Abstract
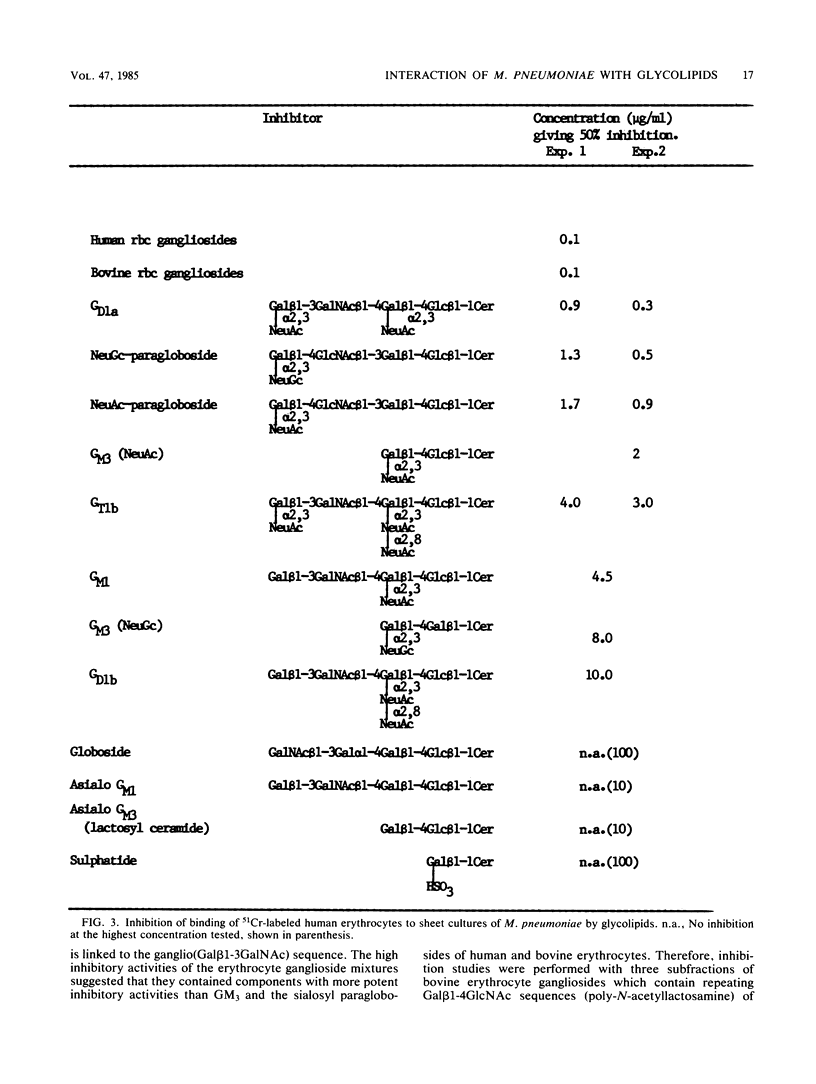
The role of sialoglycolipids (gangliosides) as receptors for the human pathogen Mycoplasma pneumoniae was investigated by using purified gangliosides of known carbohydrate structures as inhibitors of the binding of 51Cr-labeled erythrocytes to sheet cultures of M. pneumoniae. We found that sialoglycolipids with long carbohydrate backbones of the poly-N-acetyllactosamine type were more potent inhibitors of M. pneumoniae binding than those with short carbohydrate chains. This is in accord with earlier inhibition data for glycoproteins and oligosaccharides. Thus, the inhibitory activity of a fraction of bovine erythrocyte gangliosides containing long backbone structures of I antigen type was approximately 200 times greater than that of the short chain gangliosides GM3 and GT1b. The binding of M. pneumoniae to erythrocytes of I and i antigen types was found to be comparable, indicating that M. pneumoniae in its adhesive specificity may not distinguish between the branched carbohydrate backbones of I type and the linear structures of i type. Thus, the production of autoantibodies to the backbone structures of I type rather than i type after infection with this agent may simply reflect a greater abundance of branched carbohydrate receptors of I type on the surface of host cells with which the mycoplasma forms immunogenic complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. K., Grabowski M. W., Barile M. F. Mycoplasma pneumoniae attachment: competitive inhibition by mycoplasmal binding component and by sialic acid-containing glycoconjugates. Infect Immun. 1982 Nov;38(2):598–603. doi: 10.1128/iai.38.2.598-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Hakomori S. I., Powell M. E. Blood-group-Ii-active gangliosides of human erythrocyte membranes. Biochem J. 1978 Jul 1;173(1):245–254. doi: 10.1042/bj1730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Kapadia A., Yount W. J. I and i antigens of human peripheral blood lymphocytes cocap with receptors for concanavalin A. Proc Natl Acad Sci U S A. 1980 Jan;77(1):376–380. doi: 10.1073/pnas.77.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T. The blood group Ii system: a carbohydrate antigen system defined by naturally monoclonal or oligoclonal autoantibodies of man. Immunol Commun. 1981;10(2):127–156. doi: 10.3109/08820138109050693. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N., Taketomi T. Further study on cerebral sphingolipids including gangliosides in two cases of juvenile amaurotic family idiocy (Spielmeyer-Vogt type) using a new analytical procedure of sphingolipids. Jpn J Exp Med. 1975 Dec;45(6):489–500. [PubMed] [Google Scholar]
- Kinsky S. C. Antibody-complement interaction with lipid model membranes. Biochim Biophys Acta. 1972 Feb 14;265(1):1–23. doi: 10.1016/0304-4157(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Kunishita T., Uemura K., Okano A., Taketomi T. Isolation of basic protein-acidic lipid complex from myelin. Jpn J Exp Med. 1979 Dec;49(6):391–396. [PubMed] [Google Scholar]
- Lamblin G., Boersma A., Klein A., Roussel P., van Halbeek H., Vliegenthart J. F. Primary structure determination of five sialylated oligosaccharides derived from bronchial mucus glycoproteins of patients suffering from cystic fibrosis. The occurrence of the NeuAc alpha(2----3)Gal beta(1----4)[Fuc alpha(1----3)] GlcNAc beta(1----.) structural element revealed by 500-MHz 1H NMR spectroscopy. J Biol Chem. 1984 Jul 25;259(14):9051–9058. [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Momoi T., Ando S., Magai Y. High resolution preparative column chromatographic system for gangliosides using DEAE-Sephadex and a new porus silica, Iatrobeads. Biochim Biophys Acta. 1976 Sep 27;441(3):488–497. [PubMed] [Google Scholar]
- Niemann H., Watanabe K., Hakomori S. Blood group i and I activities of "lacto-N-norhexaosylceramide" and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Shumak K. H. Biologic activity of cold-reacting autoantibodies (second of two parts). N Engl J Med. 1977 Sep 15;297(11):583–589. doi: 10.1056/NEJM197709152971105. [DOI] [PubMed] [Google Scholar]
- Roelcke D., Riesen W., Geisen H. P., Ebert W. Serological identification of the new cold agglutinin specificity anti-Gd. Vox Sang. 1977;33(5):304–306. doi: 10.1111/j.1423-0410.1977.tb04480.x. [DOI] [PubMed] [Google Scholar]
- Scudder P., Hanfland P., Uemura K., Feizi T. Endo-beta-D-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J Biol Chem. 1984 May 25;259(10):6586–6592. [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A ceramide tetrasaccharide of human erythrocyte membrane reacting with anti-type XIV pneumococcal polysaccharide antiserum. Biochim Biophys Acta. 1973 Dec 13;330(2):147–155. doi: 10.1016/0005-2736(73)90219-8. [DOI] [PubMed] [Google Scholar]
- Taketomi T., Kawamura N. Fatty acids and sphingosine bases of porcine erythrocyte glycolipids and sphingomyelin. J Biochem. 1972 Oct;72(4):791–798. doi: 10.1093/oxfordjournals.jbchem.a129972. [DOI] [PubMed] [Google Scholar]
- Thorpe S. J., Feizi T. Species differences in the expression of carbohydrate differentiation antigens on mammalian blood cells revealed by immunofluorescence with monoclonal antibodies. Biosci Rep. 1984 Aug;4(8):673–685. doi: 10.1007/BF01121021. [DOI] [PubMed] [Google Scholar]
- Uemura K., Childs R. A., Hanfland P., Feizi T. A multiplicity of erythrocyte glycolipids of the neolacto series revealed by immuno-thin-layer chromatography with monoclonal anti-I and anti-i antibodies. Biosci Rep. 1983 Jun;3(6):577–588. doi: 10.1007/BF01120703. [DOI] [PubMed] [Google Scholar]
- Uemura K., Roelcke D., Nagai Y., Feizi T. The reactivities of human erythrocyte autoantibodies anti-Pr2, anti-Gd, Fl and Sa with gangliosides in a chromatogram binding assay. Biochem J. 1984 May 1;219(3):865–874. doi: 10.1042/bj2190865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K., Yuzawa M., Taketomi T. Characterization of major glycolipids in bovine erythrocyte membrane. J Biochem. 1978 Feb;83(2):463–471. doi: 10.1093/oxfordjournals.jbchem.a131933. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. I., Childs R. A., Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J Biol Chem. 1979 May 10;254(9):3221–3228. [PubMed] [Google Scholar]



