Abstract
Background/aims
Iron overload can cause liver toxicity and increase the risk of liver failure or hepatocellular carcinoma in humans. Curcumin (diferuloylmethane), a component of the food spice turmeric, has antioxidant, iron binding, and hepatoprotective properties. The aim of this study was to quantify its effects on iron overload and resulting downstream toxic effects in cultured T51B rat liver epithelial cells.
Methods
T51B cells were loaded with ferric ammonium citrate (FAC) with or without the iron delivery agent 8-hydroxyquinoline. Cytotoxicity was measured by MTT assay. Iron uptake and iron bioavailability were documented by chemical assay, quench of calcein fluorescence, and ferritin induction. Reactive oxygen species (ROS) were measured by fluorescence assay using 2′,7′-dichlorodihydrofluorescein diacetate. Oxidative stress signaling to jnk, c-jun, and p38 was measured by western blot with phospho-specific antibodies.
Results
Curcumin bound iron, but did not block iron uptake or bioavailability in T51B cells given FAC. However, it reduced cytotoxicity, blocked generation of ROS, and eliminated signaling to cellular stress pathways caused by iron. Inhibition was observed over a wide range of FAC concentrations (50 – 500 μM), with an apparent IC50 in all cases between 5 and 10 μM curcumin. In contrast, desferoxamine blocked both iron uptake and toxic effects of iron at concentrations that depended on the FAC concentration. Effects of curcumin also differed from those of α-tocopherol, which did not bind iron and was less effective at blocking iron-stimulated ROS generation.
Conclusions
Curcumin reduced iron-dependent oxidative stress and iron toxicity in T51B cells without blocking iron uptake.
Keywords: curcumin, iron, liver disease, oxidative stress, stress activated MAP kinase
Introduction
Iron is essential for many biological processes, but can be toxic when present at high levels in a free or loosely bound form (1, 2). As a redox active transition metal, iron generates reactive oxygen species (ROS) via the Fenton and Haber-Weiss reactions (3, 4). ROS react directly with proteins, lipids, and nucleic acids and induce oxidative stress by depleting cellular stores of antioxidants (2, 4). ROS also influence multiple cell signaling pathways important to cell survival, proliferation, and death (5). The liver plays a key role in regulating body iron, both by storing iron during times of excess and by stimulating uptake during deficiency (6–8). Conditions that cause excess iron uptake, including hereditary hemochromatosis, are accompanied by iron deposition in liver. Excess iron has toxic effects that may lead to fibrosis, cirrhosis, and liver failure or hepatocellular carcinoma (1, 9).
Curcumin is the principle active ingredient in turmeric, a spice derived from the rhizome of Curcuma longa. Turmeric is used in both Chinese and Indian traditional medicines (10), with applications as an anti-inflammatory agent, for peptic ulcer and dyspepsia, in skin diseases and wound healing, and in liver and urinary tract diseases (11, 12). Turmeric is a food substance in many cultures and lacks significant toxicity in vivo (13). Even very high levels of curcumin and related curcuminoids had low toxicity at oral doses up to 12 g per day in humans (14–16). Curcumin has potent antioxidant and free radical scavenging activity, and many relevant enzymatic targets have been identified (12, 17). Curcumin is also effective in preventing chemically-induced liver damage. It decreased liver toxicity in rats caused by galactosamine or carbon tetrachloride (18, 19), and prevented carcinogenic effects of the hepatocarcinogens afflatoxin or nitrosodiethylamine (12, 20–22). It reduced liver fibrosis in a rat model of nonalcoholic steatohepatitis and in rats given thioacetamide (23, 24). These findings make curcumin a strong candidate for prevention of liver damage associated with iron overload.
Curcumin is also promising because it can bind iron (25, 26). Its affinity for iron is similar to other iron chelators such as nitrilotriacetic acid (NTA), albeit lower than clinically used chelators such as desferoxamine (pM 17 for curcumin:Fe3+ vs. pM 26 for desferoxamine:Fe3+) (27). A dose of 4g curcumin can theoretically bind up to 600mg iron (25), approximately 15% of total (human) body iron and roughly half that found in normal liver (7). Although not all iron exists in forms extractable by curcumin, it is reasonable to predict that a safe, well-tolerated dose may chelate a significant fraction of body iron. In support of this idea, mice fed a diet high in curcuminoids had decreased levels of liver ferritin, indicative of decreased iron burden (27).
The purpose of this study was to determine whether curcumin prevents iron toxicity in cultured cells, and if so to investigate possible mechanisms. We found curcumin reduced or blocked several indicators of oxidative stress and iron toxicity in T51B rat liver epithelial cells. Surprisingly, this occurred without a corresponding block of iron uptake or iron bioavailability.
Materials and Methods
Materials
Ferric ammonium citrate (FAC), desferoxamine, α-tocopherol, and calcein-AM were from Sigma/Aldrich (St. Louis, MO). Newborn calf serum was from Atlanta Biologicals (Norcross GA). Other cell culture reagents were from GIBCO/Invitrogen (Carlsbad, CA). 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was from Molecular Probes/Invitrogen (Eugene, OR). Curcumin was obtained from the chemoprevention repository of the National Cancer Institute. It was 94% pure curcumin (diferuloylmethane) based on isocratic C18 HPLC in 0.1% citric acid:tetrahydrofuran (60:40) and detection at 420 nm. The small amount of non-curcumin material co-migrated with desmethoxycurcumin. For this and all reagents, however, concentrated stock solutions were prepared assuming 100% reagent purity and stored in aliquots at −20°C. Antibodies and other specialty reagents were from commercial sources as noted below. Concentrated stock reagents prepared in water and sterile filtered, or dissolved in organic solvent as indicated, were stored in aliquots at −20°C and kept on ice after thawing. As appropriate, control experiments documented that solvent alone had no significant effect.
Cell culture and iron treatment
T51B is a non-neoplastic rat liver epithelial cell line with properties similar to oval cells, a hepatocyte precurser cell in liver (28–30). They have been used for tumor promotion and carcinogenicity studies (31–33), and are particularly well suited for investigating effects of iron associated with neoplastic transformation and hepatocellular carcinoma (34). T51B cells were maintained in Eagles basal media supplemented with 10% newborn calf serum, 2mM l-glutamine, and 100 U/ml penicillin/streptomycin (complete media), at 37 °C in a 5% CO2 atmosphere. In general, cells were seeded for experiments at a density of 200,000 cells per 60 mm dish. When left untreated, they became confluent 4–6 days after plating and were subsequently quiescent (33). Treatment protocols were initiated 1 day after plating as specified in the Figure legends. FAC was added directly to the culture media and replenished as appropriate with all subsequent media renewals. Where indicated, 10 μM 8-hydroxyquinoline was given simultaneously with FAC. Ferric citrate is an important form of non-transferrin bound iron present in human blood and known to increase with iron overload in hemochromatosis (35, 36). FAC was chosen because it is less prone to in vitro formation of insoluble iron hydroxides than ferric citrate (37).
Statistical analysis
Quantitative results are presented throughout the paper as mean values, with the number of replicates and standard deviation (s) or standard error (s.e.) specified in each Figure or Legend. Where indicated, differences between treatment groups were evaluated using a 2 tailed unpaired student t-test for samples with unequal variance, and significance noted at moderate (p<0.05) and high (p<0.001 levels). Depending on the type of assay and as specified in the Figure Legends, statistical analyses were performed and presented either as a single representative experiment, after compiling all data from multiple experiments, or by treating the mean values from each experiment as a single value.
Iron affinity resin
Fe-NTA-Agarose was constructed from commercially available nickel NTA agarose (Qiagen, Inc. Valencia, CA). The Ni-NTA agarose was stripped with EDTA and recharged with iron as has been described (38). Binding and pull down experiments were performed in 50 % ethanol and assumed 100% replacement of Ni sites (defined by the manufacturer) with Fe. For the binding experiments, the indicated amounts of curcumin were incubated with or without Fe-NTA-Agarose in 50% ethanol at room temperature (roughly 25 nmol metal binding sites in 0.5 ml total volume). After 10 minutes, the resin was removed by centrifugation, and aliquots of the supernatants were diluted as needed (10-fold) to determine curcumin remaining in solution (absorbance at 435 nm). Affinity estimations were based on the method of Scatchard, assuming free curcumin equal to the amount remaining in solution in the presence of Fe-NTA-agarose, and bound curcumin equal to the amount curcumin added minus the free measured at each concentration shown.
Biochemical assays
Total iron content of cells was determined using bathophenanthroline disulfonate (39). Cell were rinsed in PBS, lysed in buffer A (0.5M KOH 0.1% triton X-100), and one volume of buffer B (10% TCA 3% thioglycolic acid 2M HCl) was added on ice. After 10 minutes the precipitated protein was removed by centrifugation, the supernatant was combined with one volume of buffer C (0.42M bathophenanthroline disulfonate 2M sodium acetate), and incubated for 30 minutes at room temperature. Absorbance at 540-570 nm was determined on duplicate samples and quantified by interpolation from a standard curve constructed from FAC diluted in buffer A and carried through the assay in parallel.
Procedures used for western blot analysis, including cell harvesting, have been described (33, 40). Equal aliquots of cell lysate protein were loaded in each gel lane. Antibodies to ferritin heavy chain and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against c-jun N-terminal kinase 1 and 2 (jnk) dually phosphorylated at thr183/tyr185, c-jun phosphorylated at ser63, p38 MAP kinase (p38) dually phosphorylated at thr180/tyr182, and the p65 subunit of NF-kappaB phosphorylated at ser536, were from Cell Signaling Technologies, Inc. (Danvers, MA), as were antibodies that recognized each of these proteins regardless of phosphorylation state (“total”). Secondary antibodies linked to horseradish peroxidase were from Jackson Immunoresearch (West Grove, PA). Chemiluminescent detection used the ECL-plus system from Amersham/GE Healthcare (Arlington Heights, IL), followed by exposure to autoradiography film. The chemiluminescence signals were quantified with an ICL UVP gel imager equipped with a Hamamatsu CCD camera using Labworks imaging software.
Cell assays
Toxicity assays used the MTT method (41) in a 96 well plate format at an initial seeding density of 10,000 cells per well. Treatments were initiated 1 day after plating and renewed in fresh complete media after 2 days and after 5 days. Dimethylformamide alone, at the levels used as solvent for curcumin, had no effect, and was generally included in FAC treatments. After the specified treatment time, cells were rinsed with PBS and incubated with 0.3 μg/ml methylthiazolyldiphenyl-tetrazolium bromide (MTT) in complete media containing 10 mM HEPES pH7.4 for 3 hours. The formazan product was solubilized in DMSO and measured by absorbance at 540-570 nm.
Quenching of calcein fluorescence in cells by iron was assessed by two methods: epifluorescence microscopy and a plate reader fluorescence assay. For microscopy, cells plated on glass coverslips were treated for 30 minutes with 0.25 μg/ml calcein-AM (Sigma) in serum-free media, incubated for an additional 30 minutes without calcein in complete media, and finally incubated with or without FAC for the times indicated in the Figure Legends. Co-incubation with curcumin or other treatments was as specified. The coverslips were rinsed 3x in PBS on ice, mounted using Vectashield (Vector Laboratories, Burlingame, CA), and viewed with a Nikon 50i inverted microscope equipped with X-cite 120 epifluorescence and a fluorescein filter set. For each treatment condition, identical fields were photographed with a Nikon Coolpix 4500 digital camera to record FITC and phase contrast views. Constant photographic parameters (exposure, contrast, magnification, etc.) were maintained for all treatment conditions presented in a given Figure. In some experiments, the green fluorescence in experimental and control (no calcein) fields were quantified from images generated by a Leica DMIRB inverted microscope equipped with a Diagnostic Instruments Pursuit CCD camera. The total pixel intensities of fields matched for cell number and treatment were determined using ImageJ software (NIH). Statistical evaluation of the data was conducted as described above.
A similar protocol was used to measure calcein fluorescence in 96 well plates. Cells were loaded with 0.25 μg/ml calcein in serum free media for 30 minutes at 37°C, rinsed, and then treated (in triplicate) as specified in complete culture media for 2 hours. The cells were rinsed three times with PBS, and green fluorescence was measured with a Victor 1420 plate reader (Wallac/Perkin Elmer Instruments) equipped with 485nm excitation and 535nm emission filters.
Measurement of reactive oxygen species using H2DCFDA fluorescence was also done in a 96 well plate format using conditions recommended by Molecular Probes, and adapted by us for other studies (DJM and KVK, unpublished). The day before the assay, the media on confluent T51B cells was changed to phenol red-free DMEM containing 10% newborn calf serum, 1g/L glucose, 2mM l-glutamine, 110 mg/L sodium pyruvate, 100 U/ml penicillin/streptomycin (ROS assay media) and supplemented with 0.2 mM oleic acid. Following an 18 hr equilibration, the cells were rinsed in Hanks Balanced Salt Solution containing calcium and magnesium (HBSS), and loaded with 20 μM H2DCFDA in HBSS containing 1% BSA. After 30 minutes the cells were rinsed and treated in ROS assay media containing 0.2 mM oleic acid, used to accentuate ROS generation by iron. Cells were assayed in triplicate using the Victor plate reader, as described above for calcein, and values reported after subtracting background fluorescence measured in parallel control wells not loaded with H2DCFDA.
Results
Curcumin binds iron
Evidence for iron binding to curcumin in solution was previously obtained using spectroscopic shift techniques (26, 42). Based on these reports, we investigated curcumin binding to iron using metal affinity chromatography. Figure 1 shows the removal of curcumin from solution by Fe-NTA-Agarose. Curcumin binding was rapid and efficient; up to 95% of the curcumin was removed after incubation for 10 minutes with the iron-containing resin in this experiment. The diketone groups of curcumin likely interact with the Fe resin in a similar fashion to the phosphate oxygens on phosphopeptides, for which this iron affinity chromatography resin was developed (38). Examination of the data shown in Figure 1 gave an affinity estimate near one μM, in agreement with the high affinity iron binding site on curcumin reported by others (26). Comparable curcumin binding was not observed when Ni was used as the metal ion, and a significant fraction of curcumin (based on A435) was recovered from Fe-NTA-Agarose upon addition of the competitive iron chelators desferoxamine or EDTA (Figure 1B). These data reinforced previous reports of iron binding to curcumin, and suggested to us that iron chelation may enable curcumin to reduce both the uptake and toxicity of iron in cells.
Figure 1.
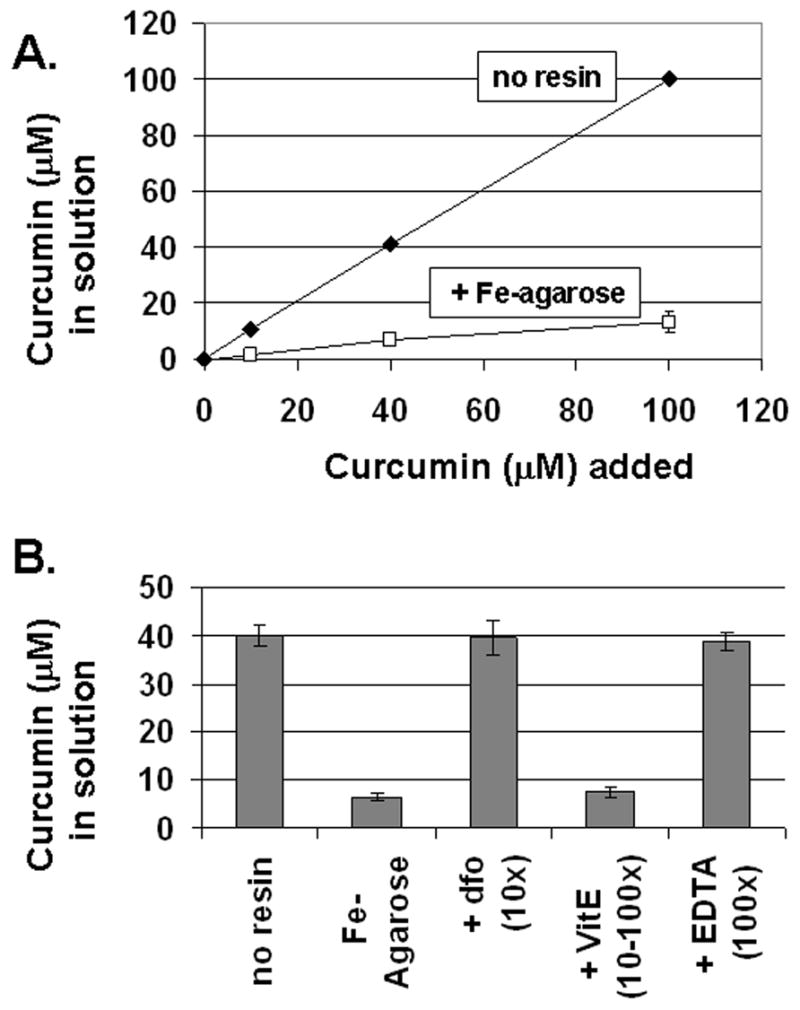
Curcumin binds to Iron. A. Removal of curcumin from solution by Fe-NTA resin. Increasing amounts of curcumin were incubated with Fe-NTA-Agarose (squares) or without (diamonds) as described under Materials and Methods. The unbound curcumin remaining in solution after removal of the resin was determined by absorbance at 435 nm. B. Inhibition of curcumin binding to iron. Curcumin (40 μM) was incubated alone (no resin), with iron resin (Fe- Agarose), or with iron resin preincubated with excess desferoxamine (dfo-10x), excess vitamin E (VitE 10-100x), or excess EDTA (100x). Unbound curcumin was determined as for panel A. The means +/− s.e. are presented from at least three independent determinations.
Curcumin inhibits iron toxicity in T51B cells
Ferric ammonium citrate (FAC), a physiologically relevant form of non-transferrin-bound iron (NTBI), inhibited the proliferation of T51B cells at high micromolar concentrations (34). To characterize this further, the time and concentration-dependent toxicity of FAC was measured by MTT assay, which reports on both the number of cells and their relative viability. As shown in Figure 2A, the growth-related increase in MTT signal was almost completely blocked by treatment with 500 μM FAC. At 200 μM FAC, the MTT signal was reduced compared to control, but the cells continued to grow. There was little or no effect at 50 μM FAC (92% of the MTT signal from control cells at 5 days, n=3). There were also negligible effects of 200 or 500 μM ammonium citrate (102% of the MTT signal from control cells at 5 days, n=2), indicating that toxicity of FAC is due specifically to iron. Figure 2 also shows that FAC toxicity was reduced by 50 μM curcumin. This was most evident after 10 days of treatment with 500 μM FAC (Figure 2A). Although 50 μM curcumin alone was toxic to T51B cells at 5 days (as were other iron chelators such as desferoxamine; both treatments resulted in no surviving cells by MTT assay), its effects were minimal in the presence of added iron. The MTT signal from cells given 50 μM FAC + 50 μM curcumin for 5 days was 79% of untreated cells (n=3 experiments). Figure 2B shows a concentration-effect analysis after 5 days treatment with 500 μM FAC and increasing curcumin concentrations. Under these conditions curcumin inhibited toxicity with an apparent IC50 near 10 μM. Protection was optimal by 50 μM and was not improved by increasing the curcumin concentration up to 200 μM.
Figure 2.
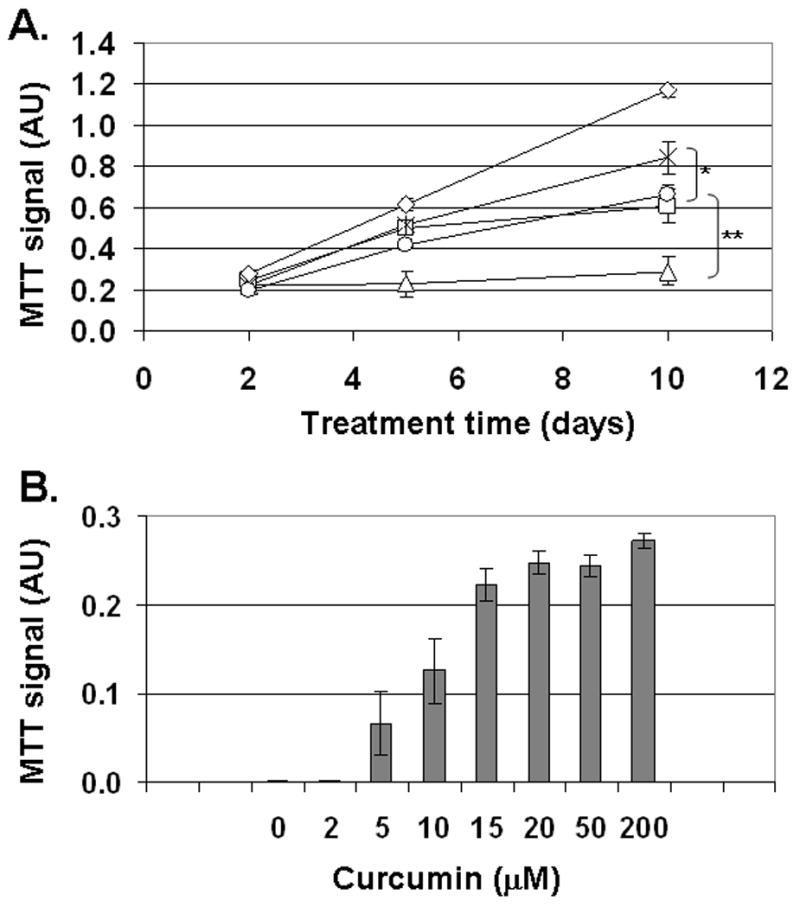
Curcumin reduces iron toxicity in T51B cells. A. Time course of cell growth in FAC. Cells treated with iron for 2, 5, or 10 days were assayed by MTT assay as described under Materials and Methods. The signals from untreated control cells (diamonds) are compared to 200 μM FAC (squares), 500 μM FAC (triangles), 200 μM FAC and 50 μM curcumin (stars), or 500 μM FAC and 50 μM curcumin (circles). Assays were performed in triplicate at the indicated times. The means +/− s.e. of data from 3 experiments are shown for each point. The significance of curcumin’s effects were calculated by two-tailed t-test at the 10 day time points (*p<0.05; **p<0.001). B. Concentration dependence of protection by curcumin. Cells were treated for 5 days with 500 μM FAC alone (500 FAC) or 500 μM FAC and curcumin (+ curc) at the indicated concentration (2–200 μM), and assayed in triplicate by MTT assay. The means +/− s.e. of data from 2–3 experiments are shown for each condition.
Curcumin does not block iron uptake in T51B cells
To begin to understand the mechanism(s) of curcumin action, we investigated its effects on iron uptake by T51B cells. Results from three approaches are shown in Figure 3, using a concentration of curcumin that was maximally effective in the toxicity assays. First, total iron was measured after 5 days treatment with 200 μM FAC (Figure 3A). Under these conditions the cells internalized approximately 4% (30 nmol) of the added iron. This was not reduced by 50 μM curcumin (Figure 3A). Co-treatment with the iron chelator desferoxamine was shown previously to reduce iron loading (34). Second, curcumin did not block the influx of free iron, as judged by quench of intracellular calcein fluorescence (Figure 3B). Finally, induction of ferritin H, another measure of free iron in cells, was not inhibited by curcumin (Figure 3C). Taken together, our results indicated that free iron was taken up normally in the presence of curcumin. The toxic effects of iron loading were greatly reduced, however.
Figure 3.
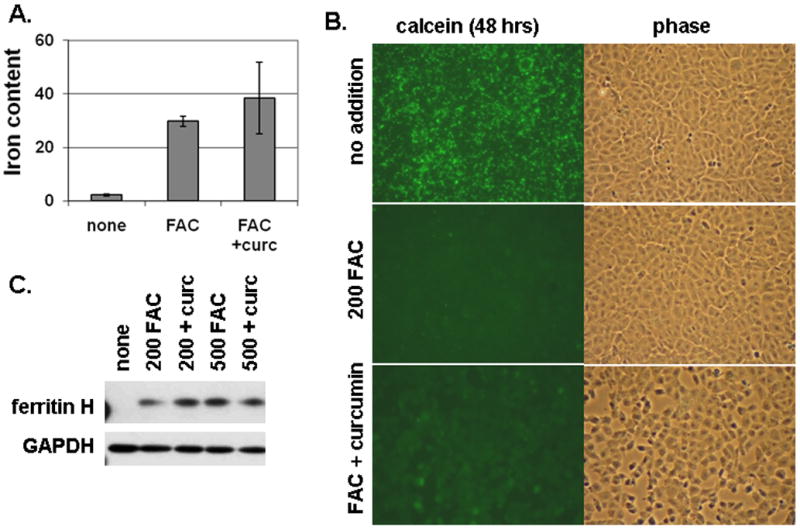
Curcumin does not block iron loading of T51B cells. A. Total iron content. Cells were untreated (none), or treated with 200 μM FAC (FAC), or 200 μM FAC with 50 μM curcumin (FAC + curc). Iron content was assayed colorimetrically as described under Materials and Methods. Mean values (nmol iron/mg cell protein) +/− s.d. of triplicates are reported. B. Quenching of intracellular calcein by iron. Cells were pulsed with calcein-AM and then incubated for 48 hours in media alone (top panels: no addition) or with addition of 200 μM FAC (middle panels: 200 FAC) or 200 μM FAC and 50 μM curcumin (bottom panels: FAC + curcumin). Identical fields viewed by FITC fluorescence (calcein) and phase contrast are shown for each. The cells were viewed without fixation. A decrease in green fluorescence indicates quenching of calcein by internalized iron. C. Ferritin induction by iron. Cells were left untreated (none) or treated with 200 μM FAC (200 FAC), 200 μM FAC and 50 μM curcumin (200 + curc), 500 μM FAC (500 FAC), or 500 μM FAC and 50 μM curcumin (500 + curc) for 5 days. Cell lysates were prepared and processed for western blot using antibodies specific for ferritin H or GAPDH as gel loading control. All results are representative of at least three independent experiments. Additional details are described under Materials and Methods.
Curcumin blocks iron-dependent generation of ROS
Generation of ROS via the Fenton reaction is thought to account for the toxicity of free iron (1, 2). To monitor this in T51B cells, we first sought a way to increase the rate of iron uptake. Lipophillic iron chelators deplete iron from cells when given alone but facilitate delivery when given with iron (43–45). We adopted this strategy using the iron chelator 8-hydroxyquinoline (46). Figure 4 shows that 8-hydroxyquinoline significantly accelerated iron uptake in T51B cells. Iron-dependent quench of calcein fluorescence was maximal by 1 hour with 8-hydroxyquinoline, compared to 24–48 hours required without (Figure 3B and data not shown). 8-hydroxyquinoline also increased the cytotoxicity of iron. As shown in Figure 5, 10 μM 8-hydroxyquinoline alone was not toxic up to 10 days of treatment, and 200 μM FAC alone had only a modest effect (30% decrease in MTT signal). This is comparable to the results shown in Figure 2. However, the combination resulted in greater than 95% loss of viability. This was reduced to less than 40% when 50 μM curcumin was added to the cells (Figure 5).
Figure 4.
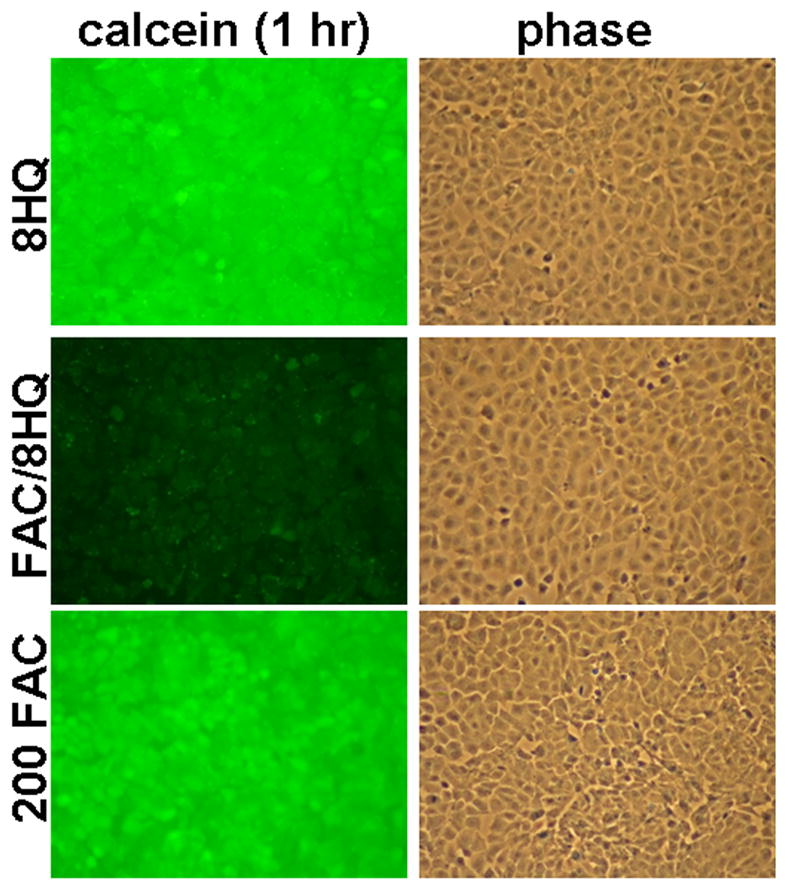
The chelator 8-hydroxyquinoline accelerates iron loading. Cells were pulsed with calcein-AM, rinsed, and incubated at 37 °C for 1 hour in complete media containing: 10 μM 8-hydroxyquinoline only (top panels: 8HQ), 10 μM 8-hydroxyquinoline and 200 μM FAC (middle panels: FAC/8HQ), or 200 μM FAC only (bottom panels: 200 FAC). The cells were rinsed on ice and viewed within 1 hour without fixation. Identical fields for FITC fluorescence (calcein) and phase contrast are shown for each treatment. This experiment was repeated twice with similar results.
Figure 5.
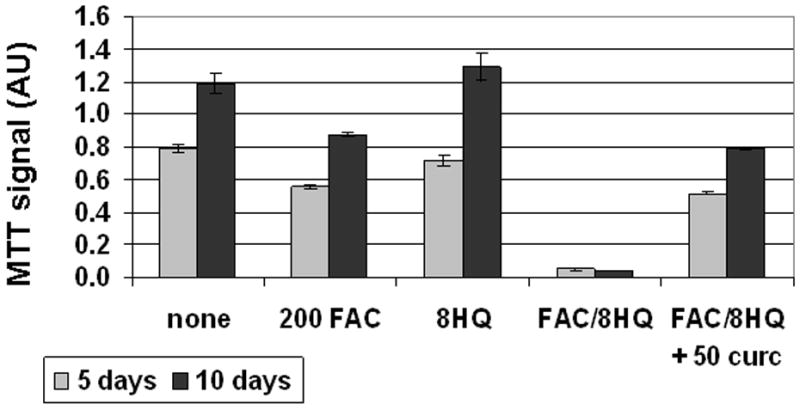
Curcumin reduces iron toxicity in the presence of 8-hydroxyquinoline. Cells were left untreated (none) or treated with 200 μM FAC (200 FAC), 10 μM 8-hydroxyquinoline (8HQ), 200 μM FAC and 10 μM 8-hydroxyquinoline and (FAC/8HQ), or 200 μM FAC and 10 μM 8-hydroxyquinoline and 50 μM curcumin (FAC/8HQ + 50 curc). They were assayed by MTT assay after 5 days (light bars) or 10 days (dark bars) as described under Materials and Methods. The mean +/− s of triplicate absorbance readings (540 nm) are presented. This experiment was repeated twice with similar results.
Figure 10.
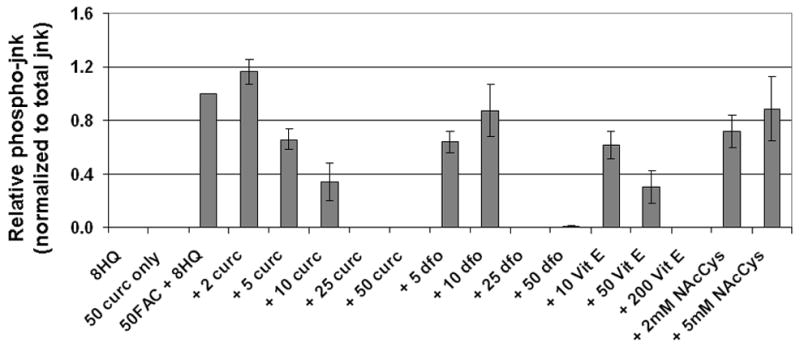
Curcumin is a potent inhibitor of jnk phospho-activation by iron. Cells treated for 2 hours were analyzed by western blot using antibodies specific for phospho-jnk and total jnk. The chemiluminescent signals were quantified as described under Materials and Methods. The relative p-jnk/total jnk ratios from 3–9 independent experiments (each normalized to the no inhibitor bands) are expressed as means +/− s.e. The treatments conditions were: 10 μM 8-hydroxyquinoline alone (8HQ), 10 μM 8-hydroxyquinoline and 50 μM curcumin (50 curc only), 10 μM 8-hydroxyquinoline and 50 μM FAC without inhibitor (50FAC + 8HQ), or 10 μM 8-hydroxyquinoline and 50 μM FAC with the inhibitors: curcumin at 2, 5, 10, 25 or 50 μM (+ 2 curc, + 5 curc, + 10 curc, + 25 curc, + 50 curc); desferoxamine at 5, 10, 25, or 50 μM (+ 5 dfo, + 10 dfo,+ 25 dfo, + 50 dfo); vitamin E at 10, 50, or 200 μM (+ 10 VitE, + 50 VitE,+ 200 VitE); or N-acetyl cysteine at 2 or 5 mM (+ 2mM NAcC, + 5mM NAcC).
To determine whether curcumin influenced iron uptake in 8-hydroxyquinoline-treated cells, we initially used fluorescence microscopy. As quantified in Figure 6, 50 μM curcumin did not interfere with the ability of iron to quench calcein or with the ability of 8-hydroxyquinoline to rapidly transport iron. To investigate a broader range of FAC and inhibitor conditions, we incorporated this basic protocol into a high throughput 96-well plate fluorescence assay. As shown in Figure 7, the decrease in fluorescence caused by 50 or 200 μM FAC was similar without or with curcumin (at 10 and 50 μM). A different pattern was seen for 50 μM dfo, which was effective at 50 μM FAC but not 200 μM FAC. It is possible that iron uptake was reduced slightly by curcumin, as there was a small amount of fluorescence of curcumin-treated cells that was not due entirely to the background measured in the absence of calcein (Figure 7). Under these same conditions, however (200 μM FAC and 50 μM curcumin), iron toxicity was greatly reduced (Figure 5). Block of iron toxicity cannot be explained by block of iron uptake.
Figure 6.
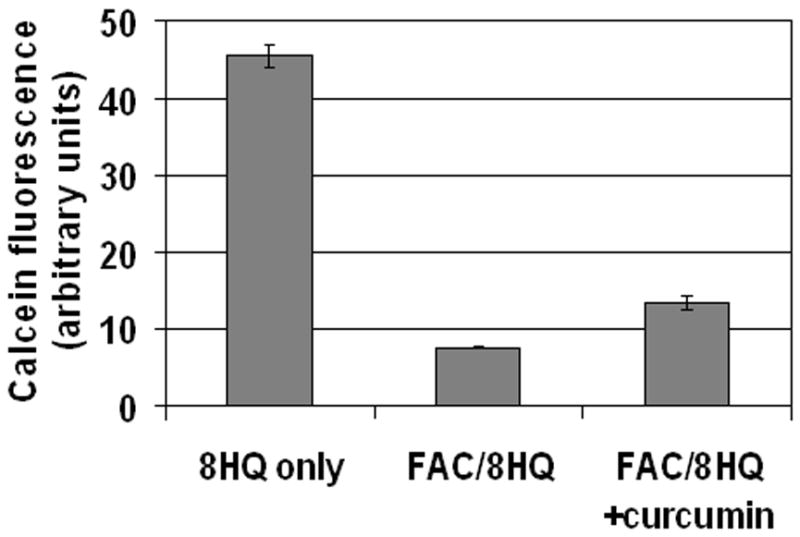
Curcumin does not block iron uptake mediated by 8-hydroxyquinoline. Cells were pulsed with calcein-AM, rinsed, and incubated at 37 °C for 2 hours in media containing 10 μM 8-hydroxyquinoline and: no iron (8HQ only), 200 μM FAC (FAC/8HQ), or 200 μM FAC and 50 μM curcumin (FAC/8HQ + curcumin). Control fields without calcein were used to define background for each condition. The calcein fluorescence from 6 distinct fields per condition was quantified as described under Materials and Methods, and mean values +/− s.e. are presented. This experiment was repeated twice with similar results.
Figure 7.
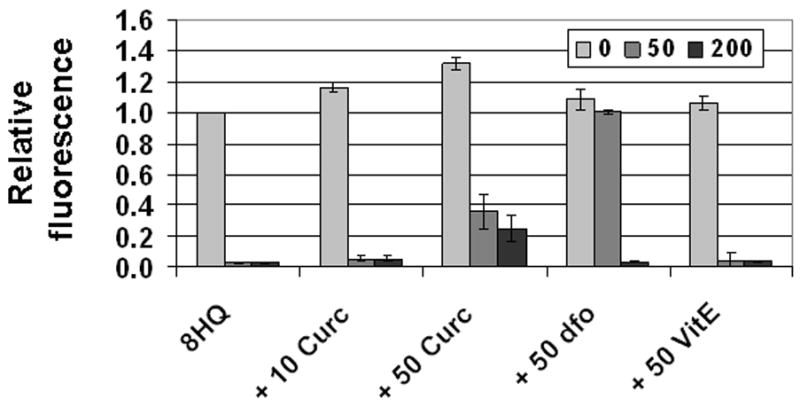
Desferoxamine, but not curcumin, blocks iron uptake mediated by 8-hydroxyquinoline. Cells in 96 well plates were pulsed with calcein-AM, and then incubated at 37 °C for 2 hours in media containing either no iron (light bars), 50 μM FAC (medium bars), or 200 μM FAC (dark bars). Test wells contained 10 μM 8-hydroxyquinoline alone (8HQ), or 10 μM 8-hydroxyquinoline and either: 10 μM curcumin (+ 10 curc), 50 μM curcumin (+ 50 curc), 50 μM desferoxamine (+ 50 dfo), or 50 μM vitamin E (+ 50 VitE). Calcein fluorescence was quantified as described under Materials and Methods. Values (normalized to cells treated with calcein and 8-hydroxyquinoline only) are reported as means +/− se, using data from 3 independent experiments.
We next examined the effect of curcumin on iron-induced ROS generation directly in T51B cells loaded with the fluorescent indicator H2DCFDA. This probe is sensitive to a variety of oxidative species, including hydroxyl radical, a toxic product of the Fenton reaction (47). We found the iron-dependent increase in ROS was completely blocked by either 50 μM curcumin or 50 μM dfo (Figure 8A). ROS generation caused by 50 μM FAC was also reduced more than 50% by 10 μM curcumin (Figure 8B). In contrast, 10 μM dfo was ineffective. These results indicate the curcumin IC50 for inhibition of ROS generation by 50 μM FAC/8HQ is below 10 μM, similar to our estimate for inhibition of cytotoxicity by 500 μM FAC (Figure 2B). These similar IC50 values over a 10-fold range of iron concentrations argue that iron chelation is not a primary mechanism of action for curcumin. A different pattern was seen for dfo, which was ineffective when the concentration of drug was below the concentration of iron. Notably, however, other compounds with antioxidant activity but without iron binding activity, including α-tocopherol (vitamin E) and N-acetyl cysteine, were significantly less potent than curcumin in this assay (Figure 8B).
Figure 8.
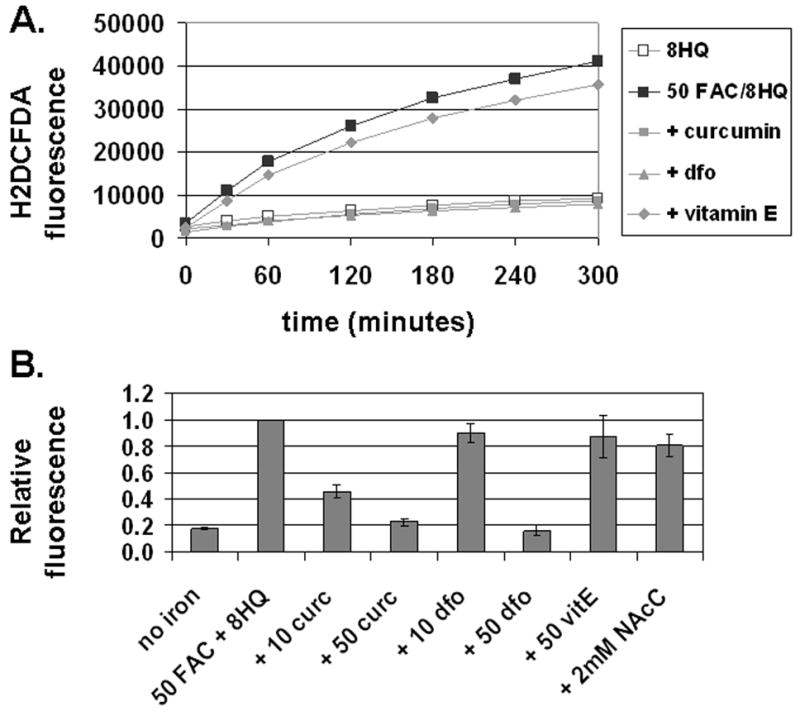
Curcumin and desferoxamine block iron-induced generation of reactive oxygen species (ROS). A. Time course of ROS generation. Cells loaded with oleic acid and H2DCFDA were treated at 37°C as specified. The fluorescent signal was measured at the indicated times as described under Materials and Methods. The treatment conditions were: 10 μM 8-hydroxyquinoline alone (open squares, 8HQ), 10 μM 8-hydroxyquinoline and 50 μM FAC without inhibitor (solid squares, 50FAC/8HQ), or 10 μM 8-hydroxyquinoline and 50 μM FAC with the inhibitors: 50 μM curcumin (+ curcumin), 50 μM desferoxamine (dfo), or 50 μM vitamin E (+ Vitamin E). Values for H2DCFDA-specific fluorescence are reported in arbitrary units as the means of triplicates. This representative experiment was repeated twice. B. Effect of potential inhibitors on ROS generation by iron. The change in H2DCFDA fluorescence from 1 to 3 hours was determined from a time course as for panel A. The treatment conditions were: 10 μM 8-hydroxyquinoline alone (no iron), 10 μM 8-hydroxyquinoline and 50 μM FAC without inhibitor (50FAC + 8HQ), or 10 μM 8-hydroxyquinoline and 50 μM FAC with the inhibitors: curcumin at 10 or 50 μM (+ 10 curc, + 50 curc), desferoxamine at 10 or 50 μM (+ 10 dfo, + 50 dfo), 50 μM vitamin E (+ 50 VitE), or 2 mM N-acetyl cysteine (+ 2mM NAcC). Signals from 2–4 independent experiments were normalized to the rate obtained without inhibitor and are expressed as means +/− s.e.
Curcumin inactivates iron signaling to redox-sensitive biochemical pathways
Several prominent ROS-sensitive signaling pathways were investigated by western blotting using antibodies specific for the phospho-activated forms of the proteins (Figure 9). By this measure, FAC and 8-hydroxyquinoline activated: (i) the stress activated MAP kinases jnk and p38, (ii) the AP-1 protein c-jun, and (iii) the p65 subunit of NF-kappa B. For the stress activated kinases jnk and p38, signaling was rapid and sustained, with strong signals remaining 4 hours after addition of FAC and 8-hydroxyquinoline. There was little or no phospho-activation by the non-toxic treatment of 50 μM FAC alone (no 8-hydroxyquinoline), and no effect in cells treated with 8-hydroxyquinoline and ammonium citrate (no iron). Figure 9 also demonstrates that phospho-activation of each of these oxidative stress responsive pathways was prevented by 50 μM curcumin. The curcumin block appeared complete over a range of iron concentrations, including a molar ratio of curcumin to iron of one-to-four. This was similar to the substoichiometric block demonstrated in the assays of cytotoxicity and ROS generation, and could not be explained by block of iron uptake.
Figure 9.
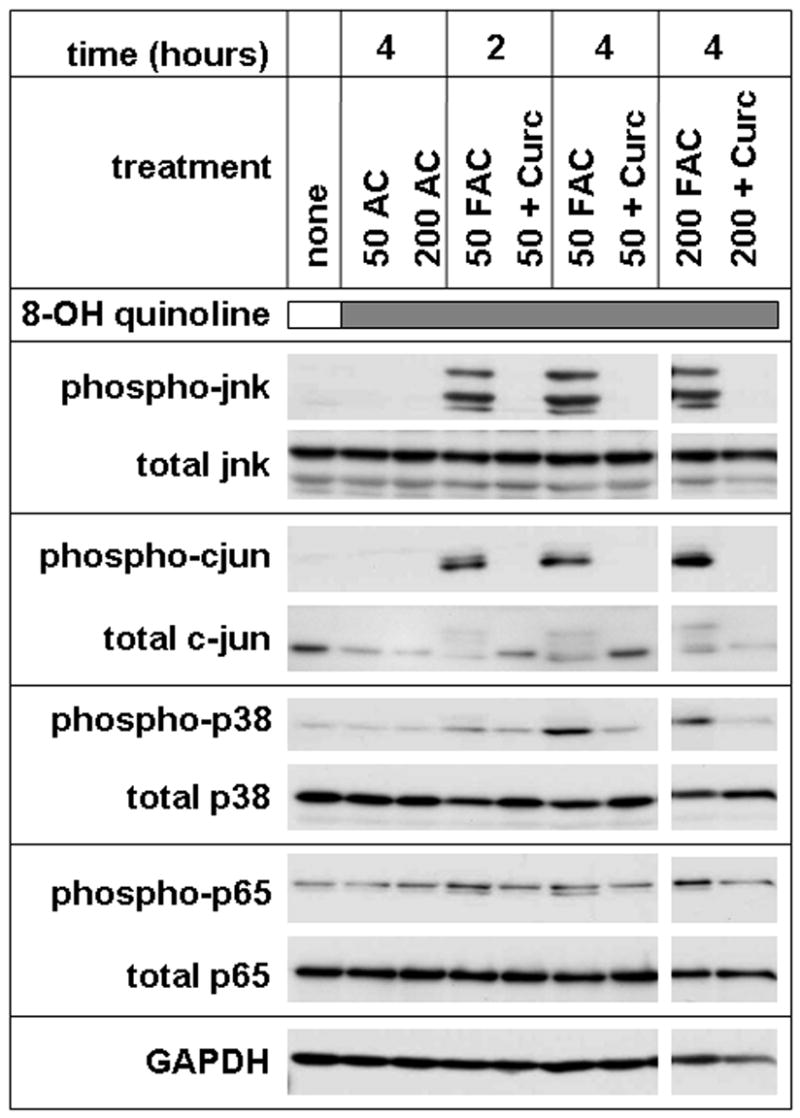
Curcumin blocks multiple iron-sensitive cell signaling pathways. Confluent cells were left untreated (none), or treated with 10 μM 8-hydroxyquinoline (shaded bar) and either 50 μM ammonium citrate (50 AC), 200 μM ammonium citrate (200 AC), 50 μM FAC (50 FAC), 50μM FAC and 50 μM curcumin (50 + curc), 200 μM FAC (200 FAC), or 200 μM FAC and 50 μM curcumin (200 + curc) for the indicated number of hours. Total cell lysates were analyzed by western blotting with antibodies to: jnk dually phosphorylated at thr183/tyr185 (phospho-jnk), c-jun phosphorylated at ser63 (phospho-cjun), p38 dually phosphorylated at thr180/tyr182 (phospho-p38), the p65 subunit of NF-kappaB phosphorylated at ser536 (phospho-p65), or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as loading control. Blots using antibodies that recognize each protein independently of phosphoylation state (total) are also shown. All panels are from a single gel. The results are representative of at least three similar experiments.
To investigate the potency of curcumin relative to other known chelators and antioxidants, we focused on the inhibition of jnk phospho-activation (Figure 10). Using a 2 hour treatment with 50μM FAC/8HQ as the activating stimulus, we found an apparent IC50 for curcumin near 5 μM, similar to our earlier results for cytotoxicity and ROS generation. The IC50 for dfo under these conditions was between 10 and 25 μM, for vitamin E near 10 μM, and for N-acetyl cysteine greater than 5 mM.
Discussion
Curcumin was an effective inhibitor of iron toxicity in T51B cells. It prevented iron-induced ROS generation and signaling of iron to several oxidative stress responsive pathways. Based on its ability to bind iron, and on similar protective effects of iron chelating drugs such as desferoxamine, we predicted initially that curcumin would significantly reduce iron loading of the cells. This was supported by a recent report in the literature that curcumin lowered iron levels in mice (27). However, we found that curcumin did not block iron uptake in T51B cells, even though we could demonstrate high affinity iron binding. In addition, the potency of curcumin as a protective agent in several assays was independent of the iron concentration. These observations indicate curcumin reduces the toxicity of intracellular iron by mechanisms other than preventing iron loading or depleting iron stores.
It remains possible that a direct interaction with iron contributes to curcumin’s effects, however. We did see evidence of a slight inhibition of the calcein-iron interaction by curcumin (Figures 6 and 7). Other support comes from comparing the effects of curcumin to those of Vitamin E, another antioxidant that does not bind iron. We observed little effect of 50 μM vitamin E on ROS generation by iron, but found an IC50 for jnk activation near 10 μM. We interpret this to mean that vitamin E acts most potently at some step downstream from ROS generation by iron but prior to jnk activation. In contrast, curcumin inhibited both ROS generation and jnk activation with an IC50 between 5 and 10 μM. This indicates curcumin interacts directly with some component of the ROS generating reaction, quenching at the earliest possible step.
Optimal protection against iron toxicity is predicted from an agent that (i) binds iron, (ii) prevents redox cycling of iron, and (iii) independently quenches free radicals formed by iron (48). Since iron chemistry is biologically critical, however, an agent that is too effective may itself be toxic. With these criteria in mind, three properties of curcumin contribute to effective neutralization of iron toxicity. First, curcumin is a hydrophobic molecule that freely enters cells. This was demonstrated in our study by curcumin’s ability to block the rapid generation of ROS and activation of oxidative stress pathways caused by iron and 8-hydroxyquinoline. Second, curcumin binds iron with relatively low affinity (near 1 μM) compared to biological transport and storage proteins (27). Because of this, curcumin-bound iron retains its bioavailability and may be readily transferred to ferritin. One curcumin molecule may undergo many cycles of binding iron, releasing it to ferritin, and then binding more iron. Third, curcumin has potent antioxidant and free radical quenching activity (12, 17). Others have also shown the redox activity of iron is significantly reduced by curcumin (49, 50). Many small molecule iron ligands, including citrate and ascorbate, do not quench the redox activity of bound iron, and may even increase it by catalyzing redox cycling. Conversely, iron is not redox active when bound to transferrin, ferritin, or chelating drugs such as desferoxamine. Curcumin may share this important iron detoxifying property, as suggested by our results using the H2DCFDA ROS assay. We speculate that curcumin’s cell permeability, moderate affinity for iron, and unique antioxidant properties all contribute to prevention of iron toxicity.
Curcumin may inhibit other biochemical targets in T51B cells. A number of curcumin-sensitive enzymes and pathways have been identified in other systems (12, 17). These include: (i) cyclooxygenase (COX)-1 and -2, (ii) inducible nitric oxide synthase (iNOS), (iii) AP-1 and NF-kappaB transcription factors, (iv) growth factor receptors, and (v) thioredoxin reductase (51). Reported pro-oxidant activity (52–54) and induction of phase II drug metabolizing enzymes (55–57) represent additional mechanisms in some cell types. The importance of specific enzymatic targets for inhibition of some cellular processes is underscored by the finding that curcumin analogs lacking antioxidant activity were still effective inhibitors of NF-kappaB and AP-1 activation in screening assays (58, 59). These reports suggest that curcumin may have effects downstream of iron in our experiments. Indeed, the available evidence suggests that curcumin acts by multiple mechanisms (17). We propose that an additional mechanism involves transient binding and redox quenching of intracellular free iron, and envision a model in which curcumin functions as a sink for free iron and/or for iron-induced free radicals in cells.
Although curcumin is exceedingly safe in humans, it is widely acknowledged to have poor bioavailability, making the in vivo relevance of our findings uncertain. However, although curcumin is rapidly metabolized and poorly absorbed, it has still been shown to be effective in many animal studies (12, 17, 18, 20–22, 50). Some of its effects may be mediated by its metabolites (60). Our studies were routinely done with 50 μM curcumin, since that concentration was consistently maximally effective. We also observed significant effects at lower concentrations, with IC50’s between 5 and 10 μM for the different assays. This approaches plasma concentrations reported in animal studies (12). Importantly, a physiologically relevant effect may not depend on complete block of iron toxicity. A related concern is that liver damage in humans results from decades of iron overload at blood levels of iron citrate that do not exceed 10– 20 μM (9, 35, 61), below the FAC concentrations studied here. However, given that curcumin binds iron with an affinity near 1 μM (Figure 1 and ref 26), it is reasonable to expect that a lower curcumin concentration would still protect against toxicity that accompanies iron citrate concentrations measured during iron overload in humans. These points all argue that our observations reveal effects that are relevant to what may occur in humans taking curcumin. Curcumin may be a safe and effective alternative for management of liver toxicity in diseases of iron overload. Based on results presented in the present study, there is little risk of iron depletion in cells or animals given curcumin for extended periods. This supports the feasibility of future studies to determine whether curcumin prevents pathologies stemming from more prolonged iron loading, including neoplastic transformation and liver cancer.
Acknowledgments
We thank Adam Claessens, Bastyr University, for preliminary experiments related to this study, and Dr. Daniel Boring, National Cancer Institute Division of Cancer Prevention, for supplying pure curcumin. This research was supported in part by NIH grant K24 DK02957 from the Institute of Diabetes and Digestive and Kidney Diseases (to KVK). DJM was supported by training grant T32 AT00815 to Bastyr University from the National Center for Complementary and Alternative Medicine.
References
- 1.Stal P. Iron as a hepatotoxin. Dig Dis. 1995;13(4):205–222. doi: 10.1159/000171503. [DOI] [PubMed] [Google Scholar]
- 2.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32(9):833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 3.Haber F, Weiss J. The catalytic decompostion of hydrogen peroxide by iron salts. Proc Royal Society London Section A. 1934;147:332–351. [Google Scholar]
- 4.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 5.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 6.Fleming RE, Sly WS. Mechanisms of iron accumulation in hereditary hemochromatosis. Annu Rev Physiol. 2002;64:663–680. doi: 10.1146/annurev.physiol.64.081501.155838. [DOI] [PubMed] [Google Scholar]
- 7.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 8.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S79–86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Luper S. A review of plants used in the treatment of liver disease: part two. Altern Med Rev. 1999;4(3):178–188. [PubMed] [Google Scholar]
- 12.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi S, Shah AH, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992;58(2):124–127. doi: 10.1055/s-2006-961412. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 15.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 16.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 18.Kiso Y, Suzuki Y, Watanabe N, Oshima Y, Hikino H. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983;49(3):185–187. doi: 10.1055/s-2007-969845. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande SS, Lalitha VS, Ingle AD, Raste AS, Gadre SG, Maru GB. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett. 1998;95(3):183–193. doi: 10.1016/s0378-4274(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 20.Soni KB, Lahiri M, Chackradeo P, Bhide SV, Kuttan R. Protective effect of food additives on aflatoxin-induced mutagenicity and hepatocarcinogenicity. Cancer Lett. 1997;115(2):129–133. doi: 10.1016/s0304-3835(97)04710-1. [DOI] [PubMed] [Google Scholar]
- 21.Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21(2):331–335. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Thapliyal R, Naresh KN, Rao KV, Maru GB. Inhibition of nitrosodiethylamine-induced hepatocarcinogenesis by dietary turmeric in rats. Toxicol Lett. 2003;139(1):45–54. doi: 10.1016/s0378-4274(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 23.Leclercq IA, Farrell GC, Sempoux C, dela Pena A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41(6):926–934. doi: 10.1016/j.jhep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H, Genina O, Helpern Z, Pines M. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27(3):373–383. doi: 10.1111/j.1478-3231.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 25.Sreejayan Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46(12):1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 26.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004;6(4):367–377. 443–369. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- 27.Jiao Y, Wilkinson Jt, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40(7):1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Swierenga SH, Whitfield JF, Boynton AL, MacManus JP, Rixon RH, Sikorska M, Tsang BK, Walker PR. Regulation of proliferation of normal and neoplastic rat liver cells by calcium and cyclic AMP. Ann N Y Acad Sci. 1980;349:294–311. doi: 10.1111/j.1749-6632.1980.tb29534.x. [DOI] [PubMed] [Google Scholar]
- 29.Blouin R, Blouin MJ, Royal I, Grenier A, Roop DR, Loranger A, Marceau N. Cytokeratin 14 expression in rat liver cells in culture and localization in vivo. Differentiation. 1992;52(1):45–54. doi: 10.1111/j.1432-0436.1992.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang RP, Peng A, Golard A, Hossain MZ, Huang R, Liu YG, Boynton AL. Hydrogen peroxide promotes transformation of rat liver non-neoplastic epithelial cells through activation of epidermal growth factor receptor. Mol Carcinog. 2001;30(4):209–217. doi: 10.1002/mc.1030. [DOI] [PubMed] [Google Scholar]
- 31.Boynton AL, Kleine LP, Whitfield JF. Relation between colony formation in calcium-deficient medium, colony formation in soft agar, and tumor formation by T51B rat liver cells. Cancer Lett. 1984;21(3):293–302. doi: 10.1016/0304-3835(84)90008-9. [DOI] [PubMed] [Google Scholar]
- 32.Huang RP, Peng A, Hossain MZ, Fan Y, Jagdale A, Boynton AL. Tumor promotion by hydrogen peroxide in rat liver epithelial cells. Carcinogenesis. 1999;20(3):485–492. doi: 10.1093/carcin/20.3.485. [DOI] [PubMed] [Google Scholar]
- 33.Messner DJ, Ao P, Jagdale AB, Boynton AL. Abbreviated cell cycle progression induced by the serine/threonine protein phosphatase inhibitor okadaic acid at concentrations that promote neoplastic transformation. Carcinogenesis. 2001;22(8):1163–1172. doi: 10.1093/carcin/22.8.1163. [DOI] [PubMed] [Google Scholar]
- 34.Messner DJ, Kowdley KV. Neoplastic transformation of rat liver epithelial cells is enhanced by non-transferrin-bound iron. BMC Gastroenterol. 2008;8(1):2. doi: 10.1186/1471-230X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989;264(8):4417–4422. [PubMed] [Google Scholar]
- 36.Wheby MS, Umpierre G. Effect of Transferrin Saturation on Iron Absorption in Man. N Engl J Med. 1964;271:1391–1395. doi: 10.1056/NEJM196412312712704. [DOI] [PubMed] [Google Scholar]
- 37.Leanderson P, Tagesson C. Iron bound to the lipophilic iron chelator, 8-hydroxyquinoline, causes DNA strand breakage in cultured lung cells. Carcinogenesis. 1996;17(3):545–550. doi: 10.1093/carcin/17.3.545. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Merrick BA, Khaledi MG, Tomer KB. Detection and sequencing of phosphopeptides affinity bound to immobilized metal ion beads by matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 2000;11(4):273–282. doi: 10.1016/s1044-0305(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 39.Scheiber-Mojdehkar B, Zimmermann I, Dresow B, Goldenberg H. Differential response of non-transferrin bound iron uptake in rat liver cells on long-term and short-term treatment with iron. J Hepatol. 1999;31(1):61–70. doi: 10.1016/s0168-8278(99)80164-0. [DOI] [PubMed] [Google Scholar]
- 40.Messner DJ, Romeo C, Boynton A, Rossie S. Inhibition of PP2A, but not PP5, mediates p53 activation by low levels of okadaic acid in rat liver epithelial cells. J Cell Biochem. 2006;99(1):241–255. doi: 10.1002/jcb.20919. [DOI] [PubMed] [Google Scholar]
- 41.Sieuwerts AM, Klijn JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995;33(11):813–823. doi: 10.1515/cclm.1995.33.11.813. [DOI] [PubMed] [Google Scholar]
- 42.Bernabe-Pineda M, Ramirez-Silva MT, Romero-Romo MA, Gonzalez-Vergara E, Rojas-Hernandez A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin-Fe(III)-water and Curcumin-Fe(II)-water. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(5):1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- 43.Caris C, Baret P, Beguin C, Serratrice G, Pierre JL, Laulhere JP. Metabolization of iron by plant cells using O-Trensox, a high-affinity abiotic iron-chelating agent. Biochem J. 1995;312(Pt 3):879–885. doi: 10.1042/bj3120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.d’Hardemare Adu M, Torelli S, Serratrice G, Pierre JL. Design of iron chelators: syntheses and iron (III) complexing abilities of tripodal tris-bidentate ligands. Biometals. 2006;19(4):349–366. doi: 10.1007/s10534-005-2997-2. [DOI] [PubMed] [Google Scholar]
- 45.Mouralian C, Buss JL, Stranix B, Chin J, Ponka P. Mobilization of iron from cells by hydroxyquinoline-based chelators. Biochem Pharmacol. 2005;71(1–2):214–222. doi: 10.1016/j.bcp.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 46.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65(8):3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 47.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43(7):995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180(1):23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 49.Reddy AC, Lokesh BR. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol Cell Biochem. 1994;137(1):1–8. doi: 10.1007/BF00926033. [DOI] [PubMed] [Google Scholar]
- 50.Reddy AC, Lokesh BR. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology. 1996;107(1):39–45. doi: 10.1016/0300-483x(95)03199-p. [DOI] [PubMed] [Google Scholar]
- 51.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280(26):25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 52.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min do S, Chang JS, Jeong YJ, Lee YH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 53.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3(9):1101–1108. [PubMed] [Google Scholar]
- 54.Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24(2B):563–569. [PubMed] [Google Scholar]
- 55.Susan M, Rao MN. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittelforschung. 1992;42(7):962–964. [PubMed] [Google Scholar]
- 56.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371(Pt 3):887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinkova-Kostova AT, Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis. 1999;20(5):911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 58.Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ, Orlando RA, Deck LM, Vander Jagt DL. TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem Pharmacol. 2006;72(8):928–940. doi: 10.1016/j.bcp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Weber WM, Hunsaker LA, Roybal CN, Bobrovnikova-Marjon EV, Abcouwer SF, Royer RE, Deck LM, Vander Jagt DL. Activation of NFkappaB is inhibited by curcumin and related enones. Bioorg Med Chem. 2006;14(7):2450–2461. doi: 10.1016/j.bmc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 60.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- 61.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18(2):277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]


