Abstract
Angiotensin-converting enzyme 2 (ACE2) preferentially forms angiotensin-(1-7) [ANG-(1-7)] from ANG II. We showed that cardiac ACE2 is elevated following treatment of coronary artery-ligated rats with AT1 receptor blockers (ARBs). Cardiac myocytes and fibroblasts were isolated from neonatal rats to determine the molecular mechanisms for the ACE2 upregulation by ARB treatment. ANG II significantly reduced ACE2 activity and downregulated ACE2 mRNA in cardiac myocytes, effects blocked by the ARB losartan, indicating that ANG II regulates ACE2. ANG II also reduced ACE2 mRNA in cardiac fibroblasts; however, no enzyme activity was detected, reflecting the limited expression of ACE2 in these cells. Endothelin-1 (ET-1) also significantly reduced myocyte ACE2 mRNA. The reduction in ACE2 mRNA by ANG II or ET-1 was blocked by inhibitors of mitogen-activated protein kinase kinase 1, suggesting that ANG II or ET-1 activates extracellular signal-regulated kinase (ERK) 1/ERK2 to reduce ACE2. Although ACE2 mRNA was not affected by ANG-(1-7), both the ANG II- and ET-1-mediated reductions in ACE2 mRNA were blocked by the heptapeptide. The ANG-(1-7) modulatory effect was prevented by the ANG-(1-7) receptor antagonist [d-Ala7]-ANG-(1-7), indicating that the ANG-(1-7) response was mediated by a specific AT(1-7) receptor. Myocyte treatment with atrial natriuretic peptide (ANP) also reversed the ACE2 mRNA downregulation by ANG II or ET-1, whereas treatment with ANP alone was ineffective. These results indicate that multiple hypertrophic and anti-hypertropic peptides regulate ACE2 production in myocytes, suggesting that ACE2 expression in the heart is dependent upon the compliment and concentration of regulatory molecules.
Keywords: angiotensin II, angiotensin-(1-7), cardiac myocytes, angiotensin-converting enzyme 2, atrial natriuretic peptide, endothelin, mitogen-activated protein kinase
angiotensin-converting enzyme 2 (ACE2) is a homolog of ACE, sharing ∼42% nucleotide sequence homology and 61% sequence similarity with the NH2-terminal catalytic domain of ACE (8, 29). ACE2 is a carboxy-monopeptidase with a preference for hydrolysis between a proline and COOH-terminal hydrophobic or basic residues and exhibits a high catalytic efficiency for the hydrolysis of dynorphin A and apelin 13. More important, ACE2 converts ANG II, a vasoconstrictor and mitogenic peptide, to angiotensin-(1-7) [ANG-(1-7)], a peptide with vasodilator and anti-proliferative properties (9, 27, 28).
ACE2 is present in the heart (2, 8, 14), and a reduction in its expression is associated with enhanced cardiac hypertrophy and reduced pumping ability (4). Although ACE2 was initially localized exclusively in cardiac endothelial cells (8), more recent studies demonstrate ACE2 immunoreactivity in both the endothelial and smooth muscle cells of myocardial vessels as well as in cardiac myocytes (2, 8, 14). Mice with a deletion of cardiac ACE2 have severely impaired cardiac function, including mild thinning of the left ventricle and a severe reduction in cardiac contractility (4). The loss of ACE2 was associated with an increase in tissue and plasma ANG II, suggesting that ANG II is a substrate for ACE2 in the heart. The age-dependent cardiomyopathy in ACE2 null mice is due to an increase in ANG II-mediated oxidative stress and infiltration of neutrophils through the ANG II-mediated activation of AT1 receptors (22). Generation of double-mutant ace/ace2 mice completely abolished the cardiac dysfunction of the ACE2 knockout mice, further suggesting that ACE and ACE2 have counterbalancing functions in cardiac tissue. Deletion of ACE2 also accelerated cardiac dysfunction in a model of pressure overload induced by transverse aortic constriction (TAC); following TAC, ACE2 knockout mice developed cardiac hypertrophy and dilation in association with decreased cardiac contractility (32). In contrast, the insertion of the ACE2 gene by lentiviral transfer provided protection from ANG II-induced cardiac hypertrophy and fibrosis, preserved cardiac function after myocardial infarction, and prevented the hypertension-association cardiac pathology in the spontaneously hypertensive rat (6, 7, 16).
We showed that ANG-(1-7) is generated from ANG II in the isolated heart of mRen2 (27) transgenic rats and that inhibition of ACE2 using the specific ACE2 inhibitor MLN-4760 significantly reduced ANG-(1-7) production, suggesting that ACE2 may play a compensatory role in response to cardiac hypertrophy (30). This is in agreement with studies by Zisman et al. (34) showing that ANG-(1-7) is made in the intact human heart and is decreased when ANG II formation is suppressed by an ACE inhibitor, suggesting that a major pathway for the formation of ANG-(1-7) was directly dependent on the availability of ANG II as a substrate. The production of ANG-(1-7) from ANG II in human heart was inhibited by the selective ACE2 inhibitor (C16, MLN-4760), providing additional evidence of a role for ACE2 in the production of ANG-(1-7) in the heart (33). ACE2 was increased in failing human heart ventricles (34) and in human idiopathic dilated cardiomyopathy and ischemic cardiomyopathy,(12) suggesting that ANG-(1-7) may serve a cardioprotective role in heart failure.
Further evidence for a modulatory role of ACE2 in heart function derives from the observation that cardiac ACE2 mRNA and activity were elevated following treatment of coronary artery-ligated rats with the AT1 receptor antagonist losartan or olmesartan (17). The increase in ACE2 correlated with an elevation in both ANG II and ANG-(1-7), suggesting that ANG II and/or ANG-(1-7) could regulate ACE2 following myocardial infarction. In this study, myocytes and fibroblasts were isolated from neonatal rat heart to investigate the regulation of cardiac ACE2 by angiotensin peptides as well as by other vasoactive peptides that are upregulated following myocardial infarction.
METHODS
Materials.
ANG II, ANG-(1-7), endothelin-1 (ET-1), atrial natriuretic peptide (ANP), and [d-Ala7]-ANG-(1-7) were obtained from Bachem California (Torrance, CA). DMEM/F-12, FBS, penicillin, and streptomycin were from GIBCO Invitrogen (Gaithersburg, MD). PD-98059 and U-0126 were obtained from Calbiochem (San Diego, CA). All other reagents were purchased from Sigma Chemical (St. Louis, MO).
Isolation of neonatal rat cardiac myocytes.
Cardiomyocytes and cardiac fibroblasts were isolated from the ventricles of neonatal (1- to 2-day old) Sprague-Dawley rats by proteolytic digestion and separated by differential plating, as previously described by us (28) and others (1). The ventricles from a litter of pups were pooled and represent an experimental n of 1. Myocytes were maintained in DMEM/F-12 containing 10% FBS, 10 μg/ml insulin, 10 μg/ml holo-transferrin, 100 μM bromodeoxyuridine (included for the first 3 days to prevent proliferation of nonmyocytes), and the antibiotics ampicillin and streptomycin. Before their use, primary cultures of myocytes were incubated for 24 h in the same media in the absence of serum and bromodeoxyuridine (serum-free media). Fibroblasts were maintained in DMEM with 10% FBS and antibiotics. Fibroblasts were passaged using trypsin/EDTA to eliminate any residual myocytes and placed in serum-free media for 24 h before their use. Myocytes isolated by this protocol routinely showed positive immunoreactive staining for anti-sarcomeric myosin (1:100; Sigma) and no labeling with antibodies to α-smooth muscle-specific actin, fibronectin, or vimentin (1:100 dilutions; Sigma), whereas fibroblasts were stained with antibodies to fibronectin, vimentin, and α-smooth muscle specific actin. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
RNA isolation and RT real-time PCR.
RNA was isolated from cultured myocytes or fibroblasts using the TRIzol reagent (GIBCO Invitrogen, Carlsbad, CA), as directed by the manufacturer. The RNA concentration and integrity were determined using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano Labchip (Agilent Technologies, Palo Alto, CA). Approximately 1 μg of total RNA was reverse transcribed using AMV reverse transcriptase in a 20-μl reaction mixture containing deoxyribonucleotides, random hexamers, and RNase inhibitor in reverse transcriptase buffer. Heating the reverse transcriptase reaction product at 95°C terminated the reaction. For RT-PCR, 2 μl of the resultant cDNA was added to TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) with an ACE2 primer/probe set (forward primer 5′-CCCAGAGAACAGTGGACCAAAA-3′; reverse primer 5′-GCTCCACCACACCAACGAT-3′; and probe 5′-FAM-CTCCCGCTTCATCTCC-3′) or a MKP1 primer/probe set (Applied Biosystems), and amplification was performed on an ABI 7000 Sequence Detection System. The mixtures were heated at 50°C for 2 min, at 95°C for 10 min followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. All reactions were performed in triplicate, and 18S ribosomal RNA, amplified using the TaqMan Ribosomal RNA Control Kit (Applied Biosystems), served as an internal control. The results were quantified as Ct values, where Ct is defined as the threshold cycle of PCR at which amplified product is first detected, and expressed as the ratio of target/control.
ACE2 activity.
ACE2 activity was measured using a fluorescence assay according to Vickers et al. (31) with modifications. Reaction mixtures containing the substrate, 50 μM 7-methoxycoumarin-4-acetyl-alanine-proline-lysine-(2,4-dinitrophenyl)-OH, cell homogenate, and 10 mM HEPES, pH 7.0, with 1.0 M NaCl were incubated at 42°C for 60 min with 2 μM amastatin, 10 μM bestatin, 10 μM chymostatin, 10 μM benzyl succinate, 0.5 mM para-chloro-mercuribenzoic acid, 10 μM lisinopril, 10 μM SCH-39370, and 10 μM Z-prolyl prolinal to block residual ACE, neprilysin, or carboxypeptidase A activity. A second set of reactions contained the selective ACE2 inhibitor MLN-4760 (C16; 10 μM) to ensure that the measured enzyme activity was ACE2. The reaction was terminated by adding 0.2% trifluoroacetic acid; the fluorescence was quantified in a Perkin Elmer LS 50B fluorometer (excitation 320 nm; emission 405 nm). The reaction product was quantified by comparison with a standard curve generated by incubation with 7-methoxycoumarin-4-acetyl-alanine-proline in a dose range of 0.125–6 nmol.
Statistics.
All data are presented as means ± SE. Statistical differences were evaluated by one-way ANOVA followed by Dunnett's post hoc test. The criterion for statistical significance was set at P < 0.05.
RESULTS
Regulation of ACE2 activity in neonatal rat myocytes and fibroblasts.
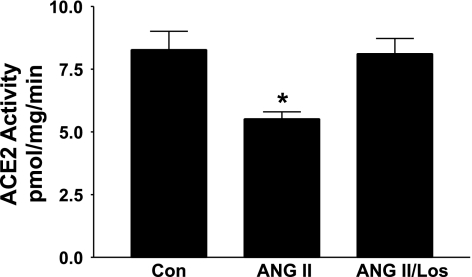
Myocytes and cardiac fibroblasts, isolated from neonatal rat heart, were incubated for 12 h with 100 nM ANG II in the presence and absence of 1 μM losartan, and ACE2 activity was measured using a fluorometric assay. In previous studies in rat astrocytes, this concentration of ANG II and time of incubation produced maximal inhibitory effects on ACE2 (11). ACE2 activity in cardiac myocytes was reduced by treatment with ANG II, and losartan blocked the ANG II-mediated decrease in catalytic activity, as shown in Fig. 1. ACE2 activity was not detected in neonatal cardiac fibroblasts using the fluorometric assay.
Fig. 1.
ANG II reduces angiotensin-converting enzyme (ACE2) activity in neonatal cardiac myocytes. Myocytes in serum-free media were incubated for 12 h with either 100 nM ANG II or 100 nM ANG II and 1 μM losartan (Los). EDTA (0.5 mM) was included to prevent the degradation of ANG II by ACE2, ACE, or neprilysin. ACE2 activity was measured using the fluorescent substrate 7-methoxycoumarin-4-acetyl-alanine-proline-lysine-(2,4dinitrophenyl)-OH; n = 3–4 rats. *P < 0.05 compared with Control (Con), in the absence of ANG II or losartan.
Identification of ACE2 mRNA in neonatal rat myocytes and cardiac fibroblasts.
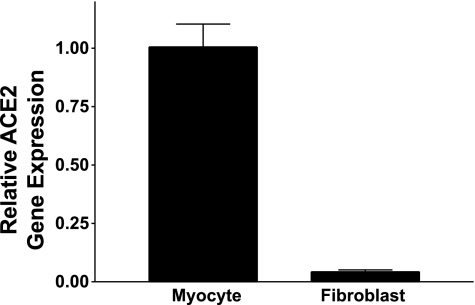
Total RNA was isolated from neonatal rat cardiac myocytes and fibroblasts to determine whether cardiac fibroblasts express ACE2 and to compare the relative distribution of the enzyme in cardiac cells. Although ACE2 mRNA was present in both myocytes and fibroblasts, as detected by RT real-time PCR, the majority of the ACE2 mRNA was present in cardiac myocytes. The relative distribution of ACE2 in myocytes compared with cardiac fibroblasts was 96:4, as shown in Fig. 2.
Fig. 2.
ACE2 mRNA in neonatal cardiac myocytes and fibroblasts. ACE2 mRNA was measured by RT real-time PCR in myocytes and fibroblasts isolated from neonatal rat heart; n = 4.
Regulation of ACE2 by ANG II and ET-1.
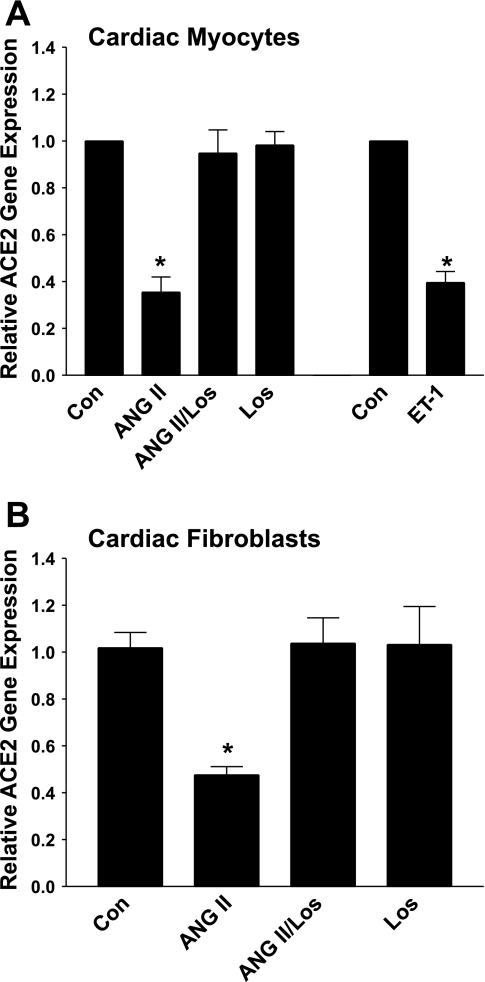
Cultured myocytes or fibroblasts isolated from neonatal rat heart were incubated for 12 h with 100 nM ANG II to determine whether the peptide regulates ACE2 mRNA. EDTA (0.5 mM) was added to the culture media to block the activity of the metalloproteases ACE, neprilysin, and ACE2 and prevent ANG II degradation, as previously described (11). After 12 h, total RNA was isolated from the cells, and ACE2 mRNA was quantified by RT real-time PCR. ACE2 mRNA was reduced markedly in myocytes or fibroblasts following incubation with ANG II, as shown in Fig. 3, A and B. Myocytes or fibroblasts were incubated with the AT1 antagonist losartan (1 μM), in the presence and absence of ANG II (100 nM), to identify the receptor mediating the reduction in ACE2 mRNA by ANG II. The addition of ANG II in the presence of losartan prevented the ANG II-mediated reduction in ACE2 mRNA, whereas losartan alone had no effect on ACE2 mRNA (Fig. 3, A and B). This study demonstrates that ANG II downregulates ACE2 mRNA and that the effect of ANG II is mediated by the AT1 receptor.
Fig. 3.
ANG II downregulates ACE2 mRNA in neonatal cardiac myocytes and fibroblasts. Myocytes (A) and cardiac fibroblasts (B) in serum-free media were incubated for 12 h with either 100 nM ANG II, in the presence or absence of 1 μM losartan (Los), with 1 μM losartan alone, or with 10 nM endothelin (ET)-1. ACE2 mRNA was measured by RT real-time PCR; n = 5. *P < 0.01 compared with Control (Con), in the absence of ANG II, losartan, or ET-1.
ET-1 is released from cardiac myocytes by stretch and induces cardiac hypertrophy, by either autocrine or paracrine mechanisms (21, 24). We determined whether ET-1 also regulates ACE2 by incubating myocytes for 12 h with 10 nM ET-1 and quantifying ACE2 mRNA by RT real-time PCR. This concentration of ET-1 was previously used by de Jonge et al. (5) to stimulate a hypertrophic response [cell growth, mitogen-activated protein (MAP) kinase activation] in neonatal cardiac myocytes. ET-1 caused a significant downregulation of ACE2, similar to our results with ANG II (Fig. 3A).
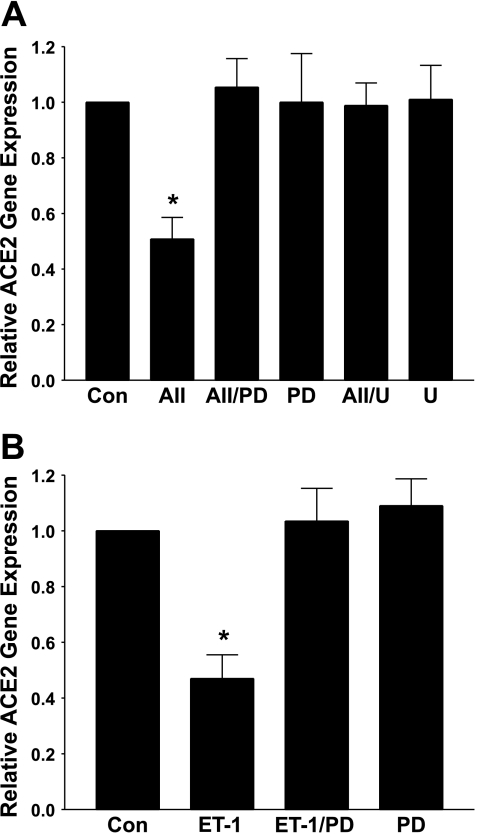
Autophosphorylation and activation of the MAP kinases extracellular signal-regulated kinase (ERK) 1 and ERK2, through agonist-mediated stimulation of the MAP kinase kinase MEK, is a major signaling pathway activated by ANG II and ET-1 to regulate gene transcription in cardiomyocytes (1, 19, 23–25). Cardiac myocytes were pretreated with the MEK inhibitors PD-98095 or U-0126 and stimulated with ANG II or ET-1, and ACE2 mRNA was quantified, to determine whether activation of ERK1 and ERK2 participates in the downregulation of ACE2 by either agonist. As shown in Fig. 4A, the ANG II-mediated reduction in ACE2 mRNA was blocked by treatment with either PD-98095 (30 μM) or U-0126 (5 μM); MEK inhibitors alone had no effect on ACE2 mRNA. The ET-1-mediated reduction in ACE2 mRNA was also completely blocked by pretreatment with the MEK inhibitor PD-98059, as shown in Fig. 4B. These results suggest a role for MEK and ERK1/ERK2 in the agonist-mediated reduction in ACE2.
Fig. 4.
Mitogen/extracellular signal-regulated kinase (MEK) inhibitors prevent the downregulation of ACE2 by ANG II or ET-1. Myocytes in serum-free media were incubated for 12 h with 100 nM ANG II (AII, A) or 10 nM ET-1 (B) in the presence and absence of the MEK inhibitor PD-98059 (PD, 30 μM) or U-0126 (U, 5 μM), as indicated. ACE2 mRNA was measured by RT real-time PCR; n = 5–6. *P < 0.01 compared with the Control (Con), in the absence of ANG II or ET-1.
Blockade of the ANG II-mediated regulation of ACE2 by ANG-(1-7) and ANP.
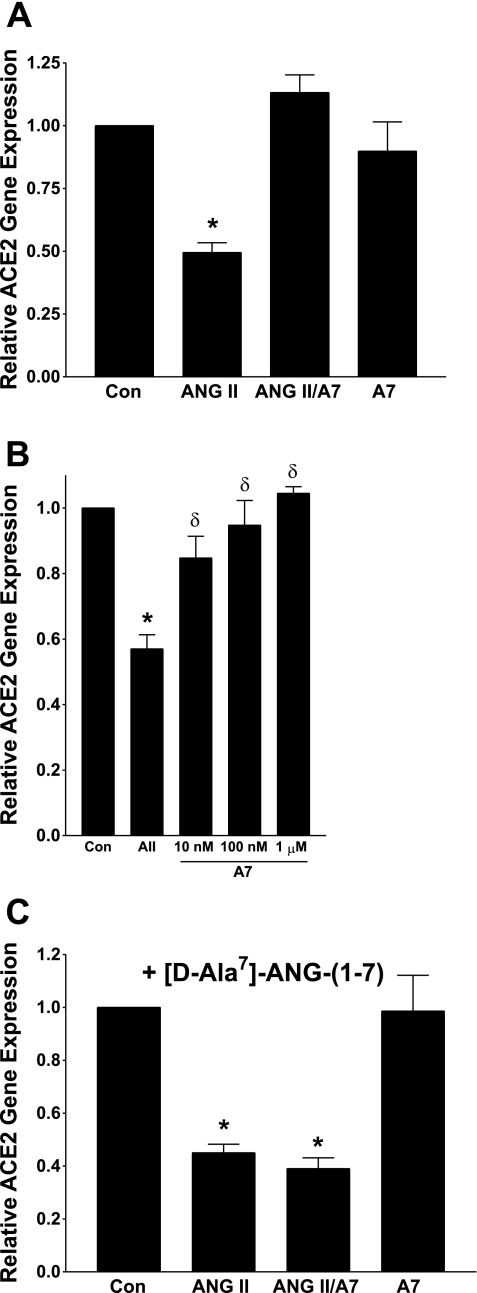
Cultured rat myocytes were incubated with 100 nM ANG-(1-7) to determine whether the heptapeptide also regulates ACE2 mRNA. We previously showed that this concentration of ANG-(1-7) caused maximal effects on protein synthesis and inhibition of MAP kinase activities in neonatal cardiomyocytes (28). As shown in Fig. 5A, ANG-(1-7) alone had no effect on ACE2 mRNA. This contrasts with the 50% reduction in ACE2 mRNA in the presence of 100 nM ANG II. However, when myocytes were incubated with both peptides, ANG-(1-7) prevented the ANG II-mediated reduction in ACE2 mRNA. The blockade was dose dependent, as shown in Fig. 5B; 10 nM ANG-(1-7) partially prevented the response to ANG II, but the response was completely blocked by 100 nM or 1 μM ANG- (1-7). The ANG-(1-7) blockade of the ANG II downregulation of ACE2 was prevented by pretreatment with 1 μM of the ANG-(1-7)-selective receptor antagonist [d-Ala7]-ANG-(1-7), as shown in Fig. 5C, indicating that the response to the heptapeptide was due to activation of an AT(1-7) receptor. These data demonstrate that ANG-(1-7) directly opposes the regulation of ACE2 by ANG II.
Fig. 5.
ANG-(1-7) prevents the downregulation of ACE2 mRNA by ANG II. Myocytes in serum-free media were incubated for 12 h with 100 nM ANG II, in the presence or absence of 1 μM ANG-(1-7) [A7], or with 1 μM ANG-(1-7) alone, as shown in A. B: myocytes in serum-free media were incubated for 12 h with 100 nM ANG II (AII) in the presence of increasing concentrations of ANG-(1-7) [A7]. For the studies shown in C, myocytes in serum-free media containing 1 μM [d-Ala7]-ANG-(1-7) were incubated for 12 h with 100 nM ANG II, in the presence or absence of 1 μM ANG-(1-7). ACE2 mRNA was measured by RT real-time PCR; n = 4–6. *P < 0.01 compared with the Control (Con), in the absence of ANG II or ANG-(1-7); δP < 0.05 compared with ANG II, in B.
The downregulation of ACE2 by ANG II was blocked by preincubation with the MEK inhibitors PD-98059 or U-0126, as shown in Fig. 4. We previously showed that ANG-(1-7) reduces the serum-stimulated activation of ERK1/ERK2 in neonatal cardiac myocytes (28) and ANG II-stimulated ERK activities in vascular smooth muscle cells (26). These results suggest that ANG-(1-7) may upregulate a MAP kinase phosphatase to reduce ANG II-stimulated ERK1/ERK2 activities and the ANG II-mediated reduction in ACE2. We determined the effect of ANG-(1-7) on the MAP kinase phosphatase MKP1 (the dual-specificity phosphatase DUSP-1), since it is one of the primary ERK phosphatases and was upregulated by ANP in cardiac myocytes (15). Myocytes were incubated with increasing concentrations of ANG-(1-7) (from 10 nM to 1 μM) and for increasing times from 1 to 24 h, and MKP1 was measured by Western blot hybridization (using an antibody from Upstate) and RT real-time PCR. ANG-(1-7) had no effect on MKP1 protein or mRNA following incubation for up to 24 h or concentrations up to 1 μM, suggesting that ANG-(1-7) does not reduce ERK activities in myocytes through an increase in MKP1.
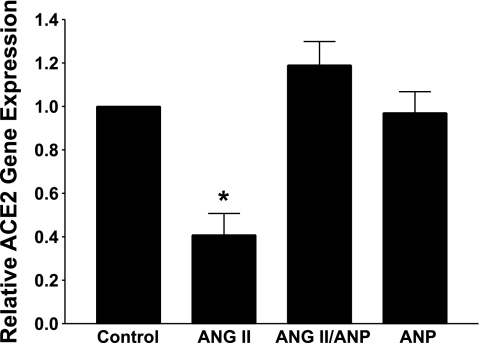
The natriuretic peptides ANP and brain natriuretic peptide (BNP) are early response genes that are upregulated in the hypertrophied heart. Although ANP is a diuretic-natriuretic peptide, it also inhibits the growth of cardiac myocytes (15). Cardiac myocytes were incubated with 100 nM ANP, to determine the effect of the peptide on ACE2 mRNA. This concentration of ANP was chosen because it caused a significant reduction in ANG II-stimulated cardiac hypertrophy and inhibited the MAP kinases ERK1/ERK2 in cardiac myocytes (15). ANP alone had no direct effect on ACE2, as shown in Fig. 6. However, incubation of ANP with ANG II prevented the ANG II-mediated reduction in ACE2, similar to our observations with ANG-(1-7).
Fig. 6.
Atrial natriuretic peptide (ANP) blocks the ANG II-mediated reduction in ACE2 mRNA. Myocytes in serum-free media containing 0.5 mM EDTA were incubated with 100 nM ANG II, in the presence or absence of 100 nM ANP. ACE2 mRNA was measured by RT real-time PCR; n = 5–6. *P < 0.01 compared with the Control, in the absence of ANG II or ANP.
DISCUSSION
ACE2 is a critical enzyme of the renin-angiotensin system that is present in various tissues important in the regulation of cardiovascular function, including the heart, the kidney, and the brain. ACE2 mRNA and activity were observed in isolated myocytes, whereas a limited amount of ACE2 mRNA and no enzyme activity was detected in cardiac fibroblasts. These data suggest that myocytes may be a source of ACE2 in the heart. This is in agreement with the immunocytochemical localization of ACE2 in myocytes in both rodent and human heart (2, 8, 14) and a previous report of no measurable ACE2 activity in cardiac fibroblasts (13). In addition, ACE2 immunoreactivity was also identified in the endothelium of cardiac capillaries, venules, arteries, and arterioles as well as in vascular smooth muscle cells and adventitia of larger coronary vessels (8).
Because ANG II is a substrate for ACE2, is present in the heart, and is increased following myocardial infarction, we investigated the regulation of ACE2 by ANG II in both cardiac myocytes and fibroblasts. Our studies show, for the first time, that ACE2 was significantly downregulated by ANG II in both isolated myocytes and cardiac fibroblasts. The decrease in ACE2 activity in myocytes and of ACE2 mRNA in both myocytes and cardiac fibroblasts was blocked by the AT1 receptor antagonist losartan, indicating that ANG II activated an AT1 receptor to reduce ACE2. This is in agreement with our previous studies showing that cardiac ACE2 mRNA was increased by administration of losartan to normotensive rats (10), by treatment of normotensive rats with the AT1 receptor antagonist losartan or olmesartan following myocardial infarction (17), or by treatment of hypertensive Lew.Tg(mRen2) congenic rats with losartan (18). In addition, administration of the ACE inhibitor lisinopril to normotensive or hypertensive rats to reduce ANG II formation caused a significant increase in ACE2 mRNA (10, 18). The increase in ACE2 mRNA by either blocking the effect of ANG II with an AT1 receptor antagonist or reducing ANG II with an ACE inhibitor is in agreement with the current study showing that ANG II downregulates ACE2.
ACE2 catalyzes the conversion of ANG II to the heptapeptide ANG-(1-7). Although ANG-(1-7) had no direct effect on ACE2 mRNA, the heptapeptide completely blocked the downregulation of ACE2 by ANG II. Treatment of hypertensive or normotensive rats with an ACE inhibitor reduced ANG II to prevent the ANG II-mediated reduction in ACE2 (10, 17, 18). On the other hand, administration of AT1 receptor antagonists prevented the ANG II-mediated reduction in ACE2 mRNA but did not reduce circulating ANG II. Treatment with either lisinopril or losartan increases ANG-(1-7) by elevating the production of ANG-(1-7) or preventing its breakdown (3). This suggests that the increase in ACE2 by treatment with ACE inhibitors or AT1 receptor antagonists could be due to a reduction in ANG II or blockade of its effects to prevent the downregulation of ACE2, an increase in ANG-(1-7) to block the response to ANG II, or a combination of responses to both peptides.
Treatment of myocytes with ET-1 also caused a significant reduction in ACE2 mRNA. ANG II and endothelin are released from cardiac myocytes in response to mechanical strain, suggesting that the two hormones have autocrine/paracrine effects in the hypertrophied heart. Because both ANG II and ET-1 downregulate ACE2, their release during cardiac hypertrophy would reduce ACE2 to prevent ANG II conversion to ANG-(1-7) and sustain the elevated levels of ANG II by a positive, feedforward mechanism.
ANG II participates in cardiac hypertrophy through the stimulation of MAP kinases, effects mediated by the AT1 receptor. Although the three major MAP kinase pathways (ERK1/ERK2, p38, and c-Jun NH2-terminal kinase or JNK) were stimulated by ANG II in cardiac myocytes, activation of ERK1 and ERK2 plays a major role in cardiac hypertrophy by regulating the expression of ANP (1). Endothelin binds to G protein-coupled endothelin receptors on cardiomyocytes to increase growth, through stimulation of MAP kinase signaling to increase nuclear transcription factors, protein kinases, and ion exchangers/channels (24, 25). Although the MAP kinases JNK, p38, and ERK1/ERK2 are also activated by ET-1, microarray analysis of cardiac myocytes stimulated with ET-1 in the presence or absence of the ERK1/ERK2 inhibitor U-0126 suggested that the majority of changes in gene expression in response to ET-1 are mediated by activation of the ERK1/ERK2 pathway (19). Treatment of myocytes with either PD-98059 or U-0126 to inhibit the MAP kinase kinase MEK and prevent ERK activation blocked both the ANG II- and ET-1-mediated downregulation of ACE2. This suggests that activation of ERK1/ERK2 and the ERK-mediated regulation of gene transcription represent the molecular mechanism for the downregulation of ACE2 by either agonist. The 5′ upstream region of the ACE2 promoter contains a number of putative sites for the binding of transcriptional factors regulated by ANG II, including sites for c-fos, c-jun, activator protein (AP)-1, AP-2, and nuclear factor-κB. In addition, there are a number of additional elements involved in the regulation of early response genes and inflammatory markers within the first 200 nucleotides of the ACE2 promoter. ANG II, through autophosphorylation and activation of ERK1 and ERK2 and their translocation to the nucleus, could negatively regulate ACE2 mRNA through interaction with these regulatory sites on the ACE2 promoter. The downregulation of ACE2 by ERK1/ERK2 in cardiac myocytes is in agreement with studies by Koka et al. (20) who showed that ANG II downregulated ACE2 in human kidney tubular cells through activation of the MAP kinases ERK1/ERK2 and p38. ANG II also stimulates p38 in cardiac myocytes and could participate in the regulation of ACE2 in cardiac myocytes.
Treatment of myocytes with ANG II reduced ACE2, whereas coadministration of ANG-(1-7) prevented the downregulation of ACE2 by ANG II. The ANG-(1-7) receptor antagonist [d-Ala7]-ANG-(1-7) blocked the response to ANG-(1-7), indicating that ANG-(1-7) mediates its effect through activation of an AT(1-7) receptor and subsequent downstream signal transduction pathways. We previously showed that ANG-(1-7) reduces the growth of cardiac myocytes and decreases activity of ERK1 and ERK2 through activation of the [d-Ala7]-ANG-(1-7)-sensitive receptor mas (28). Because ANG II activates the MAP kinases ERK1/ERK2 to reduce ACE2 mRNA, ANG-(1-7) may prevent the response to ANG II by either reducing MAP kinase kinase activity or increasing MAP kinase phosphatase activity.
The hypertrophied heart also releases the natriuretic peptides ANP and BNP. ANP has both direct and indirect effects on the heart, directly inhibiting myocyte growth and indirectly reducing hemodynamic load through its diuretic and natriuretic properties. Although ANP alone had no effect on ACE2 mRNA, it prevented the reduction mediated by either ANG II or ET-1. Hayashi et al. (15) showed that ANP prevented the increase in cell size and protein synthesis in myocytes treated with ANG II or ET-1, reduced the activation of ERK1 and ERK2, and upregulated the MAP phosphatase MKP1. Stimulation of ANP receptors (either NPR-A or NPR-B) generates cGMP through activation of a guanylate cyclase domain, and the cell-permeable analog of cGMP, 8-bromo-cGMP, mimicked the reduction in ERK1/ERK2 activity and the increase in MKP1 in cardiac myocytes, indicating that these responses are mediated by cGMP (15). This suggests that ANP may block the ANG II/ET-1-mediated reduction in ACE2 mRNA through an increase in cGMP, a decrease in ERK1/ERK2 activities, and an increase in MKP1. Although ANP increases cGMP to activate cGMP-dependent mechanisms, we showed that ANG-(1-7) inhibited growth through an increase in cAMP and activation of the cAMP-dependent protein kinase in vascular smooth muscle cells, suggesting that ANG-(1-7) may activate a cAMP-dependent protein kinase in cardiac myocytes (26). ANG-(1-7) also reduced ERK1 and ERK2 activities (26) as well as the ANG II-mediated decrease in ACE2 mRNA. However, we showed that ANG-(1-7) does not increase MKP1 (DUSP1) in cardiac myocytes. The increase in cAMP may upregulate or stimulate the activity of another MAP kinase phosphatase to dephosphorylate the MAP kinases ERK1 and ERK2 and reduce their activities, analogous to the cGMP-mediated increase in MKP1.
Our findings demonstrate that ACE2 in cardiac myocytes is a tightly regulated enzyme, responding to a number of vasoactive peptides and hormones. Both ANG II and ET-1 downregulated ACE2 mRNA to reduce ACE2 activity. Because ACE2 converts ANG II to ANG-(1-7), this represents positive feedback regulation by ANG II to prevent its degradation to ANG-(1-7) and sustain the vasocontrictive, mitogenic, and fibrotic effects of both ANG II and ET-1. The downregulation of ACE2 by ANG II or ET-1 was blocked by MEK inhibitors to reduce ERK activities, suggesting that both agonists downregulate ACE2 through activation of the MAP kinases ERK1 and/or ERK2. In contrast, both ANG-(1-7) and ANP prevented the downregulation of ACE2 by ANG II. Because ANG-(1-7) and ANP have antiproliferative and antifibrotic effects in cardiac myocytes and fibroblasts, blockade of the ANG II-mediated reduction in ACE2 by ANG-(1-7) or ANP will sustain ACE2 activity to convert ANG II to ANG-(1-7) through positive feedforward regulation. Any alteration in the physiological concentrations of ANG II, ET-1, ANP, or ANG-(1-7) following myocardial infarction and heart failure will thus affect the regulation of ACE2, thereby shifting the balance between hypertrophic hormones like ANG II and ET-1 and inhibitors of myocyte and cardiac fibroblast growth, such as ANG-(1-7) or ANP.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-51952 and by grants provided in part from Unifi, Inc., Greensboro, NC, and Farley-Hudson Foundation, Jacksonville, NC.
Acknowledgments
Losartan was a kind gift of Merck & Co., Inc. We acknowledge the technical assistance of L. Tenille Howard, Randi Leonard, and Robert Lanning.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem J 347: 275–284, 2000. [PMC free article] [PubMed] [Google Scholar]
- 2.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 114: 1–7, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension 31: 362–367, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Crackower MA, Sarao ROG, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos A, da Costa JZL, Pei YP, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yogil Y, Penninger JM. Angiotensin-converting enzme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. [DOI] [PubMed] [Google Scholar]
- 5.de Jonge HW, Dekkers DH, Houtsmuller AB, Sharma HS, Lamers JM. Differential signaling and hypertrophic responses in cyclical endothelin-1 stimulated neonatal rat cardiomyocytes. Cell Biochem Biophys 47: 21–32, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardic overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension 51: 712–718, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Diez-Freire C, Vaxquez J, de Adjounian MFC, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27: 12–19, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E9, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Diz DI, Gallagher PE, Tallant EA. angiotensin-(1-7):it's contribution to arterial pressure control mechanisms. In: Handbook of Experimental Pharmacology, edited by Unger T and Scholkens B. Springer: Heidelberg, Germany, 2004, p. 478–518.
- 10.Ferrario CM, Jessup JA, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin converting enzyme 2. Circulation 111: 2605–2610, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for Ang II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290: C420–C426, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med 2: 19–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Kotovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 113: 357–364, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hamming I, Cooper ME, Haagmans BL, Hooper NM, Osterhaus ADME, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol 212: 1–11, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi D, Kudoh S, Shiojima I, Zou Y, Harada K, Shimoyama M, Imai Y, Monzen K, Yamazaki T, Yazaki Y, Nagai R, Komuro I. Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mitogen-activated protein kinase phosphatase-1. Biochem Biophys Res Commun 322: 310–319, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Huentelman MJ, Grobe JL, Vazquez J, Steward JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90.5: 783–790, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Up-regulation of angiotensin converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 1–7, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol 291: H2166–H2172, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy RA, Kemp TJ, Sugden PH, Clerk A. Using U0126 to dissect the role of the extracellular signal-regulated kinase 1/2 (ERK1/2) cascade in the regulation of gene expression by endothelin-1 in cardiac myocytes. J Mol Cell Cardiol 41: 236–247, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Koka V, Huang XR, Chung ACK, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang F, Gardner DG. Autocrine/paracrine determinants of strain-activated brain natriuretic peptide gene expression in cultured cardiac myocytes. J Biol Chem 273: 14612–14619, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JM, Khokha R, Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 75: 29–39, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Sadoshima J, Qui Z, Morgan JP, Izumo S. Angiotensin II and other hypertrophic stimuli mediated G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca2+-dependent signaling. Circ Res 76: 1–15, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Sugden PH An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol 35: 871–886, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Sugden PH, Clerk A. Endothelin signalling in the cardiac myocyte and its pathophysiological relevance. J Vasc Pharmacol 3: 343–351, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7). Hypertension 42: 574–579, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Tallant EA, Diz DI, Ferrario CM. State-of-the-Art lecture. Antiproliferative actions of angiotensin-(1-7) in vascular smooth muscle. Hypertension 34: 950–957, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560–H1566, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1-7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol 292: H3019–H3024, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang K, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Ohishi M, Katsuya T, Ito N, Ikushima M, Kaibe M, Tatara Y, Shiota A, Sugano S, Takeda S, Rakugi H, Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47: 718–726, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation 108: 1707–1712, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Zisman LS, Meixell GE, Bristow MR, Canver CC. Angiotensin-(1-7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation 108: 1679–1681, 2003. [DOI] [PubMed] [Google Scholar]