Abstract
Introduction
One form of the hereditary long QT-syndrome, LQT3-ΔKPQ, is associated with sustained inward sodium current during membrane depolarization. Ranolazine reduces late sodium channel current, and we hypothesized that ranolazine would have beneficial effects on electrical and mechanical cardiac function in LQT3 patients with the SCN5A-ΔKPQ mutation.
Methods
We assessed the effects of 8-hour intravenous ranolazine infusions (45mg/hr for 3 hours followed by 90mg/hr for 5 hours) on ventricular repolarization and myocardial relaxation in five LQT3 patients with the SCN5A-ΔKPQ mutation. Changes in electrocardiographic QTc parameters from before to during ranolazine infusion were evaluated by time-matched, paired t-test analyses. Cardiac ultrasound recordings were obtained before ranolazine infusion and just before completion of the 8-hour ranolazine infusion.
Results
Ranolazine shortened QTc by 26±3ms (p<0.0001) in a concentration-dependent manner. At peak ranolazine infusion, there was a significant 13% shortening in left ventricular isovolumic relaxation time, a significant 25% increase in mitral E-wave velocity, and a meaningful 22% decrease in mitral E-wave deceleration time compared to baseline. No adverse effects of ranolazine were observed in the study patients.
Conclusion
Ranolazine at therapeutic concentrations shortened a prolonged QTc interval and improved diastolic relaxation in patients with the LQT3-ΔKPQ mutation, a genetic disorder that is known to cause an increase of late sodium current.
Keywords: Long QT Syndrome, QT prolongation, Ranolazine
Introduction
Long QT-syndrome (LQTS) is a familial genetic disorder in which affected family members have electrocardiographic QT prolongation and a propensity to syncope, polymorphic ventricular tachycardia (torsade de pointes) and sudden death.1 One form of LQTS, LQT3, involves mutations in the SCN5A sodium channel gene. Mutational deletion of the ΔKPQ amino-acid sequence in this channel protein is associated with sustained inward sodium current during membrane depolarization resulting in prolongation of the ventricular action potential and the QT interval.2
Ranolazine reduces late sodium channel current, shortens the action potential duration, and suppress early afterdepolarization-triggered arrhythmias in animal models of LQT3 with sustained inward sodium current.3–7 Furthermore, ranolazine reduces late sodium current-induced intracellular sodium-dependent calcium overload and improves the rate of relaxation of ventricular myocytes exposed to reactive oxygen species.8 Based on these ion-channel, electrophysiologic, and lusitropic properties of ranolazine, we investigated the electrical and mechanical cardiac effects of intravenous ranolazine infusion in five LQT3 patients with the SCN5A-ΔKPQ mutation.
Methods
The primary hypothesis of this study is that intravenous administration of the drug ranolazine that preferentially blocks the late sodium channel current will be associated with QTc shortening and increased myocardial diastolic relaxation in LQT3 subjects with the ΔKPQ deletion mutation involving the SCN5A gene. The study protocol was approved by the Western Institutional Review Board, and a Safety Monitor independently reviewed the ongoing results at regular intervals throughout the study. Five patients were studied for 3 days each in the General Clinical Research Center (GCRC) between March 19, 2007 and May 3, 2007.
Patients
Subjects were drawn from the Rochester, NY portion of the International LQTS Registry. Eligibility criteria for enrollment in the study included genotype-confirmed LQT3-ΔKPQ mutation, QTc>470ms, males or females age 18 –55 years of age, and signed informed consent. Patients already taking beta-blocking medication were continued on the drug. Patients with an implanted pacemaker or defibrillator or those with prior cervical-thoracic sympathetic ganglionectomy were not excluded. Exclusion criteria included significant comorbidity that would preclude the patient’s safe participation in this study, pregnancy or women of child-bearing age not receiving appropriate contraceptive therapy, ventricular paced rhythm, evidence of prior sensitivity to ranolazine, therapy with any other sodium-channel blocker, and use of medications that prolong QT or inhibit CYP-3A4 hepatic enzymes including diltiazem or verapamil. Thirteen patients with the LQT3-ΔKPQ mutation from two unrelated families met the eligibility criteria without exclusion, and of eight contacted patients five agreed to participate in the study.
Measurement of Electrocardiographic Parameters
12-lead ECGs and 24-hour 12-lead Holter ECGs were obtained to evaluate ventricular repolarization before, during, and after ranolazine infusion. The measured electrocardiographic parameters are presented in Tables 1 and 2. The QT interval and the Fridericia- and Bazett-corrected QT interval for heart rate (QTc) were measured according to standard criteria. Since the QTc findings were nearly identical for the Friderica and Bazett corrections, only the Fridericia QTc values are reported in the results. Digital 24-hour Holter recordings with 1000Hz sampling rate were obtained on the day before and day of ranolazine infusion. Holter repolarization parameters similar to those recorded on the 12-lead ECG were measured, and the recordings were scrutinized for atrial or ventricular arrhythmias. All electrocardiographic analyses were interpreted in a blinded manner regarding subject and sequence of before, during, and after ranolazine infusion.
Table 1.
Baseline Clinical Characteristics of 5 LQT3 Study Patients with ΔKPQ Mutation
| Patient No. | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean±sd | |
| Age, y | 55 | 35 | 47 | 24 | 39 | 40±12 |
| Sex | M | M | F | M | M | |
| Height, cm | 169 | 180 | 173 | 163 | 188 | 175±10 |
| Weight, kg | 103 | 75 | 61 | 65 | 79 | 77±16 |
| Syncopal history | N | N | N | Y | Y | |
| Beta-blocker | Y | Y | N | Y | Y | |
| Atrial pacemaker | N | N | N | N | Y | |
| ICD | N | Y | N | Y | N | |
| ECG | ||||||
| Rhythm | sinus | sinus | sinus | atrial paced | atrial paced | |
| PR, ms | 230 | 200 | 200 | 200 | 240 | 214±19 |
| QRS, ms | 110 | 80 | 90 | 80 | 80 | 88±13 |
| RR, ms | 1470 | 1400 | 900 | 1000 | 1000 | 1154±261 |
| QT, ms | 720 | 700 | 480 | 550 | 580 | 606±107 |
| QTc, Fridericia, ms | 630 | 630 | 500 | 550 | 580 | 578±55 |
| QTpeak, ms | 590 | 570 | 400 | 460 | 460 | 496±81 |
| Tpeak-Tend, ms | 130 | 130 | 80 | 90 | 120 | 110±23 |
| Tduration, ms | 250 | 240 | 170 | 170 | 210 | 208±38 |
| Tamp, mv | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | 0.28±0.04 |
Table 2.
Electrocardiographic Findings Before, During, and After Ranolazine Infusion
| Parameters | Ranolazine Infusion | ||
|---|---|---|---|
| Before* | During† | After¶ | |
| PR, ms | 206±13 | 200±21 | 212±19 |
| QRS, ms | 88±8 | 86±11 | 94±17 |
| RR, ms | 1152±253 | 1108±259 | 1168±361 |
| QT, ms | 576±94 | 544±100** | 590±109 |
| QTc, Fridericia, ms | 548±52 | 526±53†† | 562±52 |
| QTpeak, ms | 484±70 | 440±64** | 492±93 |
| Tpeak-Tend, ms | 92±27 | 106±38 | 126±60 |
| T duration, ms | 184±46 | 190±37 | 230±77 |
| T amp, mv | 0.26±0.06 | 0.24±0.06 | 0.26±0.11 |
Time-matched on Day -1 to 8th hour of Day 1 ranolazine infusion.
At 8th hour of ranolazine infusion on Day 1.
At 16 hours after ranolazine infusion on Day 1.
p<0.01 and
p<0.05change from before ranolazine infusion by paired t-test comparison.
Measurement of Cardiac Ultrasound Parameters
Cardiac ultrasound recordings were obtained on two occasions, before ranolazine infusion and just before completion of the 8-hour ranolazine infusion. Images were digitized and stored to optical disk for analysis offline. The echocardiographic and tissue Doppler analyses were made in a blinded manner and standard parameters were measured.9–11
Ranolazine Infusion
Ranolazine, approved by the Federal Food and Drug Administration for intravenous infusion, was supplied by CV Therapeutics, Inc. (Palo Alto, CA). In each of the 5 adult LQT3 patients, ranolazine was infused at 45mg/hr for 3 hours followed by 90mg/hr for 5 hours.
Protocol
Each patient had clinical and laboratory assessments including ultrasound evaluation on Day -2. Baseline studies off drug began at 8:00am on Day –1: 12-lead ECG recorded at 8:00am and every 0.5 hour for 8 hours and then at 2, 4, and 16 hours thereafter; a continuous 12-lead Holter was recorded for 48 hours. The 8-hour infusion of intravenous ranolazine was started at 8:00am on Day 1 with time-matched ECG recordings at the same times as on Day -1. The second echocardiogram was obtained just before completion of the 8-hour of ranolazine infusion on Day 1. Blood samples for plasma ranolazine concentrations were drawn at hourly intervals during the ranolazine infusion, and at 2, 4, 16 hours following completion of ranolazine infusion on Day 1.
Sample Size
The number of patients with this genetically confirmed mutation in the United States is small, in the range of 50 patients. Since this is an exploratory study to evaluate the effect of short-term intravenous ranolazine in patients with the LQT3-ΔKPQ mutation, we did not base the sample size on anticipated drug effect with power considerations. Rather, we prespecified a 5-patient study based on our experience with a prior flecainide investigation in patients with this mutation.12
Data Analysis
Changes in electrocardiographic parameters from before to during ranolazine infusion were evaluated by time-matched, paired t-test analyses, with repeated measures analysis of variance (ANOVA) where appropriate. The primary prespecified electrocardiographic QTc analysis utilized a mixed-effects linear model of the change from time-matched baseline QTc during ranolazine infusion with a fixed effect for time and a random effect for subject. A linear regression equation relating ranolazine blood level to QTc was developed from the overall data using an analysis of covariance with effects for ranolazine level, subject, and the interaction for subject with ranolazine level. The echocardiographic analyses involved evaluation of baseline parameters relative to normal standards for the laboratory as well as statistical comparisons of change in specific quantitative parameters between baseline and the peak ranolazine infusion. Two-sided p-values <0.05 were considered significant.
Results
Clinical Characteristics
The clinical characteristics of the 5 LQT3 study patients are summarized in Table 1. All 5 patients were in good health, had no medical condition other than LQTS, were asymptomatic at full activity, and their baseline physical exam and routine blood-chemistry profile were normal.
Electrocardiographic Findings and Ranolazine Infusion
All 5 LQT3 patients completed the 8-hour ranolazine infusion, and there were no adverse effects or meaningful changes in blood pressure. Ranolazine had little or no effect on the PR interval, QRS duration, or T-wave amplitude, but was associated with significant reduction in QT, QTc and QTpeak measurements (Table 2). Representative shortening of QTc with repolarization is shown in Figure 1. In the 3 patients who were in sinus rhythm, ranolazine had no meaningful effect on heart rate. At 16 hours after ranolazine infusion, QT, QTc, QTpeak, Tpeak-Tend, and Tduration parameters showed a rebound phenomenon with values non-significantly higher than before ranolazine infusion.
Figure 1.
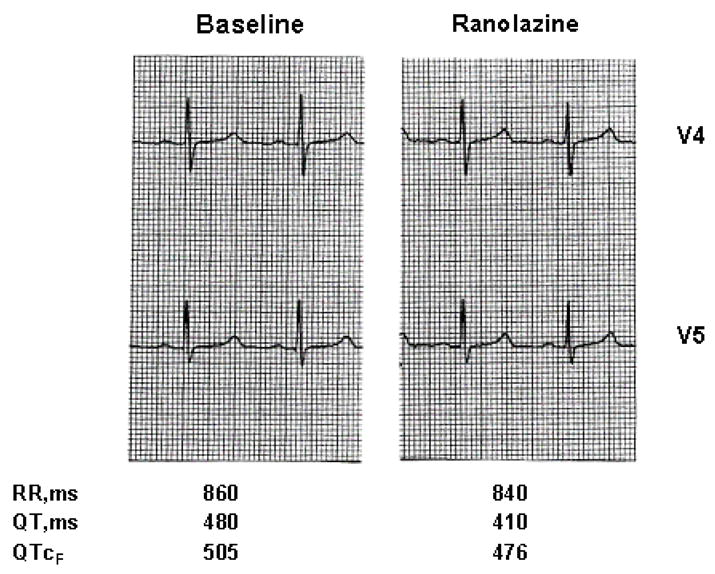
Electrocardiographic tracings (V4 and V5 leads) at baseline and at peak ranolazine infusion in a patient with the LQT3-ΔKPQ mutation. The RR, QT, and QTc (Fridericia) intervals are presented below each tracing.
Over the entire 8-hour ranolazine infusion, the mean reduction in QTc from baseline was 26±3ms (p<0.0001), i.e., from 558±55ms (baseline, Day -1) to 532±46ms (ranolazine, Day 1). During the first 3 hours of low-dose ranolazine infusion that achieved a maximum plasma concentration of 908 ng/ml, the mean QTc change from baseline ranged from −6 to −20ms. During the next 5 hours when the ranolazine infusion rate was doubled, the maximum plasma concentration increased to 2074 ng/ml and the mean change from time-matched baseline QTc values ranged from −22 to −42ms. The mean changes in QTc values during and after ranolazine infusion from time-matched QTc values before ranolazine together with ranolazine plasma concentrations are graphically presented in Figure 2. All mean changes in QTc values obtained during the higher-dose ranolazine infusion were significantly lower than time-matched values obtained on the day before ranolazine infusion. The mean correlation coefficient between plasma ranolazine concentrations and QTc change from time-matched pre-ranolazine levels was −0.7±0.22. In an overall linear regression model fit for QTc with adjustment for subject, ranolazine concentration, and the interaction of ranolazine concentration with subject, the average slope of QTc vs. ranolazine plasma concentration was −24 ms per 1000 ng/ml (p=0.008).
Figure 2.
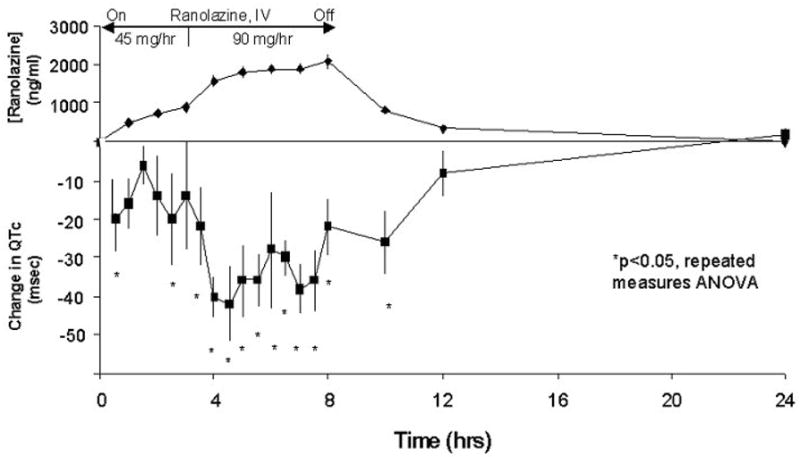
Plasma Ranolazine Concentrations and Changes in QTc from Time-Matched Baseline Values in Five LQT3 Patients During and After Ranolazine Infusion.
The quantitative Holter-ECG QTc findings were virtually identical to the 12-lead ECG findings. No arrhythmias were identified on the Holter recordings before, during, or after ranolazine infusion.
Echocardiographic Findings and Ranolazine Infusion
The pertinent echocardiographic findings recorded during baseline and peak ranolazine infusion are presented in Table 3. Baseline studies were not entirely normal. Although the left ventricular ejection fraction was normal, there was evidence of modest left ventricular chamber enlargement, mild left ventricular dyssynchrony, and grade 1 diastolic dysfunction as manifested by prolonged left ventricular isovolumic relaxation time, reduced early diastolic filling rate (mitral E-wave velocity), and increased mitral E-wave deceleration time (Table 3). These parameters were not statistically abnormal when compared to normal standards in this small study group, but the visual and quantitative pattern of diastolic dysfunction at baseline was noted and commented upon by the experienced echocardiologist (K.Q.S.). At peak ranolazine infusion, there was significant 13% shortening in left ventricular isovolumic relaxation time, a significant 25% increase in mitral E-wave velocity, and a meaningful 22% decrease in mitral E-wave deceleration time compared to baseline (Table 3).
Table 3.
Resting Echocardiographic Findings Before and During Ranolazine Infusion
| Parameter | Baseline | Ranolazine* |
|---|---|---|
| LV volumes | ||
| End diastole, ml | 126±32 | 129±42 |
| End systole, ml | 47±12 | 52±17 |
| LV systolic function | ||
| LVEF, % | 62 ± 8 | 60 ± 5 |
| Stroke volume, ml | 79 ± 25 | 78 ± 27 |
| Dyssynchrony score, ms | 100±57 | 92±50 |
| LV diastolic function | ||
| Isovolumic relaxation time, ms | 125 ± 27 | 109 ± 35† |
| Mitral E-wave velocity, cm/s | 57 ± 8 | 71 ± 9† |
| Mitral A-wave velocity, cm/s | 50 ± 10 | 63 ± 12 |
| Mitral E/A ratio | 1.2 ± 0.3 | 1.2 ± 0.3 |
| Mitral E-wave deceleration time, ms | 289 ± 80 | 226 ± 34 |
| Mitral peak annular E velocity, cm/s | 7.9 ± 3.6 | 8.7 ± 3.4 |
At 8th hour of ranolazine infusion.
p<0.05 change from baseline by paired t-test comparison.
Discussion
The major findings from this study are that ranolazine, a new antianginal agent with novel electrophysiologic properties, shortens the QTc interval and improves myocardial relaxation in LQT3 patients with the SCN5A-ΔKPQ mutation. Shortening of the QTc interval by ranolazine is concentration dependent. We do not have a good explanation for the non-significant rebound in the QT, QTc, QTpeak, Tpeak-Tend, and Tduration parameters above baseline values at 16 hours after the ranolazine infusion.
In 1995, Bennet et al. showed that the molecular mechanism for the LQT3-ΔKPQ form of LQTS was due to a persistent inward sodium current that prolongs the action potential with resultant QT prolongation in the surface electrocardiogram.2 Since ranolazine has been shown to inhibit the late sodium current with minimal effect on peak sodium current during the upstroke of the action potential in experimental LQT3 models,4 it was logical to study this drug in patients with ΔKPQ mutation. Thus, shortening of the QT interval with ranolazine in our patients with the LQT3-ΔKPQ mutation has mechanistic rationale, and the QTc reduction occurred within the therapeutic concentration of the drug used in the treatment of patients with angina. Ranolazine has only minimal effect on phase 0 of the ventricular action potential, and the QRS interval was not prolonged in the current study. The absence of a QRS prolonging effect with ranolazine is in contrast to the drug flecainide that has early and late sodium-channel blocking effects on the ventricular action potential and is associated with QRS widening in LQT3 patients with the ΔKPQ mutation.12 In a recent study, Ruan et al showed that the electrophysiologic properties of mexiletine as evaluated in cellular expression studies were correlated with the clinical QTc response in LQT3 patients.13 Prior cellular electrophysiologic studies have shown the benefit of ranolazine in experimental models of LQT314, so our in vivo QTc findings in LQT3 patients with the ΔKPQ mutation are in good alignment with the Raun findings even though we are using a different sodium-channel blocker.
Ranolazine has modest QT prolonging effects in coronary patients treated with this antianginal agent,15 possibly due to some inhibition of the delayed rectifier potassium current IKr.16 However, in large clinical trials ranolazine showed no proarrhythmic effect, but rather, had antiarrhythmic properties.15 In the current ranolazine study with LQT3 patients, there were no supraventricular or ventricular arrhythmias during the 8-hour intravenous infusion of ranolazine.
It has been hypothesized that an increase in late entry of sodium into the myocardial cell results in an increase in cytosolic calcium concentration,17 a factor that could contribute to electrical instability by triggering afterdepolarization-related ventricular arrhythmias. In addition, an increase in late sodium current causes intracelluar sodium–dependent calcium-overload that can result in a slowing of LV diastolic relaxation, i.e., a negative lusitropic effect. In the current study, the baseline resting ultrasound exam did not appear to be entirely normal in our LQT3 patients with findings suggestive of minor grade 1 diastolic dysfunction. During ranolazine infusion, left ventricular isovolumic relaxation time shortened, mitral E-wave deceleration time decreased, and mitral E-wave velocity increased when compared to baseline values, and these findings are consistent with a positive lusitropic action in this disorder.
This study was initiated as a pilot investigation to evaluate the effects of intravenous ranolazine in high-risk patients with the LQT3-ΔKPQ mutation. We only studied 5 patients in view of our prior positive study with oral flecainide in 5 patients with the same LQT3 mutation.18 Nevertheless, the small sample size, the study of only patients with the LQT3-ΔKPQ mutation, and the failure to study patients with the oral preparation are limitations that preclude any recommendation for use of ranolazine for management of patients with LQT3 mutations. A larger study with oral ranolazine needs to be done to determine the safety and efficacy of oral ranolazine for clinical use in LQT3 patients.
Conclusions
Ranolazine at therapeutic concentrations shortened a prolonged QTc interval and improved diastolic relaxation in patients with the LQT3-ΔKPQ mutation, a genetic disorder that is known to cause an increase of late sodium current. The results of this study are the first to provide evidence that ranolazine inhibits late sodium current in humans, and the findings suggest that ranolazine may increase repolarization reserve in LQT3 patients. Appropriate randomized clinical trials are indicated to evaluate the safety and efficacy of ranolazine in patients with the LQT3-ΔKPQ mutation, and possibly in patients with other LQT3 channel mutations with persistent late sodium entry into cardiac myocytes.
Acknowledgments
The authors thank Mark Andrews, Jack Schuler, and Patty Severski for setting up the data management system and Kathryn Pyykkonen for her nursing assistance. The authors appreciate the advice rendered by Drs. Luiz Belardinelli and Ewa Karwatowska-Prokopczuk from Cardiovascular Therapeutics, Inc. in the conduct of this study.
Supported in part by a General Clinical Research Center (GCRC) grant, 5 M01 RR00044, from the National Center for Research Resources, NIH; research grants HL-33843 and HL-51618 from the NIH; and by a research grant from Cardiovascular Therapeutics, Inc., Palo Alto, CA.
References
- 1.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Jr, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991 Sep;84(3):1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 2.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376(6542):683–685. doi: 10.1038/376683a0. [see comments] [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004 Aug 24;110(8):904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006 May;148(1):16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schram G, Zhang L, Derakhchan K, Ehrlich JR, Belardinelli L, Nattel S. Ranolazine: ion-channel-blocking actions and in vivo electrophysiological effects. Br J Pharmacol. 2004 Aug;142(8):1300–1308. doi: 10.1038/sj.bjp.0705879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the proarrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004 Aug;44(2):192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004 Aug;310(2):599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006 Jul;318(1):214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Otto C. Textbook of Clinical Echocardiography. 3. Philadelphia: WB Saunders; 2004. [Google Scholar]
- 11.Sogaard P, Egeblad H, Pedersen AK, Kim WY, Kristensen BO, Hansen PS, Mortensen PT. Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation. 2002 Oct 15;106(16):2078–2084. doi: 10.1161/01.cir.0000034512.90874.8e. [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, Severski P, Rosero S, Daubert JP, Qi M, Cieciorka M, Manalan AS. Safety and efficacy of flecainide in subjects with Long QT-3 syndrome (DeltaKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol. 2005 Oct;10(4 Suppl):59–66. doi: 10.1111/j.1542-474X.2005.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Y, Liu N, Bloise R, Napolitano C, Priori SG. Gating properties of SCN5A mutations and the response to mexiletine in long-QT syndrome type 3 patients. Circulation. 2007 Sep 4;116(10):1137–1144. doi: 10.1161/CIRCULATIONAHA.107.707877. [DOI] [PubMed] [Google Scholar]
- 14.Fredj F, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Brit J Pharm. 2006 doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007 Oct 9;116(15):1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 16.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Jr, Scornik F, Perez G. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004 Sep;9(Suppl 1):S65–83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 17.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999 Jun 25;84(12):1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 18.Windle JR, Geletka RC, Moss AJ, Zareba W, Atkins DL. Normalization of ventricular repolarization with flecainide in long qt syndrome patients with scn5a:deltakpq mutation. Ann Noninvasive Electrocardiol. 2001;6(2):153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


