Abstract
Loss-of-function activin receptor-like kinase 1 gene mutation (ALK1+/−) is associated with brain arteriovenous malformations (AVM) in hereditary hemorrhagic telangiectasia type 2. Other determinants of the lesional phenotype are unknown. In the present study, we investigated the influence of high vascular flow rates on ALK1+/− mice by manipulating cerebral blood flow (CBF) using vasodilators. Adult male ALK1+/− mice underwent adeno-associated viral-mediated vascular endothelial growth factor (AAVVEGF) or lacZ (AAVlacZ as a control) gene transfer into the brain. Two weeks after vector injection, hydralazine or nicardipine was infused intraventricularly for another 14 days. CBF was measured to evaluate relative tissue perfusion. We analyzed the number and morphology of capillaries. Results demonstrated that hydralazine or nicardipine infusion increased focal brain perfusion in all mice. It was noted that focal CBF increased most in AAVVEGF-injected ALK1+/− mice following hydralazine or nicardipine infusion (145 ± 23% or 150 ± 11%; P < 0.05). There were more detectable dilated and dysplastic capillaries (2.4 ± 0.3 or 2.0 ± 0.4 dysplasia index; P < 0.01) in the brains of ALK1+/− mice treated with AAVVEGF and hydralazine or nicardipine compared with the mice treated with them individually. We concluded that increased focal tissue perfusion and angiogenic factor VEGF stimulation could have a synergistic effect to promote capillary dysplasia in a genetic deficit animal model, which may have relevance to further studies of AVMs.
Keywords: activin receptor-like kinase 1, angiogenesis, vascular endothelial growth factor
brain arteriovenous malformations (AVM), an important cause of intracranial hemorrhage, are a nidus of abnormal vessels lacking a true capillary bed and are characterized by various degrees of arteriovenous shunting (14). The pathogenesis of brain AVM remains poorly understood. There is currently no animal model that mimics the hemodynamic characteristics of the disease and the clinical course of recurrent spontaneous hemorrhage.
There are two general sets of clues regarding the pathogenesis of the disease. First, lesion tissue displays an actively angiogenic pattern. Features of the brain AVM phenotype include overexpression of vascular endothelial growth factor (VEGF) (21, 27, 40, 44), increased endothelial proliferation (19), altered Tie-2/angiopoietin signaling (18), and increased matrix metalloproteinase (MMP)-9 activity (7, 20).
Finally, there is evidence that genetic alteration in transforming growth factor (TGF)-β signaling greatly predisposes patients to the formation of brain AVM (32). Hereditary hemorrhagic telangiectasia (HHT), a multisystemic focal vascular lesion, is an autosomal dominant vascular disorder that has a high incidence of solid organ AVMs, including brain tissue (15, 41). Activin receptor-like kinase 1 heterozygous (ALK1+/−) mice have been proposed as an animal model for HHT (25). Aged ALK1+/− animals display vascular lesions, such as multiple thin-walled blood vessels, multiple foci of hemorrhage, dilated capillaries, abnormally large vessels, and discontinuities in the vessel wall in many organs including the mouse brain (43).
Because human brain AVM vessels are exposed to high flow rates, we wanted to address the question of whether flow could interact with the other identified components that may be involved in brain AVM pathogenesis. We first examined the capillary density after hydralazine treatment in the adeno-associated viral-mediated (AAV)VEGF-transduced brain of ALK1+/− or wild-type (WT) mice. The capillary densities were similar in these two groups. However, capillary dysplasia was detected in the ALK1+/− mouse brain but not in the WT mouse brain. Thus we conducted further studies on vascular changes in the ALK1+/− mice. We hypothesized that increased focal perfusion and angiogenic stimulation would have a synergistic effect in the promotion of capillary dysplasia in a genetic deficit animal model.
MATERIALS AND METHODS
Animals and treatment.
Experimental procedures for using laboratory animals were approved by the Institutional Animal Care and Use Committee of the University of California San Francisco (UCSF). ALK1+/− mice were developed in Dr. Douglas Marchuk's laboratory at Duke University and bred at the UCSF animal facility. There were no observable phenotype abnormalities in these animals. Adult male ALK1+/− mice weighing 25–30 g (8–10 wk old) were used for the experiments. Animals were allowed free access to food and water with a 12-h:12-h alternating light-dark cycle.
Experimental groups.
Adult male ALK1+/− mice underwent AAVVEGF or lacZ gene (AAVlacZ) transduction. Following 14 days of AAVVEGF or AAVlacZ gene transfer, the mice received a microosmotic pump implantation. Hydralazine, nicardipine, or saline (as control) was continuously infused into the cerebral left ventricle through a minipump. These ALK1+/− mice were divided into six experimental groups based on different treatments: 1) saline infusion in AAVlacZ-transduced mice, 2) saline infusion in AAVVEGF-transduced mice, 3) hydralazine infusion in AAVlacZ-transduced mice, 4) hydralazine infusion in AAVVEGF-transduced mice, 5) nicardipine infusion in AAVlacZ-transduced mice, and 6) nicardipine infusion in AAVVEGF-transduced mice.
AAVVEGF gene transfer.
Adult male ALK1+/− mice were anesthetized using ketamine-xylazine (100/10 mg/kg body wt) intraperitoneally. The mice were placed in a stereotactic frame with a mouth holder (David Kopf Instruments, Tujunga, CA). A burr hole was drilled in the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture (Fig. 1). A 10-μl Hamilton syringe was inserted into the right parenchyma about 3.0 mm under the cortex. Two microliters of viral suspension containing 2 × 109 genome copies of AAVVEGF were slowly injected into the right caudate putamen at a rate of 0.2 μl/min. The control groups underwent AAVlacZ injection using the same procedure.
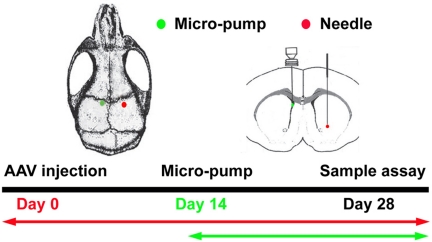
Fig. 1.
Experimental design. Adult male mice underwent adeno-associated viral-mediated (AAV)VEGF or AAVlacZ gene transfer in the parenchyma of the ipsilateral hemisphere of the brain for 2 wk. A minipump was then implanted intraventricularly in the left hemisphere of the brain. Hydralazine, nicardipine, or saline was consistently infused through the minipump for an additional 2 wk. Tissue samples were collected 4 wk after euthanizing animals. The red dot indicates the location of AAV vector injection; the green dot indicates the location of micropump implantation.
Hydralazine or nicardipine osmotic pump infusion.
To study the contribution of high cerebral blood flow (CBF) on vessel dysplasia, we used hydralazine or nicardipine, which increases CBF primarily by arteriolar vasodilation (26, 34). A 100-μl microosmotic pump (Alzet model 1002; Alza, Palo Alto, CA) was prefilled with hydralazine or nicardipine at 12 mg/ml doses; previous studies have shown that hydralazine and nicardipine increase CBF (2, 26). Two groups of mice received saline as a control. Under ketamine-xylazine (100/10 mg/kg body wt) anesthesia, a brain infusion cannula (30 gauge), attached with a microosmotic pump, was stereotactically implanted into the left lateral ventricle at 1 mm lateral to bregma and 2.6 mm depth (Fig. 1). The microosmotic pump was located between the head and neck. The pump rate was set to deliver 0.25 μl/h for 14 days.
Tissue collection.
Following 14 days of minipump infusion, the mice were anesthetized and perfused with ice-cold phosphate-buffered saline (PBS) through the left ventricle of the heart using Masterflex Pump Controller (Cole Parmer Instrument, Chicago, IL) at 4 ml/min. This flow rate approximates the murine physiological blood pressure (22). The perfusion protocol was identical among all experimental groups. The brain was removed, snap-frozen with dry ice, and stored at −80°C. A series of 20-μm cryostat coronal sections, 1 mm anterior and 1 mm posterior of the needle track, was collected and prepared to examine capillary density and vascular morphology.
Lectin staining, capillary density counting, and microvascular morphology.
Fluorescein-lycopersicin esculentum lectin (Vector Laboratories, Burlingame, CA) staining was used to identify capillaries. We chose lectin staining because our previous studies demonstrated that this method is reproducible and reliable (29, 39, 48, 50). Sections were fixed with 4% paraformaldehyde (PFA) for 30 min, blocked with 5% normal blocking serum for another 30 min, and incubated overnight with 2 μg/ml lectin at 4°C. Sections were then incubated overnight with anti-α-smooth muscle antibody actin (α-SMA; 1:1,000 dilution; Sigma, St. Louis, MO) at 4°C. The secondary antibody was Alexa 594 anti-mouse IgG (1:1,000 dilution; Molecular Probe, Carlsbad, CA). Three adjacent areas of capillaries (left, right, and bottom within ∼0.5 mm from the needle track) were chosen in two separate sections, and pictures were taken at low magnification (10×). As a surrogate for vessel counting, capillary density was determined by lectin optical density measurements using National Institutes of Health ImageJ software. The number of capillaries was calculated as the mean of the numbers obtained from the six images, as previously described (16).
Dilated vessels and dysplastic vessels were counted manually using the same images used for capillary counts. Studies have shown that the normal diameter of a capillary is between 3 and 8 μm (49). We scored a capillary as dilated if its diameter was >8 μm and the vascular wall had no smooth muscle layer. Normal capillaries have minimal change in vessel caliber between the vessels at the origin branch point and those at the termination of a vascular segment. Furthermore, the vascular wall is smooth. We scored vessels as dysplastic if 1) there was a deviation from the normal arborization pattern resulting in a visible morphology change in vessel caliber between the microvessels at the origin branch point and those at the termination of a vascular segment, 2) the microvessel had a diameter >15 μm, and 3) they lacked a smooth muscle layer. This definition encompasses the loss of normal smooth, tubular capillary structure and the primary observed patterns of irregular vascular wall or contour or focal aneurysmal dilations, either single or multiple and spiral, twisted vascular shapes.
To better observe dysplastic vessel morphology, the section was examined using Nikon ECLIOSE E1000 confocal microscopy (Nikon, Melville, NY). A section image of 1 μm was captured and reconstructed for three-dimensional observation if abnormal capillaries were detected. All capillary counting was performed blindly.
Immunofluorescent staining.
To label capillary wall cells, sections were fixed with 4% PFA for 30 min and incubated with 5% normal blocking serum for another 30 min, followed by fluorescent-lectin staining. Sections were then incubated with CD31 antibody (1:200 dilution; BD Pharmingen, San Jose, CA) and α-SMA overnight at 4°C. The secondary antibodies were Alexa 594 anti-rat IgG and Alexa 350 anti-mouse IgG (1:1,000 dilution; Molecular Probe, Carlsbad, CA).
Before the animals were euthanized, BrdU (Sigma-Aldrich, St. Louis, MO) was injected intraperitoneally daily at a dose of 100 mg per mouse per day for 7 days (46). For BrdU/CD31/α-SMA staining, sections were incubated with CD31, α-SMA (1:500 dilution; Abcam, Cambridge, MA), and BrdU antibody (1:500 diluted in mouse-on-mouse dilute working solution; Sigma-Aldrich) overnight, and Alexa Fluor 594 anti-rat IgG, 350 anti-rabbit, and 488 anti-mouse IgG were used as secondary antibodies. Sections were evaluated using a fluorescence microscope (Nikon Microphoto-SA, Melville, NY).
CBF measurement.
Local CBF adjacent to the needle track was measured before euthanizing the mice, using a laser Doppler flowmetry monitor (Model BPM2 System; Vasamedics, St. Paul, MN) equipped with a small-caliber probe (P-433; Vasamedics) that generated a beam of 740-nm wavelength light and penetrated the brain tissue ∼1 to 2 mm beyond the probe ending (11). The probe was placed on the thinned bone of the mouse skull. To standardize subsequent measurements, this site was marked with a spot drilled in the bone. Changes in local CBF were recorded as percentages of the baseline. The CBF in the same region in the contralateral hemisphere was monitored as a control.
Statistical analysis.
Parametric data in the different groups were compared using one-way ANOVA, followed by Fisher protected least-significant difference test. For AAVVEGF injection versus drug, two-way ANOVA was applied. All data are presented as means ± SD. A probability value of <5% was considered statistically significant.
RESULTS
No visible abnormal behavioral changes were detected in the animals following AAVVEGF injection and minipump implantation. Animals moved freely and ate and drank normally. No significant differences in mean artery blood pressure, PaO2, PaCO2, and blood pH were noted in the various groups of mice. This indicated that AAV-mediated gene transfer and minipump implantation did not affect general physiological parameters.
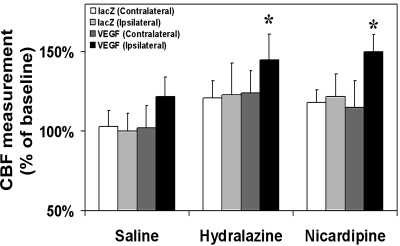
Hydralazine or nicardipine increased local CBF in the ALK1+/− mouse brain.
To determine whether hydralazine or nicardipine increased local CBF, we measured CBF before euthanizing the animals. There was no difference in CBF change in both the ipsilateral and contralateral hemispheres in AAVlacZ-transduced mice after the hydralazine or nicardipine infusion, suggesting that the minipump was accurately inserted in the lateral ventricle and contributed equally to both hemispheres. All the groups treated with hydralazine or nicardipine had a statistically relevant increase of CBF than their saline-treated counterparts (P < 0.05; Fig. 2). We noted that local CBF increased up to 145% and 150% in the AAVVEGF-transduced mice following hydralazine or nicardipine infusion compared with the AAVVEGF-transduced mice following saline infusion (P < 0.05), indicating that hydralazine or nicardipine infusion plus VEGF stimulation could maximize local CBF.
Fig. 2.
Cerebral blood flow (CBF) measurement. The changes to the local CBF in the 6 treated groups of mice are shown. The CBF was measured on the ipsilateral and contralateral hemisphere. Data are means ± SD; n = 6/group. *P < 0.05, ipsilateral hemisphere of AAVVEGF-transduced mice treated with hydralazine or nicardipine vs. the AAVVEGF-transduced mice treated with saline.
Hydralazine or nicardipine increased VEGF-induced angiogenesis.
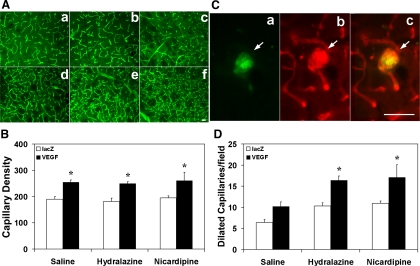
To determine whether hydralazine or nicardipine increased VEGF-induced angiogenesis, we first counted capillary numbers in the six groups of mice (Fig. 3A). We found that capillary density increased in the AAVVEGF-transduced mice compared with the AAVlacZ-transduced mice following saline infusion (254 ± 33 vs. 190 ± 25 capillary density; P < 0.05; Fig. 3B). Treatment of hydralazine or nicardipine did not further increase capillary density in AAVVEGF-transduced mice. We then counted the number of dilated capillaries. As indicated in the materials and methods section, a capillary with >8 μm diameter would be defined as a dilated capillary. We found that dilated capillaries increased in the AAVVEGF-transduced mice treated with hydralazine or nicardipine compared with the AAVVEGF-transduced mice treated with saline (P < 0.05; Fig. 3D). The average diameter of dilated capillaries is 11 μm. To determine whether these dilated capillaries were under active angiogenesis, CD31/BrdU fluorescent staining was performed. The results showed that the CD31-positive cells were well merged with BrdU-positive staining (Fig. 3C), indicating that the endothelial cells actively proliferated following hydralazine infusion in the AAVVEGF-transduced mice.
Fig. 3.
VEGF induces angiogenesis in the brain. A: photomicrographs show capillaries in the 6 groups of mice with different treatments. Scale bar = 50 μm. B: bar graph quantifies the number of capillaries in the 6 groups. Data are means ± SD; n = 6/group. *P < 0.05 vs. AAVlacZ-transduced group. C: photomicrographs show CD31 and BrdU double staining of dysplastic capillaries. Red is CD31-positive endothelial cells, green is BrdU-positive staining, and merged yellow color indicates that the endothelial cell is in the proliferating stage. Scale bar = 50 μm. D: bar graph shows the number of dilated capillary counts in the same groups above. Data are means ± SD; n = 6/group. *P < 0.05, AAVVEGF-transduced mice treated with hydralazine or nicardipine vs. AAVVEGF-transduced mice treated with saline.
Hydralazine or nicardipine enhanced capillary dysplasia in the AAVVEGF-transduced ALK1+/− mouse brain.
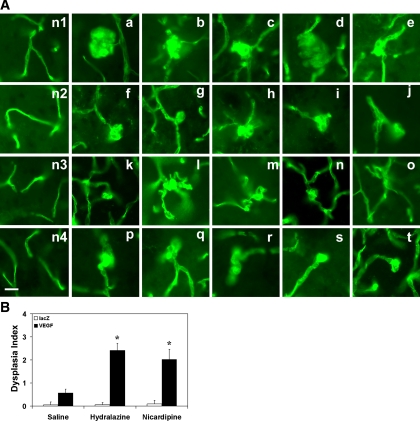
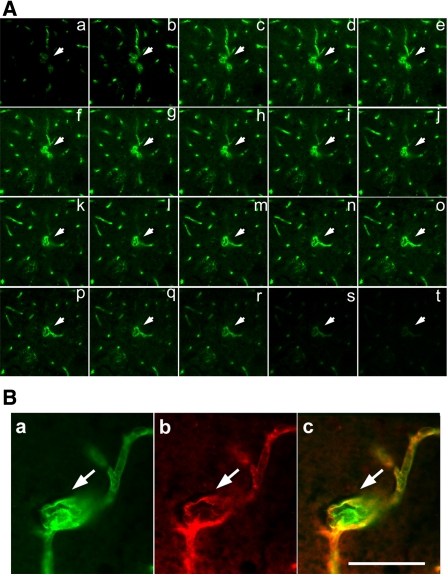
To determine whether hydralazine or nicardipine enhanced capillary dysplasia (e.g., massive or single enlargement or node and clustered, twisted, or spiral capillaries) in the AAVVEGF-transduced ALK1+/− mouse brain, hydralazine or nicardipine infusion plus AAVVEGF injection was further examined (Fig. 4A). We chose dysplastic capillary per 200 capillaries as the dysplasia index because this value approximates the average number of capillary counts in one 10× field. There were a few smooth muscle-positive stained small arteries or small veins in the mouse brain section. Most of them had normal morphology, although occasionally a change was observed. However, we focused only on capillary dysplasia in this study. We counted lectin-positive vessels that were negative for α-SMA to exclude small arteries or small veins. All the AAVVEGF-transduced mouse groups had a higher dysplasia index than the AAVlacZ-transduced groups (P < 0.01; Fig. 4B). There were more dysplastic capillaries in the AAVVEGF-transduced mouse brain treated with hydralazine or nicardipine than that treated with saline (P < 0.01; Fig. 4B). The average diameter for the dysplastic capillaries that we observed was 21 μm. These dysplastic capillaries usually developed in the ipsilateral cortex and caudate putamen, adjacent to the needle track and with abundant capillary growth.
Fig. 4.
Hydralazine or nicardipine induces capillary dysplasia in the AAVVEGF-transduced mouse brain. A: photomicrographs show the different patterns of capillary dysplasia morphology following hydralazine or nicardipine infusion in the AAVVEGF-transduced activin receptor-like kinase 1 gene mutation mice (a–t); n1–n4 are the controls (normal capillary morphology). Scale bar = 25 μm. B: bar graph shows dysplasia index in the treated groups of mice as above. Data are means ± SD; n = 6/group. *P < 0.01, AAVVEGF-transduced mice treated with hydralazine or nicardipine vs. AAVVEGF-transduced mice treated with saline.
Capillary dysplasia detection under three-dimensional confocal microscope.
In the three-dimensional reconstruction sections, the dysplastic capillary morphology could be clearly identified (Fig. 5A). Immunostaining confirmed that CD31 and lectin double positively stained the dysplastic capillary but stained negative for α-SMA (Fig. 5B). This result suggested that VEGF stimulation, along with hydralazine- or nicardipine-induced vessel dilation, triggered abnormal capillary formation in the adult ALK1+/− mouse brain.
Fig. 5.
Three-dimensional confocal image indicates capillary dysplasia. A: photomicrographs show a series of confocal images of capillary dysplasia morphology at different levels. B: photomicrographs show CD31 and lectin double-labeled immunostaining of dysplasia capillary. Red is CD31-positive endothelial cells, green is lectin-positive capillary walls, and merged yellow color indicates both CD31- and lectin-positive staining. Scale bar = 50 μm.
DISCUSSION
In the present study, we demonstrated that in the AAVVEGF-transduced ALK1+/− mouse brain 1) a continuous infusion of hydralazine or nicardipine enhanced local CBF, 2) vasodilator infusion alone did not affect capillary counts, and 3) the vasodilator enhanced VEGF-induced capillary dilation and capillary dysplasia. Our results indicated that at least three factors, VEGF stimulation, increased blood flow, and ALK1 gene haploinsufficiency, act synergistically to promote brain capillary dysplasia.
We chose laser Doppler to measure focal CBF because this method is simple and not invasive. We did not quantitatively measure focal CBF using isotopes in this study. However, for experimental purposes, absolute quantification was not necessary, since we were interested in demonstrating as proof of principle that relative increases in perfusion could affect vascular morphology.
VEGF is a well-known angiogenic factor. The vessels resulting from VEGF stimulation are often large, dilated, and leaky (4, 35). Injection of a high dosage of VEGF plasmid to the ischemic heart causes complications such as angioma formation (37). This complication has also been observed when VEGF-engineered myoblasts are implanted in the ischemic rat leg (42) and ischemic rat heart (30), indicating that the proper dose of VEGF is key to maintaining normal capillary phenotype. We use 2 × 109 genome copies of AAV in this experiment because our previous experiment indicated that injection of 2 × 109 genome copies of AAVVEGF can induce reproducible focal angiogenesis in the mature mouse brain, including the ischemic brain (16, 38). In the AAVVEGF-transduced mice, the number of capillaries increased up to 133% and dilated capillaries up to 160%, and there were more visible dysplastic capillaries compared with those of the AAVlacZ-transduced group. Therefore, VEGF is still a main factor for angiogenesis in the adult brain tissue.
Hydralazine (1, 24, 34, 36) and nicardipine (26, 45) have been widely used in treating arterial hypertension and are well-studied experimental means of manipulating cerebral perfusion. Our results indicated that both drugs dilate microvessels and increase CBF. There are no reported data indicating that hydralazine or nicardipine has intrinsic, direct dysmorphic effects, even with their long history of use. The doses of vessel dilators were selected based on the criteria that can cause a clinically relevant increase in flow rates without adverse effects, such as systemic hypotension during recirculation of a locally infused drug, or perhaps hyperemia resulting in increased intracranial pressure. Another study found that cerebral microvascular abnormality induced by chronic hypertension in rats can be reversed using hydralazine (17). However, indirect effects may be operative. Because these drugs cause vasodilation, they may enhance pressure-induced extravasation of protein into the brain, resulting in leakage at lower pressure levels (23). Increased vascular leakage is a prerequisite for inducing angiogenesis (13) although whether vasodilators increase vascular leakage needs to be further studied. One important mechanistic consideration to follow up on in future studies would be to distinguish the effect of increased vascular shear at the endothelial surface from effects caused by changes in barrier function from vasodilation.
In the present study, we found that after hydralazine and nicardipine infusion in the AAVlacZ-transduced ALK1+/− mice, dilated capillaries increased to 160% and 170%, respectively, and CBF to 120% of baseline (Fig. 3). Interestingly, after hydralazine and nicardipine infusion in AAVVEGF-transduced ALK1+/− mice, dilated capillaries markedly increased to 250% and 260%, respectively (Fig. 3), and CBF to 145% and 150% (Fig. 2) of baseline, respectively. These results suggest vasodilator and VEGF stimulation have an additive effect. Most importantly, no dysplastic capillary was found after hydralazine or nicardipine infusion alone but was occasionally found after VEGF stimulation, suggesting vasodilators alone do not induce capillary dysplasia (Fig. 4). However, hydralazine or nicardipine infusion greatly increased capillary dysplasia (4-fold) in the AAVVEGF-transduced ALK1+/− mice compared with that with VEGF stimulation alone (Fig. 4). It is possible that higher brain perfusion contributed to the development of brain capillary dysplasia in the setting of VEGF excess and abrogated ALK1 function. We used two unrelated vasodilators in this study to rule out some unknown synergism between the drugs and the VEGF stimulation against the ALK1 gene deletion to cause dysplasia.
ALK1 is a receptor for the TGF-β ligands superfamily (3) and regulates TGF-β action in endothelial cells through binding TGF-β1 and TGF-β3 (8, 31). Decrease in TGF-β1 or TGF-β1 receptors leads to unstable cellular interactions in the vessel wall, dilated vessels, and vascular abnormalities (5). These data also suggest a potential mechanism for the role of this pathway in AVM pathogenesis. ALK1 is required for endothelial cell maturation (10, 28). Disruption of this signaling pathway by mutation or possibly through physiological perturbation will result in a block in the maturation process, leading to inappropriate endothelial cell migration and proliferation. This suggests that aberrant endothelial cell migration and proliferation may be one of the earliest events in the development of an AVM, either sporadic or as a result of familial mutation.
ALK1 homozygous mice die at embryo on day 10.5 and display vascular developmental defects such as AVM (33, 47). Development of vascular lesions in ALK1+/− mice is age dependent and has only been reported in ALK1+/− mice older than 7 mo (43). The factors that initiate lesion formation and those that influence disease progression remain unknown. We used young ALK1+/− mice (8–10 wk old) to explore the factors causing vascular dysplasia development; there is no visible abnormal vascular structure in the ALK1+/− mouse brain at this age (data not shown here).
Alterations in vascular flow rates can induce vessel remodeling, and MMPs and nitric oxide/nitric oxide synthase appear to be critical in flow-induced vessel remodeling (6, 9, 12). These effects need to be further studied in the future.
One of the limitations for this line of experiments is the lack of strictly quantitative objective criteria to use as the index of vascular dysplasia. Nonetheless, the rather striking and reproducible effects we observed show that our index is sufficient to demonstrate the principle of synergism between flow, VEGF, and genetic background. The visual representation shown in Fig. 4A suggests a biological effect worthy of further quantitative study. Future longitudinal time course studies might clarify the progression from normal to enlarged capillaries.
In summary, locally increased VEGF concentration together with increased CBF may alter the balance of vascular signaling in ALK1+/− mice, which leads to what appears to be pathological capillary remodeling. This experimental vascular phenotype holds promise for modeling the phenotype seen in human brain AVM.
GRANTS
These studies were supported by National Institutes of Health Grants R01-NS27713 (to W. L. Young), R21-NS50668 (to G.-Y. Yang), and P01-NS44145 (to W. L. Young and G.-Y. Yang).
Acknowledgments
We thank Voltaire Gungab for editorial assistance and the staff of the Center for Cerebrovascular Research (http://avm.ucsf.edu/) for collaborative support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Auer LM, Sayama I, Johansson BB. Cerebrovascular effects of dihydralazine in hypertensive and normotensive rats. Acta Med Scand Suppl 678: 73–81, 1983. [DOI] [PubMed] [Google Scholar]
- 2.Barry DI, Strandgaard S, Graham DI, Svendsen UG, Braendstrup O, Paulson OB. Cerebral blood flow during dihydralazine-induced hypotension in hypertensive rats. Stroke 15: 102–107, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Berg JN, Gallione CJ, Stenzel TT, Johnson DW, Allen WP, Schwartz CE, Jackson CE, Porteous ME, Marchuk DA. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 61: 60–67, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, Achen MG, Stacker SA, Hedman M, Alitalo K, Yla-Herttuala S. Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther 14: 1451–1462, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bourdeau A, Cymerman U, Paquet ME, Meschino W, McKinnon WC, Guttmacher AE, Becker L, Letarte M. Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1. Am J Pathol 156: 911–923, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CY, Chen YS, Ma MC, Chen CF. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. J Vasc Surg 45: 804–811, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci 11: 3121–3128, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chen YG, Massague J. Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J Biol Chem 274: 3672–3677, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chien S Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007. [DOI] [PubMed] [Google Scholar]
- 10.David L, Mallet C, Vailhe B, Lamouille S, Feige JJ, Bailly S. Activin receptor-like kinase 1 inhibits human microvascular endothelial cell migration: potential roles for JNK and ERK. J Cell Physiol 213: 484–489, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab 9: 589–596, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol 27: 317–324, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039, 1995. [PMC free article] [PubMed] [Google Scholar]
- 14.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet 359: 863–873, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med 333: 918–924, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab 27: 1853–1860, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Harper SL Effects of antihypertensive treatment on the cerebral microvasculature of spontaneously hypertensive rats. Stroke 18: 450–456, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL. Abnormal balance in the angiopoietin-tie2 system in human brain arteriovenous malformations. Circ Res 89: 111–113, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Mesa-Tejada R, Quick CM, Bollen AW, Joshi S, Pile-Spellman J, Lawton MT, Young WL. Evidence of increased endothelial cell turnover in brain arteriovenous malformations. Neurosurgery 49: 124–131, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 34: 925–931, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM, Young WL. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 56: 1058–1065, 2005. [PubMed] [Google Scholar]
- 22.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287: H1618–H1624, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Johansson BB Blood-brain barrier dysfunction in acute arterial hypertension after papaverine-induced vasodilatation. Acta Neurol Scand 50: 573–580, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Johansson BB, Auer LM, Trummer UG. Pial vascular reaction to intravenous dihydralazine in the cat. Stroke 11: 369–371, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13: 189–195, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Joshi S, Young WL, Pile-Spellman J, Duong DH, Vang MC, Hacein-Bey L, Lee HT, Ostapkovich ND. The feasibility of intracarotid adenosine for the manipulation of human cerebrovascular resistance. Anesth Analg 87: 1291–1298, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Koizumi T, Shiraishi T, Hagihara N, Tabuchi K, Hayashi T, Kawano T. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 50: 117–124, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lamouille S, Mallet C, Feige JJ, Bailly S. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood 100: 4495–4501, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracranial hemorrhage. Stroke 38: 2563–2568, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 102: 898–901, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem 273: 25628–25636, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum Mol Genet 12: R97–R112, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA 97: 2626–2631, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overgaard J, Skinhoj E. A paradoxical cerebral hemodynamic effect of hydralazine. Stroke 6: 402–404, 1975. [DOI] [PubMed] [Google Scholar]
- 35.Rissanen TT, Rutanen J, Yla-Herttuala S. Gene transfer for therapeutic vascular growth in myocardial and peripheral ischemia. Adv Genet 52: 117–164, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder T, Sillesen H. Dihydralazine induces marked cerebral vasodilation in man. Eur J Clin Invest 17: 214–217, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat—angiogenesis and angioma formation. J Am Coll Cardiol 35: 1323–1330, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-regulated VEGF factor gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther 15: 30–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci 11: 3190–3198, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Shenkar R, Elliott JP, Diener K, Gault J, Hu LJ, Cohrs RJ, Phang T, Hunter L, Breeze RE, Awad IA. Differential gene expression in human cerebrovascular malformations. Neurosurgery 52: 465–478, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 91: 66–67, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell 2: 549–558, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, Marchuk DA. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet 12: 473–482, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sure U, Butz N, Siegel AM, Mennel HD, Bien S, Bertalanffy H. Treatment-induced neoangiogenesis in cerebral arteriovenous malformations. Clin Neurol Neurosurg 103: 29–32, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Takenaka T, Miyazaki I, Asano M, Higuchi S, Maeno H. Vasodilator and hypotensive effects of the optical isomers of nicardipine (YC-93), a new Ca2+-antagonist. Jpn J Pharmacol 32: 665–670, 1982. [DOI] [PubMed] [Google Scholar]
- 46.Taupin P BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 53: 198–214, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet 26: 328–331, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, Young WL, Yang GY. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab 24: 237–244, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106: 829–838, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang GY, Chen Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 39: 1254–1261, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]