters seemed quite similar to those produced by infants with TD. Our next goal is to examine ways to modify treadmill practice to enable infants with MMC to respond with more steps than in the current testing paradigm. Subsequently, we plan to examine the potential for practice stepping on a treadmill, with human support like that provided here, to generate positive outcomes—such as increasing muscle and cardiovascular strength, bone density, and the neuromotor control needed for upright locomotion—for infants with MMC.
Dr Ulrich provided concept/idea/research design, project management, fund procurement, facilities/equipment, and institutional liaisons. Dr Teulier provided writing and data analysis. Dr Smith, Dr Kubo, Dr Chang, Dr Moerchen, and Dr Ulrich provided data collection. Dr Murazko provided participants.
Approval for this study was granted by the Institutional Review Board at the University of Michigan.
This research was funded by grant R01HD047567 awarded to Dr Ulrich by the National Institute of Child Health and Human Development, National Institutes of Health.
This work was presented orally at the annual meeting of the American Academy of Physical Medicine and Rehabilitation; September 27–30, 2007; Boston, Massachusetts; at the 19th annual meeting of the European Academy of Childhood Disability; June 14–16, 2007; Groningen, the Netherlands; and at the conference of the North American Society for the Psychology of Sport and Physical Activity; June 7–9, 2007; San Diego, California.
Peak Performance Technologies, 7388 S Revere Pkwy #901, Centennial, CO 80112.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1.Spina Bifida Fact Sheet. Bethesda, MD: National Institute of Neurological Disorders and Stroke; 2007. NIH Publication No. 07–309.
- 2.Cherian A, Seena S, Bullock RK, Antony AC. Incidence of neural tube defects in the least-developed area of India: a population-based study. Lancet. 2005;366:930–931. [DOI] [PubMed] [Google Scholar]
- 3.Lary JM, Edmonds LD. Prevalence of spina bifida at birth—United States, 1983–1990: a comparison of two surveillance systems. MMWR CDC Surveill Summ. 1996;45:15–26. [PubMed] [Google Scholar]
- 4.International Clearinghouse for Birth Defects Monitoring Systems. World Atlas for Birth Defects/International Centre for Birth Defects of the International Clearinghouse for Birth Defects Monitoring Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2003.
- 5.Duffy CM, Hill AE, Cosgrove AP, et al. Three dimensional analysis in spina bifida. J Pediatr Orthop. 1996;16:786–791. [DOI] [PubMed] [Google Scholar]
- 6.Huber-Okrainec J, Dennis M, Brettschneider J, Spiegler BJ. Neuromotor speech deficits in children and adults with spina bifida and hydrocephalus. Brain Lang. 2002;80:592–602. [DOI] [PubMed] [Google Scholar]
- 7.Bartonek A, Eriksson M, Saraste H. Heart rate and walking velocity during independent walking in children with low and midlumbar myelomeningocele. Pediatr Phys Ther. 2002;14:185–190. [DOI] [PubMed] [Google Scholar]
- 8.Williams EN, Broughton NS, Menelaus MB. Age-related walking in children with spina bifida. Dev Med Child Neurol. 1999;41:446–449. [PubMed] [Google Scholar]
- 9.Iborra J, Pages E, Cuxart A. Neurological abnormalities, major orthopaedic deformities and ambulation analysis in a myelomeningocele population in Catalonia (Spain). Spinal Cord. 1999;37:351–357. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg-Emons HJ, Bussman JB, Brobbel AS, et al. Everyday physical activity in adolescents and young adults with myelomeningocele as measured with a novel activity monitor. J Pediatr. 2001;139:880–886. [DOI] [PubMed] [Google Scholar]
- 11.Christopher RJ. Woodhouse myelomeningocele: neglected aspects. Pediatr Nephrol. 2008;23:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbins JC, Grannum PA, Berkowitz RL, et al. Ultrasound in the diagnosis of congenital anomalies. Am J Obstet Gynecol. 1979;134:331–345. [DOI] [PubMed] [Google Scholar]
- 13.Korenromp MJ, van Gool JD, Bruinese HW, Kriek R. Early fetal leg movements in myelomeningocele. Lancet. 1986;1:917–918. [DOI] [PubMed] [Google Scholar]
- 14.Warsof SL, Abramowicz JS, Sayegh SK, Levy DL. Lower limb movements and urologic function in fetuses with neural tube and other central nervous system defects. Fetal Ther. 1988;3:129–134. [DOI] [PubMed] [Google Scholar]
- 15.Sival DA, Begeer JH, Staal-Schreinemachers AL, et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum Dev. 1997;50:27–37. [DOI] [PubMed] [Google Scholar]
- 16.Sival DA, van Weerden TW, Vles JS, et al. Neonatal loss of motor function in human spina bifida aperta. Pediatrics. 2004;114:427–434. [DOI] [PubMed] [Google Scholar]
- 17.Chapman D. Context effects on the spontaneous leg movements of infants with spina bifida. Pediatr Phys Ther. 2002;14:62–73. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MH. Functional brain development during infancy. In: Bremmer G, Fogel A, eds. Blackwell Handbook of Infant Development. Malden, MA: Blackwell Publishing; 2004:169–190.
- 20.Sporns O, Edelman GM. Solving Bernstein's problem: a proposal for the development of coordinated movement by selection. Child Dev. 1993;64:960–981. [PubMed] [Google Scholar]
- 21.Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge, MA: MIT Press; 1994.
- 22.Oakeshott P, Hunt GM, Whitaker RH, Kerry S. Perineal sensation: an important predictor of long-term outcome in open spina bifida. Arch Dis Child. 2007;92:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sival DA, Brouwer OF, Bruggink JLM, et al. Movement analysis in neonates with spina bifida aperta. Early Hum Dev. 2006;82:227–234. [DOI] [PubMed] [Google Scholar]
- 24.Elman J, Bates E, Johnson M, et al. Rethinking Innateness.Cambridge, MA: MIT Press; 1996.
- 25.Ulrich BD, Jensen JL, Thelen E, et al. Adaptive dynamics of the leg movement patterns of human infants, II: treadmill stepping in infants and adults. J Mot Behav. 1994;26:313–324. [DOI] [PubMed] [Google Scholar]
- 26.Thelen E, Ulrich BD. Hidden skills: a dynamic systems analysis of treadmill stepping during the first year. Monogr Soc Res Child Dev. 1991;56:1–98; discussion 99–104. [PubMed] [Google Scholar]
- 27.Thelen E, Ulrich BD, Niles D. Bilateral coordination in human infants: stepping on a split-belt treadmill. J Exp Psychol Hum Percept Perform. 1987;13:405–410. [DOI] [PubMed] [Google Scholar]
- 28.Ulrich BD, Ulrich DA, Collier DH, Cole EL. Developmental shifts in the ability of infants with Down syndrome to produce treadmill steps. Phys Ther. 1995;75:14–23. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with Down syndrome: evidence-based developmental outcomes. Pediatrics. 2001;108:84–93. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich DA, Lloyd MC, Tiernan CW, et al. Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down syndrome: a randomized trial. Phys Ther. 2008;88:114–122. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Looper J, Ulrich BD, et al. Effects of different treadmill interventions on walking onset and gait patterns in infants with Down syndrome. Dev Med Child Neurol. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich BD, Haehl V, Buzzi UH, et al. Modeling dynamic resource utilization in populations with unique constraints: preadolescents with and without Down syndrome. Hum Mov Sci. 2004;23:133–156. [DOI] [PubMed] [Google Scholar]
- 33.Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–120. [DOI] [PubMed] [Google Scholar]
- 34.Hunt GM, Oakeshott P. Outcome in people with open spina bifida at age 35: prospective community-based cohort study. BMJ. 2003;326:1365–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rademacher N, Black DP, Ulrich BD. Early spontaneous leg movements in infants born with and without myelomeningocoele. Pediatr Phys Ther. 2008;20:137–145. [DOI] [PubMed] [Google Scholar]
- 36.Lomax-Bream LE, Barnes M, Copeland K, et al. The impact of spina bifida on development across the first 3 years. Dev Neuropsychol. 2007;31:1–20. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich BD, Ulrich DA, Collier DH. Alternating stepping patterns: hidden abilities of 11-month-old infants with Down syndrome. Dev Med Child Neurol. 1992;34:233–239. [DOI] [PubMed] [Google Scholar]
- 38.Bartonek A, Saraste H. Factors influencing ambulation in myelomeningocele: a cross-sectional study. Dev Med Child Neurol. 2001;43:253–260. [DOI] [PubMed] [Google Scholar]
- 39.Angulo Barroso RM, Tiernan C. Motor systems development. In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. 2nd ed. Cambridge, MA: MIT Press. In press.
- 40.Adolph KE, Eppler MA, Gibson EJ. Crawling versus walking infants’ perception of affordances for locomotion over sloping surfaces. Child Dev. 1993;64:1158–1174. [PubMed] [Google Scholar]
- 41.Goldfield EC, Kay BA, Warren WH Jr. Infant bouncing: the assembly and tuning of action systems. Child Dev 1993;64:1128–1142. [PubMed] [Google Scholar]
- 42.Rovee-Collier C. Information pick-up by infants: what is it, and how can we tell? J Exp Child Psychol. 2001;78:35–49; discussion 98–106. [DOI] [PubMed] [Google Scholar]
- 43.Sommerville JA, Woodward AL. Pulling out the intentional structure of action: the relation between action processing and action production in infancy. Cognition. 2005;95:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavagna GA, Franzetti P, Fuchimoto T. The mechanics of walking in children. J Physiol. 1983;343:323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt KG, Saltzman E, Ho CL, et al. Discovery of the pendulum and spring dynamics in the early stages of walking. J Mot Behav. 2006;38:206–218. [DOI] [PubMed] [Google Scholar]