Abstract
In anemic patients with heart failure (HF), erythropoietin-type drugs can elicit clinical improvement. This study examined the effects of chronic monotherapy with darbepoetin-α (DARB) on left ventricular (LV) function and remodeling in nonanemic dogs with advanced HF. HF [LV ejection fraction (EF) ∼25%] was produced in 14 dogs by intracoronary microembolizations. Dogs were randomized to once a week subcutaneous injection of DARB (1.0 μg/kg, n = 7) or to no therapy (HF, n = 7). All procedures were performed during cardiac catheterization under general anesthesia and under sterile conditions. LV end-diastolic volume (EDV), end-systolic volume (ESV), and EF were measured before the initiation of therapy and at the end of 3 mo of therapy. mRNA and protein expression of caspase-3, hypoxia inducible factor-1α, and the bone marrow-derived stem cell marker c-Kit were determined in LV tissue. In HF dogs, EDV and ESV increased and EF decreased after 3 mo of followup. Treatment with DARB prevented the increase in EDV, decreased ESV, and increased EF. DARB therapy also normalized the expression of HIF-1α and active caspase-3 and enhanced the expression of c-Kit. We conclude that chronic monotherapy with DARB prevents progressive LV dysfunction and dilation in nonanemic dogs with advanced HF. These results suggest that DARB elicits beneficial effects in HF that are independent of the presence of anemia.
Keywords: erythropoietin, anemia, ventricular remodeling, stem cells
many patients with congestive heart failure (HF) are anemic or exhibit hemoglobin levels near the lower limit of normal (12). The severity of HF has been shown to positively correlate with the degree of anemia (38, 45). The kidneys represent the main site of the synthesis of erythropoietin, the most important hormone regulating erythropoiesis (18, 19). Darbepoetin-α (DARB) is a derivative of erythropoietin that contains two more carbohydrate groups than the native protein and increase its half-life by threefold compared with recombinant erythropoietin. DARB has been shown to be efficacious in maintaining hemoglobin levels in anemic patients (24). Clinical trials have shown that in anemic patients with HF, DARB increases and maintains hemoglobin and improves health-related quality of life and exercise duration (29, 44).
Erythropoietin has also been shown to possess a host of other nonerythropoietic properties including the ability to stimulate angiogenesis (20), attenuate apoptosis and hypoxia (28, 41), and mobilize bone marrow-derived stem cells (BMSCs) (1, 2). Erythropoietin has also been shown to exert direct cytoprotective effects on cardiomyocytes through stimulation of the erythropoietin receptor and the phosphatidylinositol 3-kinase/Akt signaling pathway (4, 46). In a rat model of myocardial infarction, treatment with DARB induced angiogenesis and improved left ventricular (LV) function (42). In the present study, we investigated the effects of long-term (3 mo) monotherapy with DARB on LV function and remodeling in nonanaemic dogs with chronic advanced HF produced by intracoronary microembolizations (37).
METHODS
Experimental model.
The canine model of chronic HF used in this study has been previously described in detail (37). In this preparation, LV dysfunction is produced by multiple sequential intracoronary microembolizations that result in the loss of viable myocardium. The model manifests many of the sequelae of HF observed in humans, including marked and progressive depression of LV systolic and diastolic function, reduced cardiac output (CO), and increased LV filling pressures. In the present study, 14 healthy mongrel dogs, weighing between 19 and 24 kg, underwent serial coronary microembolizations to produce HF. Embolizations were performed 1–2 wk apart and were discontinued when the LV ejection fraction (EF), determined angiographically, was ∼25%. All procedures were performed during cardiac catheterization under general anesthesia and under sterile conditions. Animals were sedated with intravenous oxymorphone hydrochloride (0.22 mg/kg) and diazepam (0.17 mg/kg), and a plane of anesthesia was maintained with 1–2% isofluorane.
Study protocol and end points.
Two weeks after the last embolization, dogs were randomized to once a week subcutaneous injection of DARB (1.0 μg/kg, n = 7) or to no therapy at all (HF, n = 7). The DARB dose used in this study was recommended by the manufacturer based on available information from preclinical testing. Hemodynamic, angiographic, echocardiographic, and Doppler measurements were made before randomization (pretreatment) and after the completion of 3 mo of therapy (posttreatment). After the final hemodynamic assessment, and while under general anesthesia, the dog's chest was opened, the heart was rapidly removed, and tissue was prepared for histological and biochemical evaluation. LV tissue samples were also obtained from six normal dogs for comparison. The primary study end points were changes in LV EF, determined angiographically, and changes in global LV remodeling based on changes in LV end-systolic volume (ESV) and end-diastolic volume (EDV), also determined angiographically. Secondary end points were changes in histomorphometric measures of cellular remodeling, namely, changes of cardiomyocyte hypertrophy, replacement fibrosis, interstitial fibrosis, capillary density (CD), and oxygen diffusion distance (ODD). The study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee and adhered to the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Hemodynamic and angiographic measurements.
Hemodynamic and angiographic measurements were made at baseline, before any microembolization, at the time of randomization, before the initiation of therapy (pretreatment), and at the end of 3 mo of therapy (posttreatment). Aortic and LV pressures were measured with catheter-tip micromanometers (Millar Instruments, Houston, TX). Left ventriculograms were obtained with the dog placed on its right side and recorded on 35-mm cinefilm at 30 frame/s during the injection of 20 ml of contrast material (RENO-M-60 Squibb, Princeton, NJ). Correction for image magnification was made with a radiopaque calibrated grid placed at the level of the LV. LV ESV and EDV were calculated from LV silhouettes using the area-length method (7), and EF was calculated as previously described (35). Stroke volume (SV) was calculated as the difference between LV EDV and ESV. CO was calculated as the product of SV and heart rate (HR).
Echocardiographic and Doppler measurements.
Echocardiographic and Doppler experiments were performed using a 77030A ultrasound system (Hewlett-Packard) with a 3.5-MHz transducer. All measurements were made with the dog placed in the right lateral decubitus position. Two chamber views were obtained to measure the LV major and minor semiaxes used in calculating LV end-diastolic circumferential wall stress (EDWS). EDWS was calculated as follows: EDWS = EDP × b/h(1 − h/2b)(1 − hb/2a2), where EDP is LV end-diastolic pressure, a is the LV major semiaxis, b is the LV minor semiaxis, and h is LV wall thickness (11).
Mitral valve inflow velocity was measured by pulsed-wave Doppler echocardiography. Velocity waveforms were used to calculate peak mitral flow velocity in early diastole (PE), peak mitral inflow velocity during left atrial contraction (PA), the ratio of PE to PA (PE/PA ratio), and early mitral inflow velocity deceleration time (DT). The presence or absence of functional mitral regurgitation (MR) was determined with Doppler color flow mapping (model 77020A Ultrasound System, Hewlett-Packard) using both apical two-chamber and apical four-chamber views. When present, the severity of functional MR was quantified based on the ratio of the regurgitant jet area to the area of the left atrium times 100.
Histomorphometric measurements.
From each heart, three transverse slices (∼3 mm thick) were obtained, one each from the basal, middle, and apical LV thirds. From each slice, transmural tissue blocks were obtained and embedded in paraffin blocks. Transmural tissue blocks were also obtained from the free wall segment of the slice, mounted on cork using Tissue-Tek embedding medium, rapidly frozen in isopentane precooled in liquid nitrogen, and stored at −70°C until use. The volume fraction of replacement fibrosis (VFRF), volume fraction of interstitial fibrosis (VFIF), myocyte cross-sectional area (MCSA; a measure of cardiomyocyte hypertrophy), CD, capillaries per fiber (an index of capillary density), and ODD were measured as previously described (36). Frozen myocardial sections were also immunostained with Griffonia simplicifolia lectin (Vector Laboratories) and photographed.
mRNA and protein expression, immunostaining, and circulating BMSC counts.
All tissue and blood samples were submitted for analysis without treatment regimen identifiers. mRNA expression of GAPDH, hypoxia inducible factor (HIF)-1α, caspase-3, and c-Kit was measured. Total RNA with an absorbance ratio (260 /280 nm) above 1.7 was isolated from frozen LV tissue, and ∼4–10 μg RNA was reverse transcribed into cDNA in an assay volume of 80 μl as previously described (31, 32). Protein levels of β-actin (to ensure equal protein loading in all groups), HIF-1α, activated caspase-3, and the stem cell marker c-Kit were measured in LV homogenates by Western blots as previously described (31, 32). Briefly, LV homogenate was prepared from ∼100 mg of LV powder. Samples were thawed in 1 ml of homogenization buffer, which consisted of 50 mM Tris·HCl (pH 7.4), 0.5 mM sodium EDTA (pH 7.0), 0.3 M sucrose, and protease inhibitors (0.8 mM benzamidine, 0.8 mg/l aprotinin, 0.8 mg/l leupeptin, and 0.4 μg/l antipain). Thawed tissue was homogenized by a polytron mixer. SDS extract was prepared from the resulting homogenate, and the protein assay was determined by Lowry's method. Approximately 20–100 μg protein of each dog sample was separated on a 4–20% SDS-polyacrylamide gel (Bio-Rad), and the separated proteins were electrophoretically transferred to a nitrocellulose membrane. The accuracy of the electrotransfer was confirmed by staining the membrane with 0.1% amido black. For identification of the desired protein, the nitrocellulose blot was incubated with the appropriately diluted primary antibody specific to each protein based on the supplier's instructions (Santa Cruz Biotechnology, Santa Cruz, CA, and Affinity Bio Reagents, Golden, CO). The antibody-binding protein(s) was visualized by autoradiography after treatment of the blot with horseradish peroxidase-conjugated secondary antibody and ECL color developing reagents according to the supplier. Band intensity was quantified using a Bio-Rad GS-670 imaging densitometer and expressed as densitometric units. In all circumstances, we made sure that the antibody was present in excess over the antigen and that the density of each protein band was in the linear scale. Band intensities of proteins were quantified in densitometric units and were normalized to β-actin.
Frozen LV myocardial sections were immunostained with c-Kit and cleaved poly ADP-ribose polymerase (PARP)-1 rabbit polyclonal antibodies (Santa Cruz Biotechnology) and double stained with propidium iodide to detect the mobilization of BMSCs and apoptosis in situ. Circulating Sca-1-positive BMSCs were isolated from whole blood as previously described (48). Sca-1-positive BMSC cells include mesenchymal, multipotent adult progenitor, and Hoechst side population cells (21). Briefly, blood samples were centrifuged over a Ficoll-Hypaque gradient, and isolated cells were blocked with 10% normal blocking serum to suppress nonspecific binding. Cells were then washed with PBS and kept in a cold room to incubate with the primary antibody for 2 h (Santa Cruz Biotechnology). Cells were once again washed with PBS and incubated with secondary antibody containing FITC for 1 h in the dark and on ice. Cells were washed, centrifuged, and then resuspended in PBS. Sca-1-positive BMSCs were fluorescent and were counted using a hemocytometer coupled to fluorescent microscope. The hemocytometer consists of nine 1-mm squares divided into smaller squares. One of the 1-mm squares represents a volume of 0.1 mm3 or 10−4 ml. Using a ×10 objective, the total number of cells and fluorescent cells in a 1-mm square area were counted. If there were <100 cells in a 1-mm square, 2 or more 1-mm square areas were counted and the results were averaged.
Statistical analysis.
All angiographic and histomorphometric analyses were performed in a blinded manner. Within-group comparisons were made using Student's paired t-test. To ensure that all study measures were similar at baseline and at the time of randomization before the initiation of therapy (pretreatment), intergroup comparisons were made using one-way ANOVA with α set to 0.05. To assess the treatment effect, the change in each measure from pretreatment to posttreatment was calculated for each study group. To determine whether significant differences in changes were present between groups, ANOVA was performed with α set to 0.05. Histomorphometric results as well as mRNA and protein expression results obtained in study groups and in a group of normal dogs were examined using ANOVA with α set to 0.05. In all instances, if significance was attained by overall ANOVA, pairwise comparisons were performed using the Student-Newman-Keuls test. For all pairwise comparisons, probability values of ≤0.05 were considered significant. All data are reported as means ± SE.
RESULTS
All 14 study animals survived the entire duration of the study. Baseline data for both groups are shown in Table 1. Baseline hemodynamic, angiographic, echocardiographic, and Doppler findings were within the normal range for mongrel dogs in our laboratory. There were no significant differences in any baseline parameters between control dogs and dogs randomized to DARB (Table 1). Similarly, there were no significant differences between study groups in any of the measures obtained at pretreatment (Tables 2 and 3).
Table 1.
Baseline hemodynamic, angiographic, echocardiographic, and Doppler measurements obtained in HF dogs subsequently randomized to no treatment at all or to treatment with DARB
| HF | HF + DARB | P Value | |
|---|---|---|---|
| HR, beats/min | 82±3 | 81±3 | 0.782 |
| Mean aortic pressure, mmHg | 73±4 | 73±3 | 0.879 |
| LV EDP, mmHg | 11±0.4 | 10±1 | 0.218 |
| LV EDV, ml | 49±2 | 48±2 | 0.842 |
| LV ESV, ml | 22±1 | 23±1 | 0.665 |
| LV EF, % | 54±2 | 52±1 | 0.468 |
| SV, ml | 26±2 | 25±1 | 0.588 |
| CO, l/min | 2.17±0.17 | 2.05±0.13 | 0.584 |
| CI, l·min−1·m−2 | 2.84±0.23 | 2.70±0.14 | 0.601 |
| MR, % | 2.5±0.9 | 1.8±0.9 | 0.587 |
| PE/PA | 2.9±0.2 | 3.3±0.2 | 0.108 |
| DT, ms | 117±11 | 121±8 | 0.775 |
| LV EDWS, g/cm2 | 49±3 | 43±2 | 0.155 |
Values are means ± SE. HF, heart failure; DARB, darbepoetin-α; HR, heart rate; LV EDP, left ventricular (LV) end-diastolic pressure; LV EDV, LV end-diastolic volume; LV ESV, LV end-systolic volume; LV EF, LV ejection fraction; SV, stroke volume; CO, cardiac output; CI, cardiac index; PE/PA, ratio of peak mitral flow velocity in early diastole (PE) to peak mitral inflow velocity during left atrial contraction (PA); MR, functional mitral regurgitation; DT, deceleration time; LV EDWS, LV end-diastolic circumferential wall stress.
Table 2.
Hemodynamic, angiographic, echocardiographic, and Doppler measurements in control dogs before (pretreatment) and after 3 mo of followup without treatment (posttreatment)
| Pretreatment | Posttreatment | P Value | |
|---|---|---|---|
| HR, beats/min | 86±6 | 84±2 | 0.716 |
| Mean aortic pressure, mmHg | 80±5 | 84±5 | 0.555 |
| LV EDP, mmHg | 16±1 | 18±0.4 | 0.025 |
| LV EDV, ml | 67±3 | 73±3 | 0.001 |
| LV ESV, ml | 50±2 | 57±2 | 0.000 |
| LV EF, % | 25±0.4 | 22±0.4 | 0.000 |
| SV, ml | 17±0.5 | 16±0.4 | 0.094 |
| CO, l/min | 1.43±0.07 | 1.34±0.05 | 0.312 |
| CI, l·min−1·m−2 | 1.75±0.08 | 1.63±0.05 | 0.258 |
| MR, % | 15.7±2.5 | 20.0±2.0 | 0.040 |
| PE/PA | 1.86±0.12 | 1.63±0.09 | 0.095 |
| DT, ms | 81.3±3.3 | 75.3±3.4 | 0.050 |
| LV EDWS, g/cm2 | 88±5 | 98±4 | 0.021 |
| Hct, % | 45±3 | 49±1 | 0.093 |
| Hgb, g/dl | 15.4±0.9 | 16.8±0.3 | 0.079 |
| ESR, mm/h | 0.14±0.14 | 0.43±0.43 | 0.57 |
Values are means ± SE. Hct, hematocrit; Hgb, hemoglobin; ESR, erythrocyte sedimentation rate.
Table 3.
Hemodynamic, angiographic, echocardiographic, and Doppler measurements obtained before (pretreatment) and after 3 mo of treatment with DARB (posttreatment)
| Pretreatment | Posttreatment | P Value | |
|---|---|---|---|
| HR, beats/min | 78±3 | 81±3 | 0.358 |
| Mean aortic pressure, mmHg | 75±3 | 87±4 | 0.024 |
| LV EDP, mmHg | 16±1 | 11±1 | 0.000 |
| LV EDV, ml | 66±4 | 66±4 | 0.736 |
| LV ESV, ml | 50±3 | 46±4 | 0.004 |
| LV EF, % | 25±0.3 | 30±1 | 0.002 |
| SV, ml | 16±1 | 20±1 | 0.000 |
| CO, l/min | 1.29±0.13 | 1.59±0.09 | 0.003 |
| CI, l·min−1·m−2 | 1.61±0.15 | 1.93±0.12 | 0.008 |
| MR, % | 17.6±1.7 | 15.5±1.9 | 0.087 |
| PE/PA | 1.93±0.10 | 2.26±0.15 | 0.076 |
| DT, ms | 77.0±5.3 | 82.4±6.0 | 0.443 |
| LV EDWS, g/cm2 | 86±6 | 72±5 | 0.043 |
| Hct, % | 44±2 | 59±5 | 0.032 |
| Hgb, g/dl | 15.1±0.6 | 19.3±2.0 | 0.096 |
| ESR, mm/h | 0.43±0.20 | 0.29±0.18 | 0.68 |
Values are means ± SE.
Effects of no therapy (HF) on the progression of LV dysfunction.
Pretreatment and posttreatment (3 mo followup without treatment) results in the HF group are shown in Table 2. HR and mean aortic pressure remained unchanged. LV EDP, EDV, and ESV increased significantly, whereas LV EF decreased significantly (Table 2). SV, CO, and the cardiac index (CI) tended to also decrease, but the reduction did not reach statistical difference. Echocardiographic and Doppler results showed a significant increase in the severity of functional MR and in LV EDWS. DT decreased significantly. The PE/PA ratio also decreased, but the reduction did not reach statistical significance. There was a modest increase in hematocrit, hemoglobin, and the erythrocyte sedimentation rate, but the changes did not reach statistical significance.
Effects of monotherapy with DARB.
Pretreatment and posttreatment (3 mo of therapy with DARB) results in this active study group are shown in Table 3. In dogs randomized to monotherapy with DARB, there was no difference in HR, but the mean aortic pressure increased modestly but significantly. The increase in EDV seen in HF dogs was prevented by treatment with DARB. LV EDP and ESV decreased significantly, whereas LV EF, SV, CO, and CI all increased significantly. Echocardiographic and Doppler results showed no significant change in the PE/PA ratio, DT, and severity of functional MR. LV EDWS decreased significantly after 3 mo of treatment. There was a modest increase of both hematocrit and hemoglobin and no change in the erythrocyte sedimentation rate.
Comparisons of treatment effect.
Treatment effect results are shown in Table 4. The changes between pretreatment and posttreatment are also shown in Table 4. Treatment effect analysis showed no differences among the two study groups with respect to HR and mean aortic pressure. Treatment with DARB resulted in a decrease in LV EDP, EDV, and ESV and a significant increase in LV EF, SV, CO, and CI. Compared with control, treatment with DARB significantly increased DT and the PE/PA ratio and significantly reduced the severity of MR and EDWS. Both hematocrit and hemoglobin tended to increase with DARB treatment, but this change was not statistically significant. There was no difference in the erythrocyte sedimentation rate between groups.
Table 4.
Comparison of changes from pretreatment to posttreatment in hemodynamic, angiographic, echocardiographic, and Doppler measurements between HF dogs and HF + DARB dogs
| HF | HF + DARB | P Value | |
|---|---|---|---|
| HR, beats/min | −3±7 | 3±3 | 0.499 |
| Mean aortic pressure, mmHg | 4±6 | 12±4 | 0.303 |
| LV EDP, mmHg | 1±0.5 | −5±1 | 0.000 |
| LV EDV, ml | 6.0±1.0 | −3.0±1.0 | 0.000 |
| LV ESV, ml | 6.0±1.0 | −4.0±1.0 | 0.000 |
| LV EF, % | −3.0±0.30 | 5.0±1.0 | 0.000 |
| SV, ml | −1±0.4 | 3±0.4 | 0.000 |
| CO, l/min | −0.09±0.09 | 0.29±0.06 | 0.003 |
| CI, l·min−1·m−2 | −0.12±0.10 | 0.33±0.08 | 0.004 |
| MR, % | 4.3±1.6 | −2.1±1.0 | 0.006 |
| PE/PA | −2.3±0.12 | 0.33±0.15 | 0.013 |
| DT, ms | −6.0±2.5 | 5.4±6.6 | 0.131 |
| LV EDWS, g/cm2 | 10±3 | −14±5 | 0.002 |
| Hct, % | 5±2 | 15±5 | 0.105 |
| Hgb, g/dl | 2±1 | 4±2 | 0.298 |
| ESR, mm/h | 0.29±0.47 | −0.14±0.34 | 0.68 |
Values are means ± SE.
Histomorphometric findings.
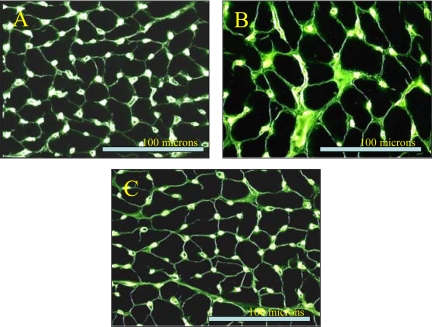
Histomorphometric data are shown in Table 5. Compared with normal dogs, HF dogs showed a significant increase in MCSA, VFRF, VFIF, and ODD along with a significant decrease CD and the capillary-to-fiber ratio. Long-term treatment with DARB significantly improved all of the above histomorphometric measures compared with HF dogs. Histological images of stained frozen sections of the LV myocardium are shown in Fig. 1.
Table 5.
Histomorphometric measurements in NL dogs, untreated HF dogs, and HF + DARB dogs
| NL | HF | HF + DARB | |
|---|---|---|---|
| MCSA, μm2 | 410±10 | 716±20* | 577±23*† |
| VFRF, % | 0±0 | 16.4±1.0* | 12.7±0.8*† |
| VFIF, % | 3.67±0.1 | 11.3±0.5* | 7.5±0.7*† |
| CD, no. of capillaries/mm2 | 2,609±80 | 1,869±77* | 2,116±49*† |
| Capillaries/fiber | 1.00±0.00 | 0.97±0.01* | 1.0±0.01† |
| ODD, μm | 8.9±0.2 | 11.4±0.4* | 10.2±0.1*† |
Values are means ± SE. NL, normal dogs; MCSA, myocyte cross-sectional area; VFRF, volume fraction of replacement fibrosis; VFIF, volume fraction of interstitial fibrosis; CD, capillary density; ODD, oxygen diffusion distance.
P < 0.05 vs. NL dogs;
P < 0.05 vs. HF dogs.
Fig. 1.
Microscopic images after immunostaining. A: frozen sections of the left ventricular (LV) myocardium stained with Griffonia simplicifolia lectin in normal dogs. B: frozen sections of the LV myocardium stained with G. simplicifolia lectin in heart failure (HF) dogs. C: frozen sections of LV myocardium stained with G. simplicifolia lectin in HF dogs treated with darbepoetin-α (DARB).
Results of mRNA and protein expression, immunostaining, and circulating BMSC counts.
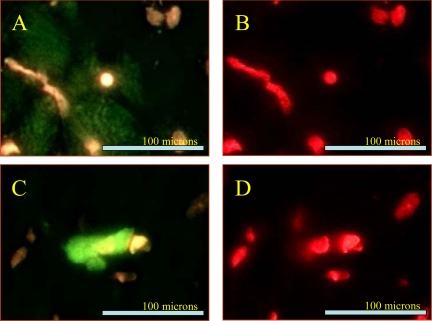
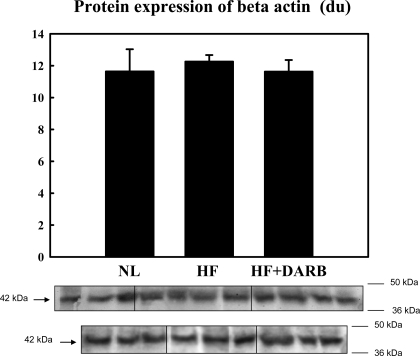
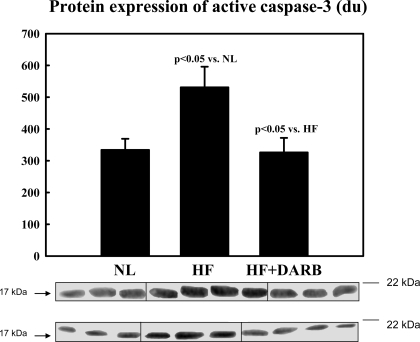
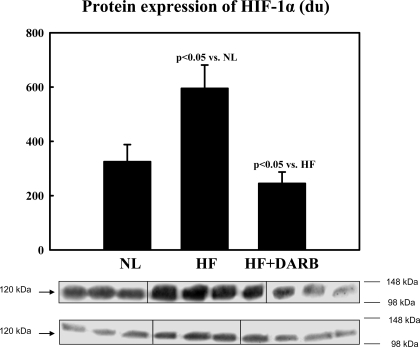
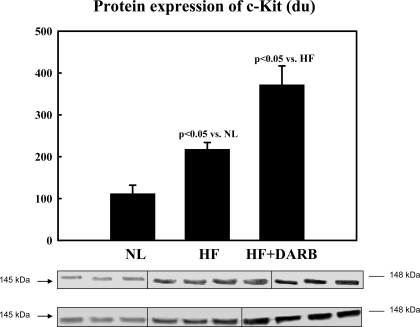
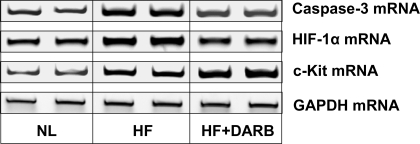
Immunohistochemical results are shown in Table 6 and Fig. 2. The number of circulating BMSCs was significantly reduced in HF dogs compared with that in normal dogs. Monotherapy with DARB normalized the number of circulating BMSCs compared with HF (Table 6). The results of protein and mRNA expression experiments are shown in Figs. 3–7 mRNA expression of GAPDH and protein expression of β-actin in the LV myocardium were not significantly different between the two study groups and that of the normal dog LV myocardium. In HF dogs, mRNA and protein expression of active caspase-3, HIF-1α, and c-Kit increased significantly compared with normal dogs. Treatment with DARB prevented the increase in active caspase-3 and HIF-1α and further increased the expression of c-Kit.
Table 6.
mRNA expression and immunohistochemical analyses of c-Kit+ve BMSCs and cleaved PARP-1 in the LV myocardium as well as numbers of circulating Sca-1-positive BMSCs in venous blood per million cells in NL dogs, untreated HF dogs, and HF + DARB dogs
| NL | HF | HF + DARB | |
|---|---|---|---|
| Immunohistochemistry | |||
| c-Kit+ve BMSCs, cells/mm2 | 1.5±0.12 | 2.1±0.19 | 2.8±0.26† |
| Cleaved PARP-1, cells/mm2 | 0.1±0.004 | 1.23±0.15* | 0.69±0.007† |
| Circulating BMSCs/million cells | 715±32 | 285±10* | 639±16† |
Values are means ± SE; n = 4 dogs/group for measurements of circulating bone marrow-derived stem cells (BMSCs)/million cells. PARP-1, poly ADP-ribose polymerase-1.
P < 0.05 versus NL dogs;
P < 0.05 vs. HF dogs.
Fig. 2.
Microscopic images after immunostaining. A: example of c-Kit staining. Frozen sections of the LV myocardium were stained with c-Kit antibody. B: c-Kit staining with double staining with propidium iodide. C: example of cleaved poly ADP-ribose polymerase (PARP)-1. Frozen sections of the LV myocardium were stained with cleaved PARP-1 antibody. D: cleaved PARP-1 staining with double staining with propidium iodide.
Fig. 3.
Top: bar graph showing band intensities [in densitometric units (du)] for the protein expression of β-actin in the LV myocardium of 6 normal (NL) dogs, 7 HF dogs, and 7 HF + DARB dogs. Bottom: bands showing the protein expression of β-actin in the LV myocardium of all dogs.
Fig. 4.
Top: bar graph showing band intensities (in densitometric units) for the protein expression of active caspase-3 in the LV myocardium of 6 NL dogs, 7 HF dogs, and 7 HF + DARB dogs. Bottom: bands showing the protein expression of active caspase-3 in the LV myocardium of all dogs.
Fig. 5.
Top: bar graph showing band intensities (in densitometric units) for the protein expression of hypoxia inducible factor (HIF)-1α in the LV myocardium of 6 NL dogs, 7 HF dogs, and 7 HF + DARB dogs. Bottom: bands showing the protein expression of HIF-1α in the LV myocardium of all dogs.
Fig. 6.
Top: bar graph showing band intensities (in densitometric units) for the protein expression of c-Kit in the LV myocardium of 6 NL dogs, 7 HF dogs, and 7 HF + DARB dogs. Bottom: bands showing the protein expression of c-Kit in the LV myocardium of all dogs.
Fig. 7.
Representative ethidium bromide-agarose gels showing mRNA encoding active caspase-3, HIF-1α, c-Kit, and GAPDH in the LV myocardium of 2 NL dogs, 2 HF dogs, and 2 HF + DARB dogs.
DISCUSSION
The results of the present study demonstrate that long-term monotherapy with DARB prevents progressive LV dysfunction and remodeling in nonanemic dogs with advanced HF. This observation is supported by the finding that monotherapy with DARB increased LV EF and prevented progressive LV enlargement. Monotherapy with DARB also decreased LV EDP and LV EDWS and increased both the PE/PA ratio and DT, suggesting that this form of therapy also improved LV diastolic function. These global benefits extended to the cellular level, as evidenced by the attenuation of replacement and interstitial fibrosis, reduced cardiomyocyte hypertrophy, and overall improvement in oxygen supply to the contractile units, as evidenced by increased CD, reduced ODD, and reduced expression of HIF-1α. All of these favorable observations provide support for the concept that DARB prevents worsening of the HF state even in the absence of associated anemia.
Effects of DARB on LV function and global remodeling.
The observation of preserved cardiac function and attenuation of LV global remodeling in dogs randomized to DARB is in agreement with previous studies of erythropoietin in experimental models of myocardial infarction and HF. In these animal models, acute and chronic therapy with erythropoietin was associated with a reduction in infarct size and with an improvement in cardiac function and remodeling (3, 13, 16, 17, 22, 30, 42, 47). In patients with myocardial infarction and chronic HF, DARB was both safe and well tolerated and showed a trend toward lowering the risk of morbidity and mortality (6, 10, 23). Ponikowski et al. (29) observed a sustained increase of hemoglobin, improved health-related quality of life, and a trend toward increased exercise time in a randomized, double-blind, placebo-controlled study of anemic patients with HF that were treated with DARB. In another randomized, double-blind, placebo-controlled study in anemic patients with HF treated with DARB, Veldhuisen et al. (44) observed a significant improvement in the Kansas City Cardiomyopathy Questionnaire total symptom score. Palazzuoli et al. (27) showed that treatment of patients with HF using erythropoietin increases LV EF and modestly reduces LV systolic diameter and volume.
Effects of DARB on myocardial fibrosis and cardiomyocyte hypertrophy.
Structural maladaptations such as cardiomyocyte hypertrophy and the accumulation of collagen in the cardiac interstitium occurs in HF and can lead to LV diastolic dysfunction (34, 39). In the present study, VFIF and MCSA, a measure of cardiac hypertrophy, were significantly reduced in dogs treated with DARB compared with HF dogs. A decrease in CD and an increase in ODD frequently accompany reactive interstitial fibrosis and hypertrophy in the setting of HF (34). In the present study, an increase in CD and a decrease in ODD were observed in HF dogs treated with DARB compared with HF dogs. The increase of CD observed in the present study may also be the result of proangiogenic activity of DARB. Kertesz et al. (20) demonstrated that deletion of erythropoietin in knockout animals leads to major angiogenic defects.
Effects of DARB on hypoxia, apoptosis, and BMSC mobilization.
In addition to improving LV function and attenuating progressive global LV remodeling, dogs randomized to DARB showed a significant decrease in the mRNA and protein expression of active caspase-3 and HIF-1α compared with HF dogs. Ongoing loss of cardiomyocytes through necrosis and/or apoptosis has been implicated, albeit in part, in the progressive worsening of LV function and remodeling in HF (33). Central to this process of programmed cell death is the activation of downstream procaspase-3, which cleaves caspase-activated deoxyribonuclease from its inhibitor. This inactivates proteins involved in DNA repair and replication and disassembles cell structures (40). Evidence from recent animal studies has shown that erythropoietin reduces apoptosis via an Akt-mediated pathway involving a decrease in active caspase-3 (5, 28, 41). Thus, the downregulation in the expression of active caspase-3 in HF dogs treated with DARB argues in favor of a reduction or amelioration of ongoing apoptosis in the failing myocardium. Gao et al. (9) demonstrated that DARB protects the heart against ischemia-reperfusion injury and improves cardiac function through a reduction of myocyte apoptosis.
In the present study, chronic treatment with DARB through its effects on myocardial fibrosis, CD, and cardiomyocyte hypertrophy may have attenuated hypoxia and, in turn, apoptosis. The latter is evidenced by a clear reduction in cleaved PARP-1 cells and a reduction in the expression of active caspase-3, a major contributor to ongoing programmed cell death. An important component of the hypoxic response is the activation of HIF-1α, a transcription factor essential for cells’ adaptation to low oxygen levels (15, 43). In the present study, the reduction in the expression of HIF-1α in the LV myocardium of HF dogs treated with DARB may reflect a reduction in the hypoxic burden on the LV myocardium.
Another important finding of this study was the observation of further enhancement in the mRNA and protein expression of c-Kit and mobilization of cKit+ve cells in the LV myocardium and normalization of circulating BMSC in venous blood of dogs randomized to DARB compared with HF dogs. c-Kit is a key stem cell marker that, when observed in tissue, provides evidence for the presence of BMSCs. In animal models of myocardial infarction and HF, erythropoietin was shown to mobilize BMSCs (14, 16, 47). Heeschen et al. (14) showed that erythropoietin stimulates postnatal neovascularization, in part, by enhancing stem cell mobilization from the bone marrow. In patients with myocardial infarction, Lipsic et al. (23) observed that treatment with DARB stimulates stem cell mobilization. In the present study, the observed upregulation of the key stem cell marker c-Kit and mobilization of cKit+ve cells suggests the possibility of BMSCs mobilization and potentially the subsequent colonization of the myocardium by circulating BMSCs. It is reasonable to assume that these positive adaptations, among others, account, in part, for the improvement in LV function seen in HF after therapy with DARB.
Potential clinical implications and conclusions.
The results of this study support the concept that the beneficial effects of DARB in HF are independent of erythropoiesis. This independence from erythropoesis is consistent with observations made by others in acute myocardial infarction (28). More recently, nonhematopoietic derivatives of erythropoietin have been developed, and some have shown the same cytoprotective effects as erythropoietin in brain and heart ischemia (8, 25, 26). The results of the present study provide a biological rationale for the use of DARB or perhaps derivatives of erythropoietin, after proper evaluation, as an adjunctive therapy in patients with HF even in the absence of anemia. At present, two large randomized clinical trials are underway to investigate the effects of DARB in patients with HF. One trial is The Reduction of Events with Darbepoetin-α in Heart Failure Trial (RED-HF), which will examine the efficacy of treatment of anemia with DARB compared with placebo on the composite of time to death from any cause or first hospital admission for worsening HF in subjects with symptomatic LV systolic dysfunction and anemia. The other trial is a substudy of the RED-HF trial, which will examine the effects of DARB on cardiac function and disease-specific biomarkers in patients with HF and anemia.
In conclusion, long-term monotherapy with DARB in dogs with advanced HF and without anemia prevents progressive LV dysfunction and attenuates global and structural LV myocardial remodeling. These preclinical findings suggest that DARB may have potential beneficial effects in HF beyond that related to the correction of underlying anemia. The beneficial effects of DARB in HF are likely mediated by a combination of factors that include, but are not limited to, a decrease in apoptosis and hypoxia and an increase in c-Kit-positive stem cells. All of these factors, individually or acting in concert, can have a direct effect on improving cardiac function.
Additional studies are needed to determine whether DARB or other derivatives of erythropoietin have added benefit when used in combination with standard HF therapies such as angiotensin-converting enzyme inhibitors and β-adrenergic receptor blockers.
GRANTS
This work was supported in part by research grants from Amgen Incorportated and National Heart, Lung, and Blood Institute Grant PO1-HL-074237-05.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood 103: 921–926, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bahlmann FH, DeGroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int 64: 1648–1652, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Dor I, Hardy B, Fuchs S, Kaganovsky E, Kadmon E, Sagie A, Coleman R, Mansur M, Politi B, Fraser A, Harell D, Okon E, Battler A, Haim M. Repeated low-dose of erythropoietin is associated with improved left ventricular function in rat acute myocardial infarction model. Cardiovasc Drugs Ther 21: 339–346, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation 109: 2050–2053, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA 100: 4802–4806, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleland JG, Sullivan JT, Ball S, Horowitz JD, Agoram B, Rosser D, Yates W, Tin L, Fuentealba P, Burton PB. Once-monthly administration of darbepoetin alfa for the treatment of patients with chronic heart failure and anemia: a pharmacokinetic and pharmacodynamic investigation. J Cardiovasc Pharmacol 46: 155–161, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Dodge HT, Sandler H, Baxley WA, Hawley RR. Usefulness and limitations of radiographic methods for determining left ventricular volume. Am J Cardiol 18: 10–24, 1966. [DOI] [PubMed] [Google Scholar]
- 8.Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, Doni M, Mengozzi M, Tonelli R, Ghezzi P, Coleman T, Brines M, Cerami A, Latini R. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci USA 102: 2046–2051, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ. Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol 293: H60–H68, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun Y, Knusel B, Armstrong P. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 117: 526–535, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Grossman W Cardiac Catheterization and Angiography (3rd ed.). Philadelphia, PA: Lea & Febiger, 1986, p. 293.
- 12.Haber HL, Leavy JA, Kessler PD, Kukin ML, Gottlieb SS, Packer M. The erythrocyte sedimentation rate in congestive heart failure. N Engl J Med 324: 353–358, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Hamed S, Barshack I, Luboshits G, Wexler D, Deutsch V, Keren G, George J. Erythropoietin improves myocardial performance in doxorubicin-induced cardiomyopathy. Eur Heart J 27: 1876–1883, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102: 1340–1346, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hewitson KS, McNeill LA, Schofield CJ. Modulating the hypoxia-inducible factor signaling pathway: applications from cardiovascular disease to cancer. Curr Pharm Des 10: 821–833, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, Tsukamoto O, Okada K, Koyama H, Komamura K, Takashima S, Shinozaki Y, Mori H, Shiraga M, Kitakaze M, Hori M. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol 48: 176–184, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hirata A, Minamino T, Asanuma H, Sanada S, Fujita M, Tsukamoto O, Wakeno M, Myoishi M, Okada K, Koyama H, Komamura K, Takashima S, Shinozaki Y, Mori H, Tomoike H, Hori M, Kitakaze M. Erythropoietin just before reperfusion reduces both lethal arrhythmias and infarct size via the phosphatidylinositol-3 kinase-dependent pathway in canine hearts. Cardiovasc Drugs Ther 19: 33–40, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen LOG, Fried E, Plzak WL. Role of kidney in erythropoesis. Nature 179: 2, 1957. [DOI] [PubMed] [Google Scholar]
- 19.Jensen JD, Eiskjaer H, Bagger JP, Pedersen EB. Elevated level of erythropoietin in congestive heart failure relationship to renal perfusion and plasma renin. J Intern Med 233: 125–130, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol 276: 101–110, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kotton DN, Summer RS, Sun X, Ma BY, Fine A. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol 284: L990–L996, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation 113: 535–543, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, van Zonneveld AJ, Schoemaker RG, van Gilst WH, Zijlstra F, van Veldhuisen DJ. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther 20: 135–141, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Macdougall IC An overview of the efficacy and safety of novel erythropoiesis stimulating protein (NESP). Nephrol Dial Transplant 16, Suppl 3: 14–21, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, Mengozzi M, Viviani B, Corsini E, Marinovich M, Torup L, Van Beek J, Leist M, Brines M, Cerami A, Ghezzi P. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med 12: 153–160, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci USA 100: 11612–11617, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palazzuoli A, Silverberg DS, Iovine F, Calabro A, Campagna MS, Gallotta M, Nuti R.Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J 154: 645 e649–615, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 112: 999–1007, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponikowski P, Anker SD, Szachniewicz J, Okonko D, Ledwidge M, Zymlinski R, Ryan E, Wasserman SM, Baker N, Rosser D, Rosen SD, Poole-Wilson PA, Banasiak W, Coats AJ, McDonald K. Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 49: 753–762, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Prunier F, Pfister O, Hadri L, Liang L, Del Monte F, Liao R, Hajjar RJ. Delayed erythropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol 292: H522–H529, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rastogi S, Mishra S, Zaca V, Alesh I, Gupta RC, Goldstein S, Sabbah HN. Effect of long-term monotherapy with the aldosterone receptor blocker eplerenone on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiovasc Drugs Ther 21: 415–422, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Rastogi S, Mishra S, Zaca V, Mika Y, Rousso B, Sabbah HN. Effects of chronic therapy with cardiac contractility modulation electrical signals on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiology 110: 230–237, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Sabbah HN, Sharov VG, Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev 5: 131–138, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 147: 29–34, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 89: 2852–2859, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 102: 1990–1995, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 35: 1737–1744, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki G, Morita H, Mishima T, Sharov VG, Todor A, Tanhehco EJ, Rudolph AE, McMahon EG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation 106: 2967–2972, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 281: 1312–1316, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, and El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun 308: 990–994, 2003. [DOI] [PubMed] [Google Scholar]
- 42.van der Meer P, Lipsic E, Henning RH, Boddeus K, van der Velden J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol 46: 125–133, 2005. [DOI] [PubMed] [Google Scholar]
- 43.van der Meer P, Voors AA, Lipsic E, van Gilst WH, van Veldhuisen DJ. Erythropoietin in cardiovascular diseases. Eur Heart J 25: 285–291, 2004. [DOI] [PubMed] [Google Scholar]
- 44.van Veldhuisen DJ, Dickstein K, Cohen-Solal A, Lok DJ, Wasserman SM, Baker N, Rosser D, Cleland JG, Ponikowski P. Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Eur Heart J 28: 2208–2216, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Volpe M, Tritto C, Testa U, Rao MA, Martucci R, Mirante A, Enea I, Russo R, Rubattu S, Condorelli GL, et al. Blood levels of erythropoietin in congestive heart failure and correlation with clinical, hemodynamic, and hormonal profiles. Am J Cardiol 74: 468–473, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Wald M, Gutnisky A, Borda E, Sterin-Borda L. Erythropoietin modified the cardiac action of ouabain in chronically anaemic-uraemic rats. Nephron 71: 190–196, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, Koster J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J 28: 2018–2027, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Zaca V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, Goldstein S, Sabbah HN. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol 50: 551–557, 2007. [DOI] [PubMed] [Google Scholar]