Abstract
Insulin resistance is an increasingly prevalent condition in humans that frequently clusters with disorders characterized by left ventricular (LV) pressure overload, such as systemic hypertension. To investigate the impact of insulin resistance on LV remodeling and functional response to pressure overload, C57BL6 male mice were fed a high-fat (HFD) or a standard diet (SD) for 9 days and then underwent transverse aortic constriction (TAC). LV size and function were assessed in SD- and HFD-fed mice using serial echocardiography before and 7, 21, and 28 days after TAC. Serial echocardiography was also performed on nonoperated SD- and HFD-fed mice over a period of 6 wk. LV perfusion was assessed before and 7 and 28 days after TAC. Nine days of HFD induced systemic and myocardial insulin resistance (assessed by myocardial 18F-fluorodeoxyglucose uptake), and myocardial perfusion response to acetylcholine was impaired. High-fat feeding for 28 days did not change LV size and function in nonbanded mice; however, TAC induced greater hypertrophy, more marked LV systolic and diastolic dysfunction, and decreased survival in HFD-fed compared with SD-fed mice. Compared with SD-fed mice, myocardial perfusion reserve was decreased 7 days after TAC, and capillary density was decreased 28 days after TAC in HFD-fed mice. A short duration of HFD induces insulin resistance in mice. These metabolic changes are accompanied by increased LV remodeling and dysfunction after TAC, highlighting the impact of insulin resistance in the development of pressure-overload-induced heart failure.
Keywords: heart failure, myocardial perfusion
insulin resistance, a state in which a given concentration of insulin is associated with a subnormal glucose response, is an increasingly prevalent health problem in the Western societies, affecting at least 54 million Americans (19). Insulin resistance predicts the subsequent development of symptomatic heart failure, independently of other risk factors (13). Whether the association of insulin resistance and heart failure can be attributed to the increased prevalence of coronary artery disease in insulin-resistant patients or whether insulin resistance itself contributes to heart failure is controversial (28).
Insulin resistance frequently clusters with hemodynamic stresses, such as systemic hypertension. Systemic hypertension induces chronic left ventricular (LV) pressure overload and is a recognized contributor to heart failure, responsible for >30% of the cases of heart failure in African-Americans (1). Adverse LV remodeling is characterized by a change of LV size and geometry, including LV hypertrophy, and progressive dilation. This process plays a crucial role in the cardiac decompensation resulting from chronic pressure overload (15, 26). Whether insulin resistance augments adverse LV remodeling induced by chronic pressure overload, thereby leading to heart failure, is unknown.
The purpose of this study was to investigate the role of insulin resistance in the modulation of LV remodeling and function after pressure overload. In mice, chronic feeding using a high-fat diet (HFD) has been shown to produce diabetes and obesity, while a HFD for shorter periods (1–2 wk) results in insulin resistance and minimal weight gain (22, 27). Mice were subjected to transverse aortic constriction (TAC) after they were fed a HFD or standard diet (SD) for 9 days. Mice fed a HFD developed systemic and myocardial insulin resistance and were predisposed to adverse LV remodeling, LV dysfunction, and decreased survival in a setting of chronic LV pressure overload.
METHODS
All animal procedures were conducted in C57BL6 male mice, in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996), and were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Diets were started at 7 wk of age. Mice were fed either a SD (10% fat, D12450B, Research Diets, New Brunswick, NJ) or HFD (60% fat from lard, D12492, Research Diets).
Metabolic blood measurements.
Mice fed with SD or HFD for 9 days were either fasted for 16 h (n = 8 in each group) or fed (12 SD-fed mice and 11 HFD-fed mice). Blood was drawn from the tail vein, and glucose level was measured by the glucose oxidase method (One Touch Ultra, Life Scan). Plasma insulin concentration was determined using an enzyme-linked immunosorbent assay (Crystal Chem, Chicago, IL). Blood glucose and insulin levels were also measured 5 days after TAC in seven nonfasted mice fed a SD and seven nonfasted mice fed a HFD.
In nonfasted mice fed a SD and nonfasted mice fed a HFD, blood was drawn from an arterial line, and plasma free fatty acids [HR Series NEFA-HR (2), Wako Pure Chemical Industries, Osaka, Japan; SD: n = 5, HFD: n = 4], leptin (Crystal Chem, Downers Grove, IL; SD: n = 8, HFD: n = 8), and adiponectin (B-Bridge International, Mountainview, CA; SD: n = 9, HFD: n = 10) concentrations were measured according to manufacturer's instructions.
Glucose and insulin tolerance tests.
Seven mice fed a SD and eight mice fed a HFD were fasted for 6 h, and glucose (2 mg/g body wt) was injected intraperitoneally (4). Blood glucose levels were serially measured at baseline and 15, 30, 60, and 120 min after injection. Twelve mice fed a SD and nine mice fed a HFD were fasted for 6 h, and 0.3 U/kg body wt of insulin was injected intraperitoneally. Blood glucose levels were serially measured at baseline and 15, 30, and 45 min after injection.
Micro-positron emission tomography.
Cardiac micro-positron emission tomography (PET) was performed on five nonfasted mice before and after 9 days of HFD using a micro-PET P4 instrument (Concord Microsystems). Mice were anesthetized using isoflurane, and 18F-fluorodeoxyglucose (18F-FDG; 100–150 μCi) was injected in the tail vein. Dynamic imaging acquisition started simultaneously with the tracer injection and lasted 45 min. Time-myocardial tracer activity curves were obtained by tracing a region of interest over the LV myocardium. 18F-FDG uptake was measured 45 min after injection and expressed as percentage of the injected dose per milliliter of tissue.
Micro-PET was repeated in six mice before and after 9 days of HFD. In this group, after 2-h fasting, mice received insulin (0.75 U/kg, intraperitoneal injection). Immediately after insulin injection, 18F-FDG was intravenously injected, and micro-PET images were acquired every minute. The duration of acquisition was 120 min, to allow for stabilization of FDG uptake after insulin injection. Myocardial 18F-FDG uptake was averaged between 100 and 120 min after injection and expressed as percentage of the injected dose of 18F-FDG per milliliter of tissue.
TAC.
TAC was performed after 9 days of SD or HFD, as previously described (7, 12). Briefly, mice were anesthetized with intraperitoneal administration of 100 mg/kg ketamine and 5 mg/kg xylazine and subsequently ventilated. TAC was performed by ligation (7–0 prolene) of the aorta between the inominate and left common carotid arteries, with an overlying 27-gauge needle, followed by removal of the needle. The mice were kept on HFD or SD for the reminder of the experiment.
Echocardiography.
Echocardiography was performed in five SD- and five HFD-fed mice before starting the diet, and weekly thereafter for 6 wk. Echocardiography was obtained before and 7, 21, and 28 days after TAC, in 12 surviving SD-fed mice and 7 surviving HFD-fed mice. In a separate experiment, the early functional response after TAC was assessed using echocardiography in six SD-fed and eight HFD-fed mice 3 days after TAC.
Echocardiography was performed using a 13-MHz linear-array transducer with a digital ultrasound system (Vivid 7, GE Medical Systems, Milwaukee, WI), as previously described (12). LV end-diastolic diameter (LVEDD), fractional shortening (FS), anterior wall thickness (AWT), and posterior wall thickness (PWT) were obtained from M-mode tracings at the level of the papillary muscles. LV mass was calculated using the following formula: 1.05 × [(AWT + PWT + LVEDD)3 − LVEDD3] (17).
Invasive hemodynamic assessment of LV function.
Hemodynamic measurements were acquired in separate groups of mice before TAC (5 mice fed a HFD or SD for 9 days) and 28 days after TAC (10 mice fed a SD and 10 mice fed a HFD).
Hemodynamic parameters were measured as previously described (7). Heart rate, LV end-systolic pressure, LV end-diastolic pressure, maximum first derivative of the developed LV pressure (dP/dtmax), minimum first derivative of the developed LV pressure (dP/dtmin), and arterial elastance were obtained with a 1.4-Fr high-fidelity SPR-839 Millar pressure catheter (Millar Instruments, Houston, TX) advanced into the LV. The time constant of isovolumic relaxation was calculated according to the method of Weiss. A left carotid arterial catheter was placed to measure systolic arterial pressure and to calculate the transstenotic pressure gradient.
After the last acquisition of hemodynamic measurements, the mice were euthanized, and their LV was harvested, weighed, and used for histology.
Histological analysis.
Paraffin-embedded 5-μm cross-sectional myocardial sections were stained using FITC-conjugated lectin from Triticum Vulgaris (wheat) (Sigma-Aldrich, St. Louis, MO) to determine myocyte width in SD- and HFD-fed mice 28 days after TAC. Twenty measurements were obtained at the level of the nucleus in longitudinally sectioned myocytes in each section (viewed at a magnification of ×600). Myocardial collagen was assessed qualitatively on midventricular sections stained with Sirius Red. Lipid deposits were assessed using Oil red O stain in paraffin-embedded midventricular sections. To visualize capillaries, LV sections were incubated with biotinylated Griffonia simplicifolia lectin I and stained with the Vectastain ABC immunoperoxidase system (Vector Laboratories, Burlingame, CA) in SD- and HFD-fed mice, 7 and 28 days after TAC. The number of capillaries per square millimeter and per cardiomyocyte fiber was counted. Five fields were analyzed for each mouse at a magnification of ×400.
Cardiac cell death was detected 3 days after TAC with the use of the terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) technique (DeadEnd Fluorometric TUNEL System, Promega, Madison, WI), as previously described (20).
Measurement of gene expression.
RNA was extracted using TRIZOL reagent (Invitrogen, La Jolla, CA) in the myocardium of five mice fed a SD for 10 days, five mice fed a HFD for 10 days, and 7 days after TAC in four mice fed a SD and seven mice fed a HFD. cDNA was synthesized with Moloney murine leukemia virus reverse transcriptase (Invitrogen). Transcript levels of peroxisome proliferator activated receptor-α (PPAR-α), carnitine palmitoyltransferase I (CPT-1), acetyl-coenzyme A dehydrogenase medium chain (ACADM), cluster of differentiation 36 (CD36), malonyl-CoA decarboxylase (MCD), vascular endothelial growth factor A (VEGF-A), and 18S ribosomal RNA (rRNA) were measured by real-time PCR with a Mastercycler ep realplex 2 (Eppendorf, Westbury, NY) and primers for PPAR-α (5-TCGGCGAACTATTCGGCTG-3, 5-GCACTTGTGAAAACGGCAGT-3), CPT-1 (5-GCACACCAGGCAGTAGCTTT-3, 5-CAGGAGTTGATTCCAGACAGGTA-3), ACADM (5-CCGAAGAGTTGGCGTATGGG-3, 5-GGGCTCTGTCACACAGTAAGC-3), CD36 (5-ATGGGCTGTGATCGGAACTG-3, 5-GTCTTCCCAATAAGCATGTCTCC-3), MCD (5-GCACGTCCGGGAAATGAAC-3, 5-GCCTCACACTCGCTGATCTT-3), VEGF-A (5-CTTGTTCAGAGCGGAGAAAGC-3, 5-ACATCTGCAAGTACGTTCGTT-3), and 18s rRNA (5-CGGCTACCACATCCAAGGAA-3, 5-GCTGGAATTACCGCGGCT-3). Changes in gene expression normalized to levels of 18S rRNA were determined with the relative threshold cycle method (Applied Biosystems).
Myocardial contrast echocardiography.
Myocardial perfusion and its response to acetylcholine were assessed using MCE in four mice fed a SD and four mice fed a HFD. Seven days after TAC, myocardial perfusion response to dobutamine was studied in five mice fed a SD and six mice fed a HFD.
Myocardial contrast echocardiography (MCE) was performed using a linear transducer (14 MHz; Acuson Sequoia C512 system; Siemens, Mountain View, CA), as previously described (23). Briefly, perflutren-filled lipid microbubbles (Definity, Bristol-Myers Squibb Medical Imaging, Billerica, MA) were infused intravenously (dilution: 1/10 in sterile saline, infusion rate: 20 μl/min). Perfusion images were acquired using a parasternal long-axis view, in real-time mode, after a sequence of 10 high-energy frames. After acquiring baseline images, either acetylcholine (2 μg·kg−1·min−1, 10 μl/min, Sigma Aldrich, St. Louis, MO) or dobutamine (10 μg·kg−1·min−1) was infused intravenously. After 5 min of continuous infusion, MCE images were obtained. Analysis of MCE was performed as described previously (23). Briefly, a region of interest was positioned within the posterolateral wall. Time signal intensity myocardial replenishment curves were obtained, and β (the initial slope of the replenishment curve estimating blood velocity), A (the plateau intensity reflecting myocardial blood volume), and the product Aβ [reflecting myocardial blood flow (MBF)] were obtained.
Statistical analysis.
Values are expressed as means ± SE. Statistical analysis was done with the JMP statistical package (SAS Institute, Cary, NC). After testing for inequality of variances, the differences in blood tests between the SD- and HFD-fed mice were tested using Student's unpaired t-tests. Myocardial glucose uptake before and after HFD was compared using Student's paired t-test. For comparison of body weights, insulin and glucose tolerance tests, and echocardiographic parameters over time, results were analyzed with an ANOVA for repeated measurements. If the interaction of time and diet was significant, unpaired Student's t-tests were used to compare echocardiographic parameters between groups at the same time point. Analysis of survival rates after banding was performed with the log-rank test. A probability value of <0.05 was considered significant.
RESULTS
HFD induces systemic and myocardial insulin resistance.
Blood glucose and plasma insulin levels were greater in fasted and nonfasted mice fed a HFD for 9 days than in mice fed a SD (Table 1). Mice fed a HFD had a lower adiponectin level than mice fed a SD (Table 1). Free fatty acids levels were similar in SD- and HFD-fed mice, as were leptin levels (Table 1).
Table 1.
Metabolic blood measurements obtained after 9 days of HFD or SD
| SD | HFD | |
|---|---|---|
| Fasted glucose level, mg/dl | 76±5 | 118±9* |
| Fasted insulin level, ng/ml | 0.43±0.11 | 0.89±0.09* |
| Unfasted glucose level, mg/dl | 165±6 | 193±9* |
| Unfasted insulin level, ng/ml | 0.7±0.1 | 1.3±0.2* |
| Free fatty acids, meq/l | 0.80±0.04 | 0.82±0.04 |
| Leptin, ng/ml | 8.2±0.7 | 10.5±0.7 |
| Adiponectin, ng/ml | 1.8±0.1 | 1.3±0.1* |
Values are means ± SE; n = 8–12 mice in each group. SD, standard diet; HFD, high-fat diet.
P < 0.05 vs. SD.
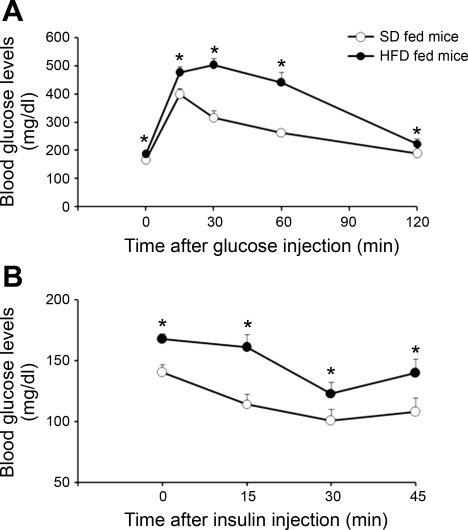
After 9–10 days of diet, HFD-fed mice had an impaired tolerance to glucose as determined by an intraperitoneal glucose tolerance test (Fig. 1A). Moreover, HFD-fed mice demonstrated an impaired insulin tolerance test (Fig. 1B).
Fig. 1.
Systemic insulin sensitivity in standard diet (SD; ○) and high-fat diet (HFD; •) fed mice. A: intraperitoneal glucose tolerance test obtained in 7 SD- and 8 HFD-fed mice. B: intraperitoneal insulin tolerance test obtained in 12 SD- and 9 HFD-fed mice. Values are means ± SE. *P < 0.05 vs. SD.
Five days after TAC, blood glucose level was higher in HFD- compared with SD-fed mice (134 ± 8 vs. 99 ± 5 mg/dl; P < 0.05). This higher blood glucose level was accompanied by a trend toward an increase in plasma insulin in HFD-fed mice compared with SD-fed mice (0.9 ± 0.2 vs. 0.6 ± 0.2 ng/ml; P = 0.10).
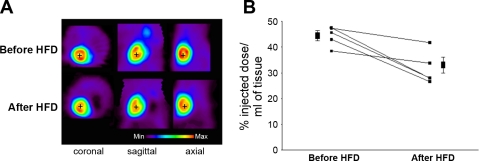
To assess myocardial glucose uptake, a myocardial micro-PET was conducted before and after 9 days of HFD in nonbanded mice. Representative myocardial micro-PET images acquired 45 min after 18F-FDG injection before and after 9 days of HFD are shown in Fig. 2A. Before HFD, 45 min after 18F-FDG injection, myocardial 18F-FDG uptake was 44 ± 2% of the injected 18F-FDG dose per milliliter of tissue. After 9 days of HFD, myocardial 18F-FDG uptake decreased to 32 ± 3% of the injected 18F-FDG dose per milliliter of tissue (P < 0.02 vs. before the HFD, Fig. 2B), despite the increased plasma insulin levels.
Fig. 2.
Myocardial 18F-fluorodeoxyglucose (18F-FDG) uptake on micro-positron emission tomography (micro-PET) before and after HFD. A: representative myocardial 18F-FDG uptake 45 min after 18F-FDG injection in a mouse before (Before HFD) and after 9 days of HFD (After HFD). B: myocardial 18F-FDG uptake 45 min after 18F-FDG injection in 5 mice before and after 9 days of HFD. Myocardial 18F-FDG uptake is expressed in percentage of injected dose/ml tissue. Myocardial 18F-FDG uptake decreased after 9 days of HFD feeding.
18F-FDG micro-PET performed on fasted mice after insulin injection showed that insulin-stimulated myocardial 18F-FDG uptake decreased after 9 days of HFD compared with baseline (25 ± 4 vs. 33 ± 3% of the injected 18F-FDG dose per milliliter tissue; P < 0.04).
HFD increases LV remodeling and decreases LV function after TAC.
No differences were found in LV size and function over a follow-up period of 6 wk in nonoperated mice fed a SD or a HFD (data not shown).
In separate experiments, mice were fed a SD or HFD for 9 days. TAC was performed, and these mice (12 surviving SD-fed mice and 7 surviving HFD-fed mice) were followed for 28 days using echocardiograms. After 9 days on a SD or HFD, mice weighed 27 ± 0.2 and 27 ± 0.4 g, respectively (P = nonsignificant). When these mice were studied after 37 days on the diet and 28 days after TAC, the groups weighed 28 ± 0.4 and 28 ± 0.3 g (Table 2). Heart rates were similar between the two groups of mice before and 7, 21, and 28 days after TAC (Table 2).
Table 2.
Body weights and echocardiographic analysis
| Before TAC |
After TAC |
|||
|---|---|---|---|---|
| Day 7 | Day 21 | Day 28 | ||
| Body weight, g | ||||
| SD | 26.5±0.2 | 24.9±0.5* | 26.5±0.3 | 27.5±0.4 |
| HFD | 26.7±0.4 | 23.7±0.7* | 27.1±0.3 | 28±0.3 |
| HR, beats/min | ||||
| SD | 643±14 | 582±21 | 593±12 | 636±11 |
| HFD | 638±24 | 638±29 | 608±15 | 608±35 |
| LVIDED, mm | ||||
| SD | 3.1±0.1 | 3.4±0.1 | 3.5±0.1 | 3.4±0.1 |
| HFD | 3.3±0.1 | 3.6±0.1* | 3.6±0.1* | 3.9±0.1*† |
| LVIDES, mm | ||||
| SD | 1.4±0.0 | 1.8±0.1 | 1.8±0.1 | 1.9±0.1* |
| HFD | 1.5±0.1 | 2.3±0.1*† | 2.4±0.1*† | 2.7±0.2*† |
| FS, % | ||||
| SD | 54±1 | 47±2 | 47±2 | 45±2* |
| HFD | 56±1 | 36±2*† | 35±2*† | 31±3*† |
| PWT, mm | ||||
| SD | 0.8±0.02 | 0.9±0.03 | 1.1±0.03* | 1.0±0.04* |
| HFD | 0.8±0.00 | 1.0±0.07* | 1.1±0.06* | 1.2±0.06* |
| LV mass, mg | ||||
| SD | 72±2 | 102±5* | 135±7* | 121±7* |
| HFD | 82±4 | 109±10* | 149±16* | 177±15*† |
Values are means ± SE; n = 12 for SD-fed mice and n = 7 for HFD-fed mice. TAC, transverse aortic constriction; HR, heart rate; LVIDED, left ventricular end-diastolic internal diameter; LVIDES, left ventricular end-systolic internal diameter; FS, fractional shortening; PWT, left ventricular posterior wall thickness; LV mass, left ventricular mass.
P < 0.05 vs. before TAC.
P < 0.05 vs. SD at the same time point.
Before TAC, echocardiographic parameters of LV size and function did not differ between groups. In SD-fed mice, TAC induced concentric LV hypertrophy without LV dilation and a decrease in LV systolic function, as assessed by FS (Table 2). In HFD-fed mice, TAC induced concentric and eccentric hypertrophy with LV dilation and posterior wall thickening (Table 2). The TAC-induced increase in echocardiographically measured LV mass was greater in HFD-fed mice than in SD-fed mice (P < 0.02).
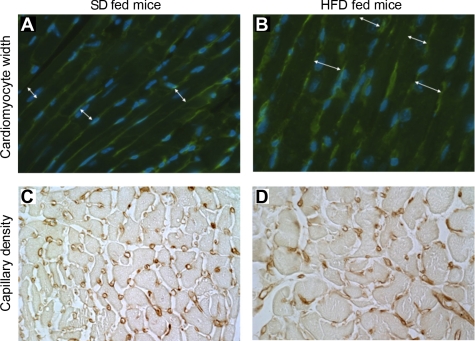
Twenty-eight days after TAC, LV mass per body weight was greater in HFD- than in SD-fed mice (5.0 ± 0.3 vs. 4.3 ± 0.2 mg/g, P < 0.05). Cellular hypertrophy, as measured by cardiomyocyte width, was greater in mice fed a HFD than mice fed a SD (17 ± 1 vs. 15 ± 1 μm, P < 0.05, Fig. 3, A and B).
Fig. 3.
A and B: cardiomyocyte width (white arrows) measured in a SD- (A) and a HFD-fed (B) mouse, 28 days after transverse aortic constriction (TAC). C and D: capillary density measured in a SD- (C) and a HFD-fed (D) mouse 28 days after TAC. Cardiomyocyte width was greater, and capillary density was decreased, 28 days after TAC in mice fed a HFD. LF, low fat.
HFD feeding augmented the decrease in LV function observed after TAC. The decrease in FS induced by TAC was greater in the mice fed a HFD than in mice fed a SD (P < 0.001). In a follow-up study, the echocardiogram performed 3 days after TAC revealed that FS was already less in HFD- than in SD-fed mice (FS: 41 ± 12 vs. 60 ± 4%; P < 0.03).
After 9 days of diet, hemodynamic parameters were similar in mice fed a SD or HFD (data not shown). The hemodynamic measurements obtained 28 days after TAC confirmed that the LV systolic function, as evaluated by dP/dtmax, ratio of dP/dtmax to instantaneous pressure, and preload adjusted maximum power, was decreased in HFD-fed mice compared with SD-fed mice (Table 3). Diastolic function, as evaluated by dP/dtmin and time constant of isovolumic relaxation, was impaired to a greater extent in HFD-fed than in SD-fed mice.
Table 3.
Hemodynamic parameters 28 days after TAC in SD- and HFD-fed mice
| SD | HFD | |
|---|---|---|
| HR, beats/min | 636±15 | 585±15 |
| LVESP, mmHg | 174±4 | 126±7* |
| LVEDP, mmHg | 5±1 | 5±1 |
| SAPL, mmHg | 103±6 | 83±7* |
| TSPG, mmHg | 70±4 | 43±9* |
| dP/dtmax, mmHg/s | 13,993±1,094 | 8,246±565* |
| dP/dtmin, mmHg/s | −13,636±787 | −8,575±695* |
| dP/dtmax/IP, s−1 | 174±10 | 141±6* |
| PAMP, nW/μl2 × 104 | 360±65 | 180±27* |
| Ea, mmHg/μl | 9.4±1.6 | 10.2±1.8 |
| τ, ms | 5.1±0.3 | 6.6±0.3* |
Values are means ± SE; n = 10 for SD mice and n = 10 for HFD mice 28 days after TAC. LVESP, LV end-systolic pressure; LVEDP, LV end-diastolic pressure; SAPL, systolic arterial pressure measured in the left carotid artery; TSPG, transstenotic pressure gradient; dP/dtmax, maximum rate of developed LV pressure; dP/dtmin, minimum rate of developed LV pressure; dP/dtmax/IP, ratio of maximum rate of developed LV pressure to the instantaneous pressure at this time point; PAMP, preload adjusted maximum power; Ea, arterial elastance; τ, time constant of isovolumic relaxation.
P < 0.05 vs. SD.
HFD did not affect fibrosis, apoptosis, or myocardial lipid deposits after TAC.
Staining for collagen using Sirius Red revealed a small qualitative increase in cardiac collagen in the interstitial and perivascular space 28 days after TAC. No difference was observed between HFD- and SD-fed mice (5 mice in each group).
The ratio of the number of TUNEL-positive cardiac nuclei per cardiomyocytes, reflecting cardiac cell death, was not different in three SD- and seven HFD-fed mice 3 days after TAC (0.3 ± 0.07 and 0.5 ± 0.3% of cardiomyocytes, respectively).
No lipid deposits were detected by Oil red O stain in the myocardium of five HFD-fed mice 28 days after TAC.
HFD decreases survival after TAC.
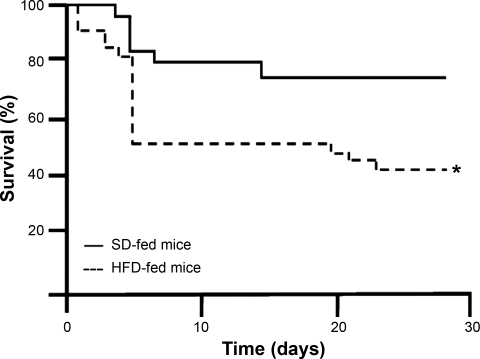
Survival after TAC was decreased in HFD- compared with SD-fed mice (43 vs. 75%, P < 0.02, Fig. 4).
Fig. 4.
Survival after TAC in mice fed a SD or HFD. Survival after TAC was decreased in HFD-fed mice compared with SD-fed mice. *P < 0.02.
HFD modifies myocardial metabolism after TAC.
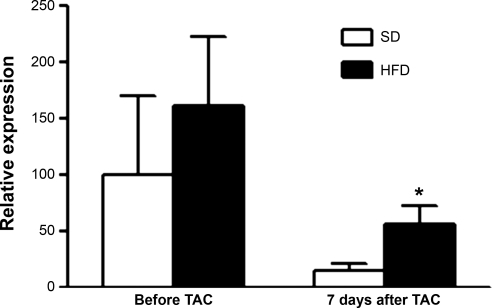
No effect of HFD or TAC was found on cardiac levels of mRNA encoding PPAR-α, CPT-1β, CD36, or levels of mRNA encoding medium-chain acyl CoA dehydrogenase. However, an increase in levels of mRNA encoding MCD in the myocardium of HFD-fed mice was noted 7 days after TAC compared with the myocardium of SD-fed mice (Fig. 5).
Fig. 5.
Expression of malonyl CoA decarboxylase before and 7 days after TAC in mice fed a SD or a HFD. *P < 0.05 vs. SD 7 days after TAC.
HFD impairs myocardial perfusion response to acetylcholine.
Mice fed a SD and HFD for 9 days had similar heart rates and systolic blood pressure before acetylcholine (Table 4). Infusion of acetylcholine to SD- and HFD-fed mice lowered the heart rate and systolic blood pressure similarly in both groups.
Table 4.
Myocardial perfusion estimated by myocardial contrast echocardiography at baseline and during acetylcholine infusion in mice fed either a SD or a HFD for 9 days
| Baseline | Acetylcholine | Ratio | |
|---|---|---|---|
| HR, beats/min | |||
| SD | 550±17 | 529±19* | |
| HFD | 558±21 | 542±21* | |
| SAPL | |||
| SD | 118±6 | 113±6* | |
| HFD | 119±6 | 113±6* | |
| A, dB | |||
| SD | 8±1 | 10±1 | 1.3±0.2 |
| HFD | 8±1 | 8±1 | 1.0±0.1† |
| β, s−1 | |||
| SD | 0.8±0.1 | 1.2±0.1* | 1.7±0.4 |
| HFD | 0.9±0.0 | 1.0±0.1† | 1.1±0.1† |
| Aβ, dB/s | |||
| SD | 6±1 | 12±2* | 2.0±0.4 |
| HFD | 8±1* | 8±1† | 1.0±0.0† |
Values are means ± SE; n = 4 for SD mice and n = 4 for HFD mice. SAPS, ??; A, plateau of the myocardial contrast signal intensity, β, initial slope of the replenishment curve; A β, product of A and β.
P < 0.05 vs. baseline.
P < 0.05 vs. SD after acetylcholine infusion.
At baseline, there was no difference in MCE-derived myocardial blood velocity (assessed by the slope of myocardial microbubble replenishment β), myocardial blood volume (assessed by the plateau of myocardial microbubble A), or MBF (estimated by the product of A and β) between SD- and HFD-fed mice (Table 4). Acetylcholine induced an increase in MBF (Aβ) in SD-fed mice (2.0 ± 0.4-fold, P < 0.05), but no response was noted in HFD-fed mice.
HFD impairs coronary vasodilator response to dobutamine after TAC.
Seven days after TAC, SD- and HFD-fed mice had similar heart rates and systolic blood pressure (Table 5). Infusion of dobutamine increased the heart rate without change in systolic blood pressure in both groups (Table 5).
Table 5.
Myocardial perfusion estimated by myocardial contrast echocardiography at baseline and during dobutamine infusion in mice 7 days after TAC
| Baseline | Dobutamine | Ratio | |
|---|---|---|---|
| HR, beats/min | |||
| SD | 430±16 | 580±17* | |
| HFD | 467±30 | 556±29* | |
| SAPL | |||
| SD | 122±6 | 113±7 | |
| HFD | 110±8 | 113±12 | |
| A, dB | |||
| SD | 8±1 | 9±1 | 1.1±0.1 |
| HFD | 9±1 | 10±1 | 1.1±0.1 |
| β, s−1 | |||
| SD | 0.7±0.1 | 1.4±0.1* | 2.1±0.3 |
| HFD | 0.8±0.1 | 1.2±0.1* | 1.4±0.1† |
| Aβ, dB/s | |||
| SD | 6±1 | 13±1* | 2.4±0.2 |
| HFD | 8±1 | 12±1* | 1.6±0.1† |
Values are means ± SE; n = 5 for SD mice and n = 6 for HFD mice.
P < 0.05 vs. baseline.
P < 0.05 vs. SD after dobutamine infusion.
Compared with baseline, infusion of dobutamine increased LV contractility (measuring by FS) in SD-fed (33 ± 6 vs. 46 ± 6%, P < 0.02) but not in HFD-fed mice (25 ± 4 vs. 32 ± 5%). MBF (as assessed by Aβ) increased in the two groups of mice compared with baseline (Table 5). The dobutamine-induced increase in MBF was less in HFD- than in SD-fed mice (1.6 ± 01- vs. 2.4 ± 0.2-fold, P < 0.05). Interestingly, the coronary vasodilator response to dobutamine was correlated with both the FS after dobutamine and the change of FS after dobutamine (r = 0.63 and r = 0.61, both P < 0.05).
HFD decreases myocardial capillary density 28 days after TAC.
Seven days after TAC, no difference was noted in the capillary density and in the ratio of capillaries/myocardial fibers, between the HFD- and the SD-fed mice (2.7 ± 0.1 vs. 2.7 ± 0.1 × 103 capillaries/mm2; 1.4 ± 0.1 capillaries/myocardial fibers vs. 1.4 ± 0.1 capillaries/myocardial fibers). No difference was seen in levels of myocardial mRNA encoding VEGF between groups 7 days after TAC. Twenty-eight days after TAC, capillary density was less in HFD-fed than in SD-fed mice (2.1 ± 0.3 vs. 2.8 ± 0.2 × 103 capillaries/mm2; P < 0.05; Fig. 3, C and D). The ratio of capillaries/myocardial fiber was similar in both groups (1.4 ± 0.2 capillaries/myocardial fiber in the HFD-fed mice and 1.6 ± 0.2 capillaries/myocardial fiber in the SD-fed mice).
DISCUSSION
A short duration of HFD feeding in C57BL6 wild-type mice induces systemic and myocardial insulin resistance. Although this altered metabolic state does not affect baseline LV structure and function, it is associated with increased LV remodeling and dysfunction after chronic pressure overload. Impaired coronary vasodilatory capacity may participate in the increased LV remodeling and dysfunction in insulin-resistant mice.
The high-fat-fed wild-type mouse is a recognized model of environmentally induced Type 2 diabetes and obesity (27). Early responses to HFD include systemic insulin resistance with minimal weight gain (22, 27). An increase in glucose and insulin levels was noted in mice fed a HFD for 9 days, in both the fasted and fed state. These metabolic disorders were associated with impaired glucose and insulin tolerance, demonstrating that 9 days of HFD feeding induces systemic insulin resistance. As systemic and myocardial insulin resistance may not necessarily coexist (14), we used micro-PET to assess myocardial glucose uptake. Myocardial glucose uptake was decreased after 9 days of HFD, both in the basal state and after insulin injection, suggesting myocardial insulin resistance. We did not normalize myocardial 18F-FDG uptake to plasma glucose. Although we would expect HF-fed mice to have higher blood glucose level than SD-fed mice, and this normalization to emphasize the decreased 18F-FDG uptake was noted after HFD, the absence of plasma glucose measurements during the micro-PET experiments is a limitation of the study.
We did not find any effect of HFD on LV structure and function, as determined by hemodynamic measurements obtained after 9 days of diet and echocardiography performed serially up to 6 wk. Similarly, Park et al. (22) reported that mice fed 3 wk of a diet containing 55% of fat had similar shortening fraction to mice fed a SD. Other investigators reported no differences in LV mass or cardiomyocyte function between mice fed 9 wk of SD or of a diet containing 45% fat (10). These findings suggest that short durations of HFD and the associated insulin resistance do not adversely affect baseline LV structure and function in a detectable way.
In the presence of chronic pressure overload induced by TAC, however, mice fed a HFD developed more pronounced LV remodeling than did mice fed a SD. LV cavity size was greater in mice fed a HFD than in mice fed a SD. Although chronic pressure overload increased LV mass in mice fed a SD or a HFD, the increase was greater in the latter. More strikingly, HFD was associated with a decrease in LV systolic and diastolic function after TAC, as documented by echocardiography and hemodynamic measurements. Furthermore, indexes normalized for load, such as the ratio of dP/dtmax to instantaneous pressure and preload-adjusted maximal power were also decreased in HFD- compared with SD-fed mice. The impaired systolic function in the HFD-fed mice was accompanied by a lower LV end-systolic pressure and lower transtenotic gradients, which most likely reflect a failure of the heart to maintain an elevated systolic pressure in the face of an aortic constriction.
The decreased LV function and increased LV remodeling seen in the HFD-fed mice after TAC was associated with a greater mortality than was observed in SD-fed mice after TAC. Although the cause of the mortality is unknown, the cardiac dysfunction assessed 3 days after TAC was already greater in HFD-fed mice than in SD-fed mice, suggesting that some of the mice in heart failure may have died before they could be analyzed using echocardiography.
Our observations appear to conflict with the findings of Okere et al. (21), who reported that hypertensive rats fed a HFD developed less LV remodeling and dysfunction than SD-fed rats (21), and the results of Chess et al. (8) who reported that, in mice, HFD compared with SD feeding led to similar hypertrophy and LV dysfunction after TAC. However, the composition of the HFD used by these investigators (fat derived from cocoa butter) differed from that used in the present study (fat derived from lard). In these studies, cocoa-derived HFD feeding did not affect plasma glucose and insulin levels (21). Furthermore, because of its differing fatty acid profile, lard may favor the development of vascular disease more than cocoa butter.
A variety of mechanisms may contribute to the adverse remodeling seen in HFD-fed mice subjected to TAC. In the present study, mice fed a short duration of HFD displayed coronary endothelial dysfunction, as demonstrated by a blunted response to acetylcholine. Because endothelial dysfunction has been proposed as a mechanism underlying impaired coronary response to dobutamine (3), we hypothesized that myocardial ischemia may play a role in the decreased LV function and increased remodeling observed after TAC in HFD-fed mice. To explore this hypothesis, dobutamine MCE was performed 7 days after TAC, a time point when the difference in cardiac phenotype between HFD- and SD-fed mice is already noted. MBF did not increase as much in response to dobutamine in mice fed a HFD than in mice fed a SD. Interestingly, the coronary vasodilator response to dobutamine was correlated to systolic function after dobutamine and to the contractile reserve (dobutamine-induced increase in FS). These findings suggest that mechanisms implicated in the impaired LV function after TAC in mice fed a HFD may include a decreased coronary vasodilator response to hemodynamic stress and subsequent ischemia.
Other mechanisms that may be involved in the adverse LV remodeling and dysfunction seen in HFD-fed mice after TAC include shifts in the direction of cardiac substrate selection. Seven days after TAC, an increase in the expression of MCD was found in the myocardium of mice fed a HFD compared with the myocardium of mice fed a SD. MCD catalyzes the decarboxylation of malonyl-CoA; an increase in MCD activity is accompanied by elevated rates of fatty acid oxidation (5, 29). It is recognized that high rates of fatty acid oxidation reduce the external power of the heart for a given myocardial oxygen consumption (6, 18). In pressure overload, a situation in which oxygen demand is already increased, this cardiac inefficiency may lead to a compromise in contractile function. The measurement of the MCD gene expression is, however, insufficient to conclude that free fatty acid oxidation is modified. Indeed, gene expression may be dissociated from protein levels and enzymatic activity. Further experiments, such as direct free fatty acid oxidation measurements in isolated hearts, would be needed to study this hypothesis.
Finally, decreased adiponectin could, by itself, participate in a greater hypertrophic response to TAC (24) and precipitate cardiac dysfunction (16).
Recently, it has been reported that the endoplasmic reticulum responds to metabolic stressors such as insulin resistance through a well-coordinated molecular response that involves multiple gene pathways and the onset of apoptosis (25). In the present study, no difference in apoptosis was seen 3 days after TAC, a time point when maximal apoptosis has been reported (11). Direct lipotoxicity, produced by fatty acid uptake/utilization mismatch, has been associated initially with cardiac hypertrophy, followed by the development of LV dysfunction, increased fibrosis, apoptosis, and premature death (9). Although we cannot eliminate the effect of lipotoxicity at a mitochondrial level, we did not see any intramyocardial lipid accumulation.
This study has important clinical implications. Heart failure is a major public health concern in the US, impacting more than 5.2 millions American adults (2). Insulin resistance is an independent predictor of heart failure, and its prevalence has greatly increased over the last decades, making the contribution of this condition to cardiac dysfunction especially relevant. Insulin resistance is frequently associated with chronic LV pressure overload, such as that found in systemic hypertension. If the findings in mice can be extrapolated to humans, the present study demonstrates that HFD and its associated insulin resistance significantly increase the impact of chronic LV pressure overload, by accelerating adverse LV remodeling and the progression of chronic pressure overload toward heart failure.
GRANTS
The present study was supported by a grant of the Harvard Clinical Nutrition Research Center (M. Scherrer-Crosbie), a grant of the Fédération Française de Cardiologie (H. B. Thibault), National Institutes of Health (NIH) Grant DK 58127 (M. Kaneki), Shriners Hospitals Philanthropy (M. Kaneki), and NIH Grant R01 HL70896 (K. D. Bloch).
Acknowledgments
The authors are most grateful to Dr. Arthur E. Weyman for insightful comments and careful reading of the manuscript, Dr. Ahmed Tawakol for insights in the field of positron emission tomography, and for Sarah Fostello for technical assistance with echocardiography.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.[Anon]. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med 327: 685–691, 1992. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics (Online). http://www.americanheart.org/downloadable/heart/1166712318459HS_StatsInsideText.pdf [2006].
- 3.Barbato E, Bartunek J, Wyffels E, Wijns W, Heyndrickx GR, De Bruyne B. Effects of intravenous dobutamine on coronary vasomotion in humans. J Am Coll Cardiol 42: 1596–1601, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118: 789–800, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR. Malonyl coenzyme A decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57: 1508–1516, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol Heart Circ Physiol 261: H741–H750, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Buys E, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri J, Llano M, Perez-Sanz TM, Ichinose F, Janssens S, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol 293: H620–H627, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail 14: 82–88, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107: 813–822, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, Culver B, Ren J. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol 188: 25–36, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hang T, Jiang S, Wang C, Xie D, Ren H, Zhuge H. Apoptosis and expression of uncoupling protein-2 in pressure overload-induced left ventricular hypertrophy. Acta Cardiol 62: 461–465, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am J Physiol Heart Circ Physiol 286: H1070–H1075, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA 294: 334–341, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH. Effect of noninsulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation 103: 1734–1739, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114: 345–352, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 67: 705–713, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Manning WJ, Wei JY, Katz SE, Litwin SE, Douglas PS. In vivo assessment of LV mass in mice using high-frequency cardiac ultrasound: necropsy validation. Am J Physiol Heart Circ Physiol 266: H1672–H1675, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Mjos OD Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest 50: 1386–1389, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Diabetes, and Digestive and Kidney Diseases (NIDDK). National Diabetes Information Clearinghouse (NDIC) (Online). http://diabetes.niddk.nih.gov/dm/pubs/statistics/#3 [2006].
- 20.Neilan T, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, Furutani E, Perez-Sanz TM, Graveline A, Janssens SP, Picard MH, Scherrer-Crosbie M, Bloch KD. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 116: 506–514, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 48: 1116–1123, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion using myocardial contrast echocardiography: validation and application in nitric oxide synthase 3-deficient mice. Circulation 116: 1250–1257, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10: 1384–1389, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsiotra PC, Tsigos C. Stress, the endoplasmic reticulum, and insulin resistance. Ann N Y Acad Sci 1083: 63–76, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 97: 48–54, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy. J Am Coll Cardiol 51: 93–102, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, Goodwin GW, Ying J, Guthrie P, Wilson CR, Laws FA, Taegtmeyer H. Regulation of cardiac and skeletal muscle malonyl-CoA decarboxylase by fatty acids. Am J Physiol Endocrinol Metab 280: E471–E479, 2001. [DOI] [PubMed] [Google Scholar]