Abstract
The need to regenerate tissue is paramount, especially for the heart that lacks the ability to regenerate after injury. The urinary bladder extracellular matrix (ECM), when used to repair a right ventricular defect, successfully regenerated some mechanical function. The objective of the current study was to determine whether the regenerative effect of ECM could be improved by seeding the patch with human mesenchymal stem cells (hMSCs) enhanced to differentiate down a cardiac linage. hMSCs were used to form three-dimensional spheroids. The expression of cardiac proteins was determined in cells exposed to the spheroid formation and compared with nonmanipulated hMSCs. To determine whether functional calcium channels were present, the cells were patch clamped. To evaluate the ability of these cells to regenerate mechanical function, the spheroids were seeded on ECM and then implanted into the canine heart to repair a full-thickness right ventricular defect. As a result, many of the cells spreading from the spheroids expressed cardiac-specific proteins, including sarcomeric α-actinin, cardiotin, and atrial natriuretic peptide, as well as the cell cycle markers cyclin D1 and proliferating cell nuclear antigen. A calcium current similar in amplitude to that of ventricular myocytes was present in 16% of the cells. The cardiogenic cell-seeded scaffolds increased the regional mechanical function in the canine heart compared with the unmanipulated hMSC-seeded scaffolds. In addition, the cells prelabeled with fluorescent markers demonstrated myocyte-specific actinin staining with sarcomere spacing similar to that of normal myocytes. In conclusion, the spheroid-derived cells express cardiac-specific proteins and demonstrate a calcium current similar to adult ventricular myocytes. When these cells are implanted into the canine heart, some of these cells appear striated and mechanical function is improved compared with the unmanipulated hMSCs. Further investigation will be required to determine whether the increased mechanical function is due to a differentiation of the cardiogenic cells to myocytes or to other effects.
Keywords: human mesenchymal stem cells, myocyte structure, cardiac contractile function, myogenesis, cardiac regeneration, myocardium, heart failure
stem cells possess great potential to treat many different forms of disease; however, regenerating mechanical function following damage to the adult mammalian myocardium has remained an elusive goal. Skeletal myoblasts, mesenchymal stem cells, resident cardiac stem cells, and embryonic stem cells have all been employed with some degree of success (18). We have previously reported an experimental model to investigate the mechanical regeneration in the canine heart by creating a right ventricular (RV) defect, patching it, and then studying both regional mechanical function and histology postsurgery (3, 10). After 8 wk with an extracellular matrix (ECM) patch, the patched region regenerated about 20% of the regional systolic contraction and was accompanied by myocytes within the patch borders. The same recovery was absent when Dacron was used as the patch material. The current studies were developed to test the hypothesis that seeding the patch with human mesenchymal stem cells (hMSCs) that were committed to a cardiac lineage might result in the recovery of mechanical function to a greater degree. It was also aimed at determining whether these cells might differentiate in vivo.
Most investigators agree that embryonic stem cells can differentiate into contractile myocytes. hMSCs also exhibit some cardiogenic potential, but the frequency with which cardiac differentiation occurs without added inducements is small (5, 15). In results, we demonstrate that cardiogenic choice can be enhanced in vitro and that “cardiogenic cells” can be delivered to the canine RV on an ECM patch where their placement is associated with an increased regional mechanical function. Furthermore, when these “cardiogenic cells” are tracked with quantum dots (QDs) (17), some of these cells can be demonstrated to differentiate to mature myocytes with normal sarcomere spacing.
MATERIALS AND METHODS
Formation of three-dimensional aggregates.
hMSCs were purchased from Cambrex Bio Science Walkersville and cultured at 37°C in a humidified atmosphere of 5% CO2 (balance room air) in mesenchymal stem cell growth medium (Cambrex Bio Science Walkersville). The cells were maintained according to Cambrex Bio Science protocols. For in vitro experiments, cells from passages 2 to 8 were used.
Spheroids were formed from hMSCs based on the hanging drop method (7, 9) with modifications. hMSCs grown to 70–80% confluence were washed with Dulbecco's phosphate-buffered saline (PBS; Sigma) and harvested with 0.25% trypsin-EDTA solution (Cambrex Bio Sciences). The hMSCs were collected by centrifugation and resuspended in high-glucose Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO), supplemented with penicillin-streptomycin (Sigma) and 30% fetal bovine serum (Sigma). Hanging drops were prepared using 40-μl droplets, each containing 250,000 hMSCs. The hMSCs were maintained in hanging drops for 3 days, and the spheroids were then plated on Lab-Tek II CC2 glass chamber slides or ECM patches and cultured in identical media for an additional 7–10 days. The media was changed twice a week.
Immunocytochemistry.
The cells plated on Lab-Tek II CC2 glass chamber slides were fixed with 10% buffered formalin (Fisher Diagnostic) at room temperature for 10 min. The slides were washed once with PBS, and the cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. The slides were washed three times, and the cells were quenched in fresh 0.1% sodium borohydride in PBS for 5 min and washed with PBS. The slides were blocked with 5% bovine serum albumin (BSA) in PBS for 30 min. The cells were then incubated overnight at 4°C with the following primary antibodies: anti-sarcomeric α-actinin clone EA-53 (1:200; Sigma), anti-α-atrial natriuretic peptide (1:200; Chemicon International), anti-voltage-gated calcium channel 1.2 (Cav1.2; 1:200; Alomone), anti-cardiotin (1:100; Chemicon International), anti-troponin-T (1:200; Santa Cruz Biotechnology), anti-cyclin D1 (1:100; Santa Cruz Biotechnology), or anti-proliferating cell nuclear antigen (PCNA; 1:250; Amcam). The cells were then washed three times with PBS and incubated with FITC or tetramethylrhodamine isothiocyanate-conjugated F(ab′)2 secondary antibodies (1:200–1:400; Santa Cruz Biotechnology) for 1 h at room temperature in the dark. The antibodies were diluted in PBS with 1% BSA according to the manufacturer's recommendations. The slides were washed three times with PBS, the nuclei were counterstained with 4,6-diamidino-2-phenylindole (Molecular Probes) according to the manufacturer's protocol, and the chamber slides were mounted on coverslips with Vectashield mounting media (Vector).
Immunofluorescence was analyzed by deconvolution microscopy using the Axiovision 4.1 imaging software package coupled to an Axiovert 200M fluorescence microscope (Carl Zeiss). The cross-sectional images were obtained with 250-nm Z-stack steps and processed using the Axiovision 4.1 constrained iterative algorithm. For a determination of the percentage of cells positive for actinin or cyclin D1, a Leica DM LB2 upright microscope was used.
Whole cell patch clamping.
Whole cell patch-clamp experiments were performed to determine whether spheroid-derived cells possess L-type calcium currents similar to those reported for adult ventricular myocytes. The spheroids were plated on polylysine-coated coverslips for 3–8 days. The cells spreading from the spheroids were impaled with electrodes for whole cell patch clamping. The unmanipulated hMSCs were used for comparison. An Axopatch 1A amplifier and the whole cell patch-clamp technique were used to measure cell membrane current. The patch pipette resistances were 1–3 MΩ before sealing. The pipette solution contained (in mmol/l) 50 KCl, 80 potassium aspartate, 1 MgCl2, 3 Mg-ATP, 10 EGTA, and 10 HEPES (pH adjusted to 7.2 with KOH). The extracellular solution contained (in mmol/l) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH adjusted to 7.4 with NaOH). Our protocol was to hold the cell at −80 mV, depolarize to −35 mV to inactivate sodium current (if present), and then depolarize for 300 ms in 10-mV increments to a maximum test voltage of +65 mV. After whole cell current-voltage data were obtained, 1 mmol/l CdCl2 was perfused with the external Tyrode solution. After 5 min of perfusion, the current-voltage data were again obtained and CdCl2 was washed out with normal Tyrode solution. Control current-voltage relationships were again determined after wash out. A current-clamp step of −50 pA was used to determine the input capacitance of the cell, and the membrane current was normalized to its capacitance.
Myocardial scaffolds.
The spheroids formed from hMSCs (passage 4) were seeded on ECM from the porcine urinary bladder (ACell, Jessup, MD) for 3–10 days before implantation. On average, eight aggregates were cultured on an ECM scaffold that was ∼17 × 25 mm in size. The media was replaced every 3 days. For comparison, two million nonmanipulated hMSCs (passages 4 to 8) were also cultured on ECM (17 × 25 mm in size) for 3–10 days before implanting.
QD nanoparticles were used to track the fate of the implanted cells. We previously demonstrated the efficacy of this approach as the hMSC-tracking technique in vivo (17). Four ECM patches were seeded with QD-labeled spheroids. In these experiments, hMSCs were labeled with QD nanoparticles before the formation of spheroids using a method described previously (17). Briefly, hMSCs were rinsed in PBS and incubated in QD-containing mesenchymal stem cell growth medium (Cambrex Bio Science Walkersville) for up to 48 h at 37°C in humidified 5% CO2 (balance room air). At the end of the loading period, QD hMSCs were rinsed twice in PBS and prepared for spheroid formation as described in Formation of three-dimensional aggregates.
Animal surgery.
Humane care was provided in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The experimental protocol was approved by the Institutional Animal Care and Use Committee at Stony Brook University.
Adult mongrel dogs weighing 20–30 kg were medicated with glycopyrrolate (0.01 mg/kg), acepromazine (0.05 mg/kg), and thiopental sodium (15 mg/kg); intubated; and placed under general anesthesia using inhalational isoflurane. To monitor systemic blood pressure, a fluid-filled pressure transducer was inserted into the femoral artery. A right thoracotomy was performed. An atraumatic clamp was used to isolate a full-thickness section of myocardium (∼15 by 10 mm) from the RV. An ∼1 × 1 cm full-thickness piece of RV tissue was excised from within the clamped region. A scaffold containing either spheroid-derived cells (n = 8) or hMSCs (n = 5) was used to repair the defect with a running 5-0 prolene suture. The clamp was released. If needed, gentle pressure or 5-0 prolene stitches were used to acquire adequate hemostasis. The chest was closed in layers, and the systemic pressure catheter was removed.
Determination of regional contractile function.
Eight weeks after implant surgery, the animal was returned to the operating room, medicated, intubated, and placed under general anesthesia (as described in Animal surgery). Systemic pressure was again monitored through an arterial line in the femoral artery. The chest was opened via a bilateral thoracotomy. Adhesions were carefully dissected off the heart to expose the patch and its suture line. A pressure transducer (Millar Instruments, Houston, TX) was placed into the RV through the right atrium.
The regional function was determined by high-density mapping (HDM) as described in detail elsewhere (2, 4, 8). This method allows for the accurate determination of deformation within a well-defined area. Briefly, the silicon carbide particles (∼40 μm in diameter) were applied to the epicardial surface of the heart to create a random light intensity pattern. A complimentary metal-oxide semiconductor camera (Photron, San Diego, CA) was focused on the region of interest (ROI) that contained the patch as defined by the 5-0 prolene sutures. Images were acquired at 125 frames/s over 7 s. Using a subpixel extended-phase correlation algorithm, the displacements of the epicardial surface during the cardiac cycle at multiple locations within the ROI were obtained. Based on these displacements, the change in the regional area within the ROI was determined.
To quantify regional mechanical function within the patch region, the systolic area contraction and regional stroke work (RSW) were determined. The systolic area contraction was defined as the normalized change in the regional area from diastole to systole, normalized to the end-diastolic area. RSW was calculated by integrating the RV pressure with respect to the area change. RSW was normalized with respect to the developed pressure and the end-diastolic regional area, yielding a unitless value. RSW is an index of the amount of work being performed by the region. Since the closed-line integral is path dependent, a positive value of RSW suggests that the region is contracting in synchrony with the normal myocardium and thereby producing work. A negative value suggests that the environment is performing work on the region (i.e., deforming it out of synchrony with the normal myocardium).
All data are expressed as means ± SE. Statistical analyses between groups were performed by one-way analysis of variance with a post hoc Tukey test. Differences were considered significant for P values < 0.05.
Histology and immunocytochemistry for explanted patches.
Tissue samples from the implant region and native myocardium were obtained after all functional data were acquired, placed in 4% paraformaldehyde, later transferred to a 30% sucrose solution, embedded in Jung tissue freezing medium (Leica Microsystems Nussloch, Wetzlar, Germany), and frozen to −20°C. The samples were cut in 10 μm-thick sections. The sections were stained with hematoxylin and eosin to identify the histological structure. To assay for myocytes, the tissue sections were stained for sarcomeric α-actinin. To reduce the potential errors that may result from the misinterpretation of the fluorescent signal, we modified the staining protocol to reduce the effects of autofluorescence. The sections were permeabilized, blocked in normal serum, and incubated in primary antibody directed against sarcomeric α-actinin (Sigma-Aldrich). The sections were then incubated with a biotinylated secondary antibody (Vector) and, finally, incubated with streptavidin-conjugated near infrared-emitting QDs (705-nm emission wavelength, Invitrogen).
Hematoxylin and eosin staining of some of the sections was performed according to standard protocols. The sections were imaged on an Olympus IX51 inverted microscope with an attached digital camera. To reconstruct the entire tissue section, a mosaic was created by capturing ∼25 overlapping images of the section at low power (using a ×4 objective). The photomerge feature of Adobe Photoshop (version 8.0, Adobe Systems) was used to combine the images into a large mosaic image of the entire tissue section.
Fluorescent images were acquired with a Zeiss Axiovert 200M fluorescent microscope in Z-stack mode and were deconvoluted with the Axiovision software package (Carl Zeiss) to obtain near-confocal quality optical sections. Some image stacks were postprocessed using ImageJ (NIH, Bethesda, MD). Custom filters were used to image QD fluorescence (Omega Optical, Brattleboro, VT).
RESULTS
Spheroid-derived cells express cardiac proteins in vitro.
Through the use of hanging droplets, clearly defined cell aggregates were detected within 24 h. Following 3 days in hanging droplets, over 90% of the spheroids adhered to the glass slides within 24 h.
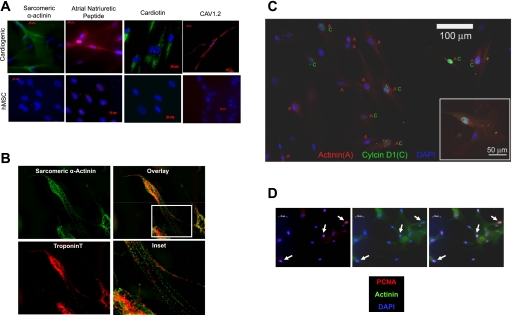
The cells spreading from the spheroids were examined 4–7 days after plating for evidence of cardiac-specific markers. We applied immunocytochemistry to probe for the expression of four proteins associated with cardiac-specific differentiation (Fig. 1) : 1) sarcomeric α-actinin, 2) atrial natriuretic peptide, 3) cardiotin, and 4) the α-subunit of the Cav1.2. In each case, the nonmanipulated hMSCs were employed as a control and the application of secondary antibody in the absence of a primary antibody was used to control for nonspecific staining. Each of the four proteins was expressed in a fraction of spreading cells (the cells that were moving away from the spheroids) and was largely absent in the nonmanipulated hMSCs (Fig. 1A). In a fraction of the spreading cells that were stained for sarcomeric α-actinin and cardiac troponin-T, a myofibrillar organization could be detected (Fig. 1B).
Fig. 1.
Spheroid formation favors cardiac commitment of human mesenchymal stem cells (hMSCs). A: cardiac proteins are present in cardiogenic cells (top) but are not expressed in detectable amounts in hMSCs (bottom). Cav1.2, voltage-gated calcium channel 1.2. B: sarcomeric α-actinin and troponin-T colocalize and appear to form primitive myofibrils. C: colocalization of sarcomeric α-actinin (A) and cyclin D1 (C) indicates that some of the cardiogenic cells also express cyclin D1. D: nuclear proliferating cell nuclear antigen (PCNA) staining of sarcomeric α-actinin positive cells (arrows) confirmed the mitotic activity of some of these cells. DAPI, 4,6-diamidino-2-phenylindole. Bar represents 50 μm.
The formation of embryoid bodies with the embryonic stem cells usually results in a fraction of cells choosing a cardiac lineage. We attempted to determine the fraction of spreading cells that committed to a cardiac lineage by staining for sarcomeric α-actinin. A total of 1,277 cells spreading from spheroids was viewed for the actinin expression. Some 57% of the spreading cells were sarcomeric actinin positive (a total of 7 spheroids were imaged). The staining for cyclin D1, a protein that is upregulated and translocated to the nucleus in mitotically competent cells, showed that 62% of those cells that stained positive for sarcomeric α-actinin also stained positive for cyclin D1 in the nucleus (Fig. 1C). In another series of experiments aimed at confirming the mitotic competence of the cardiogenic cells, PCNA was also shown to colocalize with sarcomeric actinin (Fig. 1D).
Spheroid-derived cells have a functional L-type calcium current in vitro.
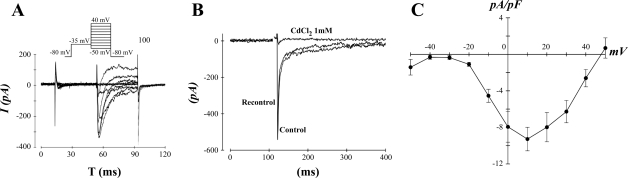
Given the positive staining for Cav1.2, we determined whether the spheroid-derived cells express functional L-type calcium channels. The time course and amplitude of the currents were consistent with those of L-type calcium channels being their source (Fig. 2A). To further confirm their identity, we used cadmium (1 mM), which blocks L-type calcium channels. Cadmium blocked the current, and its effect was reversible upon washout (Fig. 2B). Five cells spreading from spheroids (of 31 cells patched) demonstrated a substantial L-type calcium current. We constructed the current-voltage relationship of the L-type calcium current from the cardiogenic cells (Fig. 2C); voltage dependence and amplitude were consistent with those observed in adult human ventricular myocytes (12). In the same solutions, we never observed a measurable L-type calcium current in 35 undifferentiated hMSCs.
Fig. 2.
Cardiogenic cells express a robust L-type calcium current. A, top: voltage step protocol used to elicit calcium currents. A, bottom: representative responses to the voltage steps in a cardiogenic cell. I, current; T, time. B: the calcium current exhibited by cardiogenic cells is inhibited by CdCl2. C: the average current-voltage relationship for 5 cardiogenic cells that expressed a measurable calcium current. Five out of 31 cardiogenic cells expressed a measurable calcium current, whereas there was no detectable current in the 35 hMSCs that were studied.
Spheroid-derived cells improve function in vivo compared with nonmanipulated hMSCs.
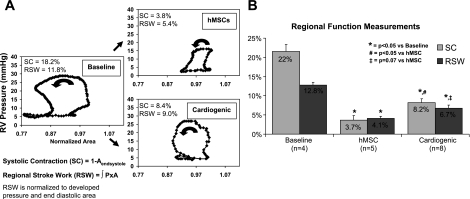
To determine the regenerative potential of the cells in vivo, 4–10 spheroids were seeded onto an acellular scaffold of ECM for 3–8 days. The cell-seeded scaffolds were implanted into the canine heart to repair surgically induced full-thickness ventricular defects using a protocol previously described (10). The systolic area contraction was significantly less in hMSC-seeded patches compared with spheroid-derived cell-seeded patches (P < 0.05; Fig. 3). The positive systolic contraction noted in the implant region confirms that it is contracting in synchrony with the rest of the heart, suggesting the added presence of contractile cells in the implant region. All pressure-area loops were in the counterclockwise direction (Fig. 3), signifying that positive regional work was performed in synchrony with the normal myocardium. Eight weeks postimplantation, the spheroid-derived cell-seeded scaffold region demonstrated an isovolumic systolic phase, followed by a decrease in regional area that continued into diastole. This was followed by an isobaric filling phase. The hMSC-seeded scaffolds also demonstrated positive RSW, as evident by the counterclockwise rotation of the pressure-area loop (Fig. 3A). The RSW in the cardiogenic cell-seeded patches was appreciably greater than that in hMSC-seeded patches (P = 0.07; Fig. 3B).
Fig. 3.
Implantation of cardiogenic cells on an extracellular matrix (ECM) patch in the canine right ventricle (RV) enhances mechanical function. A: representative plots of regional stroke work (RSW) from baseline, scaffold seeded with hMSCs, and scaffold seeded with cardiogenic cells (Cardiogenic). The direction of the work loop is indicated by the arrow. B: regional mechanical function, as assessed by systolic contraction (SC) and RSW, was improved in cardiogenic-seeded scaffolds compared with hMSC-seeded scaffolds. Aendsystole, area at the end of systole; P × A, RV pressure × regional area; n, sample size.
Spheroid-derived cells demonstrate organized sarcomeres in vivo.
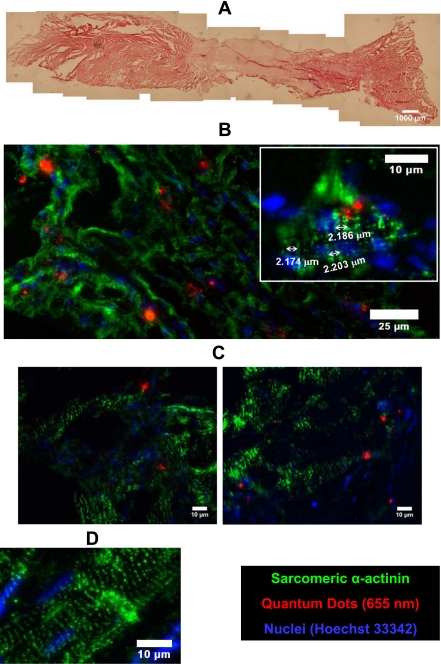
The partial restoration of regional contractile function suggests that the implant region contains contractile cells. Hematoxylin and eosin staining demonstrated myocardial tissue at the edges of the specimen with regions protruding into the implant region. Morphologically, similar tissue was interspersed with stromal fibrous material at the patch border, and the middle of the patch consisted primarily of fibrotic tissue (Fig. 4A). A microscopic examination of the implant zone revealed several islands of myocytes staining positive for sarcomeric α-actinin. In some myocytes, clear striations could be detected with actinin staining that employed a secondary antibody conjugated to a near-infrared QD tag, a fluorophore that greatly reduces problems associated with autoflourescence of the host tissue (Fig. 4).
Fig. 4.
Cardiogenic cells loaded with quantum dots (QDs) and implanted on an ECM patch in the canine RV colocalize with sarcomeric α-actinin and exhibit sarcomere structures. A: low-power hematoxylin and eosin image of the patch implant region. Myocardium exists at the edges of the sample and intermixes with fibrous tissue toward the edge of the patch. B: QD 655-labeled cardiogenic cells were found in sarcomeric α-actinin-positive (QD 705) cells in the interior of the patch region. B, inset: QD 655-labeled cardiogenic cells with striations detected by sarcomeric α-actinin staining. The approximate sarcomere length in a cardiogenic cell is labeled. C: QD 655-labeled cardiogenic cells were also found in the border region and appeared to have an alignment similar to neighboring myocytes. D: an example of positive sarcomeric α-actinin staining (QD 705) in normal myocardium.
To determine whether the spheroid-derived cells that we delivered were the source of some of the myocytes in vivo, we loaded hMSCs with QDs before the hanging drop procedure (17). Unlike the QD tag used to detect actinin, the QDs used for tracking are not conjugated. The intracellular QD label colocalized within cells staining positive for sarcomeric α-actinin in regions well within the patch boundaries (Fig. 4B). Furthermore, some of these cells showed striations spaced ∼2 μm apart (Fig. 4B, inset). At the border of the patch implant, myocytes containing QDs aligned with unlabeled myocytes (Fig. 4C) and showed similar morphology to native myocardium located at a distance from the border zone (Fig. 4D).
DISCUSSION
Using our RV defect model, we were able to demonstrate the enhanced regeneration of the regional mechanical function when the ECM patches were seeded with the spheroid-derived cells. No such improvement was observed with hMSCs that were not subjected to our differentiation protocol. This suggests that a commitment of hMSCs to the cardiogenic cell line is necessary to optimize the regeneration in our model of cardiac damage. Our novel method for partially differentiating hMSCs results in the expression of several cardiac markers. We consider these cells to be “cardiogenic,” since they express cardiac markers but do not contract in vitro and have not fully differentiated into functional cardiac myocytes. Ideally, a full differentiation should not occur until the cells are transplanted into the heart, since different regions of the heart have unique ion currents and the expression of these currents may be affected by the local environment (19). In addition, these cells appear to retain the ability to enter the cell cycle, which may be necessary to repopulate the heart with functional myocytes.
Contractile myocytes must possess large calcium currents. Our cardiogenic cells demonstrated an L-type calcium current with a current that peaks at 0 to +10 mV and a density between 5 and 10 pA/pF, similar to values reported in cardiac myocytes in the human (12, 16). We were unable to detect a sizable L-type calcium current in nonmanipulated hMSCs that were not subjected to the hanging droplet method. Others groups, using solutions designed to optimize L-type calcium currents, have measured currents of ∼10% of the amplitude observed in our present experiments (6, 13).
Another important step in developing a cell for myocardial regeneration is the organization of sarcomeres in the differentiated cells. When cardiogenic cells were seeded onto a biological scaffold and implanted into the heart for 8 wk, some of these cells formed sarcomeres. The region containing these implanted cardiogenic cells showed improved regional mechanical function compared with nonmanipulated hMSCs. The use of HDM allows for the regional myocardial function to be determined with a high-spatial resolution within the implant region. However, to truly confirm the contractile properties of these cells, they should be isolated and their fractional shortening determined.
Since these cardiogenic cells did not exhibit the complete cardiac phenotype in vitro, it remained possible that the “committed” cells retained the capacity to divide. The presence of cyclin D1 and PCNA in cardiogenic cells does not confirm cell proliferation; it suggests that the cells are mitotically competent. Mitotic competence would be a distinct advantage for in vivo regeneration since it is estimated that a cell number on the order of one billion would need to be replaced in patients with heart failure (14).
This study was not designed to test the regenerative capacity of cardiogenic cells in an infarcted environment. Rather, it was designed to test the ability of these cells to enhance the recovery of regional mechanical function and differentiate into myocytes in vivo. However, similar to infarcted tissue, the patch region with seeded cells was devoid of blood flow. Without an established vascular system, the cells seeded on the scaffold may have experienced a period of ischemia. However, given that the scaffold is only 100–150 μm thick, diffusion from the blood in the ventricular chamber may be possible. Thus the scaffold may have given the cells an environment more conducive to regeneration than that of the myocardial infarction sites. An advantage of this model over a myocardial infarction model is the clearly defined region for investigating myocardial regeneration. The region is identifiable by the nondegradable sutures used to implant the patch. This provides a clearly demarked region of interest for our optical technique, HDM (8, 10), to determine the regional mechanical function. In addition, the region for histological analysis is also clearly defined.
An interesting finding in this study was the lack of mechanical improvement in the hMSC-seeded scaffolds over levels that we previous documented in unseeded scaffolds (1, 10). We previously reported a low but significant level of functional regeneration for the same scaffold without cell seeding (systolic contraction = 4.1 ± 0.9%; and RSW = 4.3 ± 1.0%) that is very similar to the functional regeneration reported here for hMSCs (systolic contraction = 3.7 ± 1.2%; and RSW = 4.1 ± 0.5%). The ECM scaffold itself contains components that are chemoattractants to endogeneous stem cells (11). We interpret the mechanical data to suggest that the favorable effects of the seeded hMSCs do not add significantly to those already produced by endogenous stem cells attracted to the ECM scaffold. It also corroborates recent evidence suggesting that nonmanipulated hMSCs infrequently differentiate into functional myocytes (5, 15).
To further enhance the myocardial regeneration in this model, it may be possible to isolate a pure population of cardiogenic cells and then seed them onto the scaffold. Eight spheroids were incubated on each scaffold before implantation, yet our in vitro results suggest that ∼50% of the cells spreading from the spheroids were positive for cardiac markers. Upon implantation, most of the spheroids detached from the scaffold, leaving only the spreading cells. Thus the total number of cardiogenic cells delivered on the scaffold was less than the two million cells originally incubated on the scaffold.
An important consideration for tracking cells delivered to the heart is the inherent autofluorescence found in the sections of myocardial tissue. To overcome this problem, we recently developed a novel approach to uniformly load a population of hMSCs with QDs (17). The loaded hMSCs retain the label for at least 6 wk in vitro and 8 wk in vivo. Furthermore, in that study, we ruled out the likely false positives that might arise by showing that 1) the QDs do not cross gap junctions and 2) should the QD loaded cells die, the QDs are not taken up by cardiac myocytes either in vitro or in vivo. In the present study we found cells within the implant region that were positive for both sarcomeric α-actinin and QDs. Of even greater significance is that some of these QD-positive cells possessed sarcomeric α-actinin organized in a striated pattern with normal sarcomere spacing. This strongly suggests that some of our cardiogenic cells have become mature myocytes in vivo.
In summary, adult mesenchymal stem cells can be partially differentiated in vitro, express calcium currents similar to adult myocytes, and organize sarcomeres. When delivered to the canine heart, they lead to increased regional function and formation of myocytes in vivo. It remains to be determined whether the cardiogenic cells become myocytes in sufficient numbers to account for the increased regional mechanical function observed and whether this same cellular substrate would work equally well in the postinfarction ventricle or in the abnormal signaling environment of heart failure.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-67101, HL-28958, and HL-20558; American Heart Association National Scientist Development grants (to S. V. Doronin and G. R. Gaudette); the Medical Scientist Training Program at Stony Brook University (to A. B. Rosen and A. J. T. Schuldt), postdoctoral training Grant DK-07521 (to D. J. Kelly); and a grant from the Institute for Molecular Cardiology at Stony Brook University.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Azeloglu EU, Kochupura PV, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Acellularized urinary bladder matrix: a functional myocardial patch. Proc Biomed Eng Soc Ann Fall Meet, 2004.
- 2.Azeloglu EU, Yun YH, Saltman AE, Krukenkamp IB, Chiang FP, Chen W, Gaudette GR. High resolution mechanical function in the intact porcine heart: mechanical effects of pacemaker location. J Biomech 39: 717–725, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, Kelly DJ, Ignotz RA, Gaudette GR. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant 15, Suppl 1: S29–S40, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Gaudette GR, Todaro J, Krukenkamp IB, Chiang FP. Computer aided speckle interferometry: a technique for measuring deformation of the surface of the heart. Ann Biomed Eng 29: 775–780, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol 554: 659–672, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller GM In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol 7: 862–869, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Kelly DJ, Azeloglu EU, Kochupura PV, Sharma GS, Gaudette GR. Accuracy and reproducibility of a subpixel extended phase correlation method to determine micron level displacements in the heart. Med Eng Phys 29: 154–162, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 83: 173–180, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation 112: I144–I149, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 11: 199–206, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Li GR, Lau CP, Leung TK, Nattel S. Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm 1: 460–468, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Li GR, Sun H, Deng X, Lau CP. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells 23: 371–382, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol 47: 1777–1785, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 14: 840–850, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Pelzmann B, Schaffer P, Bernhart E, Lang P, Machler H, Rigler B, Koidl B. L-type calcium current in human ventricular myocytes at a physiological temperature from children with tetralogy of Fallot. Cardiovasc Res 38: 424–432, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells 25: 2128–2138, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Schuldt AJ, Rosen MR, Gaudette GR, Cohen IS. Repairing damaged myocardium: evaluating cells used for cardiac regeneration. Current Treat Opt Cardiovasc Med 10: 59–72, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS. Effects of the renin-angiotensin system on the current Ito in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res 86: 1062–1068, 2000. [DOI] [PubMed] [Google Scholar]