Abstract
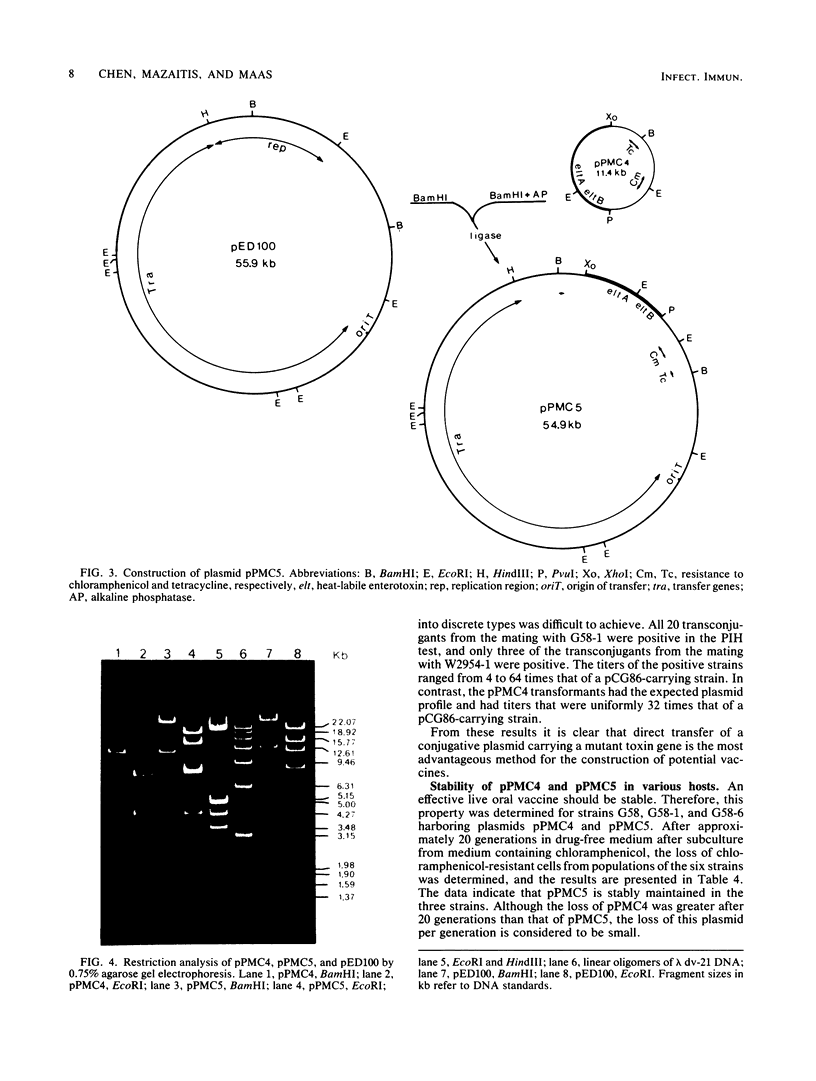
A conjugative plasmid with potential usefulness for vaccine strains was constructed. In the first step, a 5.9-kilobase DNA segment containing the two loci for the A and B subunits of heat-labile enterotoxin with a mutation in the gene for the A subunit was joined to the cloning vehicle pGA22, generating the nonconjugative plasmid pPMC4 with genes for resistance to tetracycline and chloramphenicol. In the second step, a segment of pPMC4 containing the genes for the A and B subunits, the gene for chloramphenicol resistance, and the replication genes of pGA22 was ligated to the genes for conjugal transfer of the F plasmid, generating the 54.9-kb plasmid pPMC5. Eleven porcine Escherichia coli isolates were tested as recipients for pPMC4 and pPMC5. For pPMC4, transformation and mobilization with a conjugative R plasmid were used to effect plasmid transfer. Only 1 of the 11 strains acted as a recipient in transformation. Mobilization with the R plasmid occurred with two strains, but the plasmids were altered during transfer. In contrast, pPMC5 was transferred with high frequency and unaltered to 9 of the 11 E. coli strains. Transconjugants from these nine matings produced high titers of the B subunit and no active heat-labile enterotoxin. Plasmid pPMC5 was stable in three porcine E. coli strains tested; plasmid pPMC4 was somewhat less stable in these strains. The method we describe for the construction of conjugative chimeric plasmids offers an opportunity for introducing genes with potential for immunization into bacterial strains that are suitable for colonizing the appropriate host sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bramucci M. G., Holmes R. K. Radial passive immune hemolysis assay for detection of heat-labile enterotoxin produced by individual colonies of Escherichia coli or Vibrio cholerae. J Clin Microbiol. 1978 Aug;8(2):252–255. doi: 10.1128/jcm.8.2.252-255.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Direct serological assay for the heat-labile enterotoxin of Escherichia coli, using passive immune hemolysis. Infect Immun. 1977 May;16(2):604–609. doi: 10.1128/iai.16.2.604-609.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaitis A. J., Maas R., Maas W. K. Structure of a naturally occurring plasmid with genes for enterotoxin production and drug resistance. J Bacteriol. 1981 Jan;145(1):97–105. doi: 10.1128/jb.145.1.97-105.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. I. Kinetics of loss of plasmid derivatives deleted of the par region. Plasmid. 1980 Sep;4(2):215–227. doi: 10.1016/0147-619x(80)90011-6. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken R. N., Mazaitis A. J., Maas W. K., Rey M., Heyneker H. Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect Immun. 1983 Oct;42(1):269–275. doi: 10.1128/iai.42.1.269-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D. S., Palchaudhuri S., Maas W. K. Genetic and physical characteristics of an enterotoxin plasmid. J Bacteriol. 1975 Dec;124(3):1240–1247. doi: 10.1128/jb.124.3.1240-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafim M. B., Pestana de Castro A. F., Lemos dos Reis M. H., Trabulsi L. R. Passive immune hemolysis for detection of heat-labile enterotoxin produced by Escherichia coli isolated from different sources. Infect Immun. 1979 Jun;24(3):606–610. doi: 10.1128/iai.24.3.606-610.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. L., Maas W. K., Gyles C. L. Isolation and characterization of enterotoxin-deficient mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1384–1388. doi: 10.1073/pnas.75.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Dallas W. S., Falkow S. Characterization of an Escherichia coli plasmid encoding for synthesis of heat-labile toxin: molecular cloning of the toxin determinant. Infect Immun. 1978 Aug;21(2):405–411. doi: 10.1128/iai.21.2.405-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Noble J. A. Escherichia coli heat-labile enterotoxin. Nucleotide sequence of the A subunit gene. J Biol Chem. 1982 May 25;257(10):5716–5721. [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Johnson D. pED100, a conjugative F plasmid derivative without insertion sequences. Mol Gen Genet. 1981;182(3):520–522. doi: 10.1007/BF00293948. [DOI] [PubMed] [Google Scholar]




