Abstract
Hypertension is a major risk factor for stroke, but the factors that contribute to the increased incidence and severity of ischemic stroke in hypertension remain to be determined. 20-hydroxyeicosatetraenoic acid (20-HETE) has been reported to be a potent constrictor of cerebral arteries, and inhibitors of 20-HETE formation reduce infarct size following cerebral ischemia. The present study examined whether elevated production of 20-HETE in the cerebral vasculature could contribute to the larger infarct size previously reported after transient middle cerebral artery occlusion (MCAO) in hypertensive strains of rat [spontaneously hypertensive rat (SHR) and spontaneously hypertensive stroke-prone rat (SHRSP)]. The synthesis of 20-HETE in the cerebral vasculature of SHRSP measured by liquid chromatography-tandem mass spectrometry was about twice that seen in Wistar-Kyoto (WKY) rats. This was associated with the elevated expression of cytochrome P-450 (CYP)4A protein and CYP4A1 and CYP4A8 mRNA. Infarct volume after transient MCAO was greater in SHRSP (36 ± 4% of hemisphere volume) than in SHR (19 ± 5%) or WKY rats (5 ± 2%). This was associated with a significantly greater reduction in regional cerebral blood flow (rCBF) in SHR and SHRSP than in WKY rats during the ischemic period (78% vs. 62%). In WKY rats, rCBF returned to 75% of control following reperfusion. In contrast, SHR and SHRSP exhibited a large (166 ± 18% of baseline) and sustained (1 h) postischemic hyperperfusion. Acute blockade of the synthesis of 20-HETE with N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016; 1 mg/kg) reduced infarct size by 59% in SHR and 87% in SHRSP. HET0016 had no effect on the fall in rCBF during MCAO but eliminated the hyperemic response. HET0016 also attenuated vascular O2•− formation and restored endothelium-dependent dilation in cerebral arteries of SHRSP. These results indicate the production of 20-HETE is elevated in the cerebral vasculature of SHRSP and contributes to oxidative stress, endothelial dysfunction, and the enhanced sensitivity to ischemic stroke in this hypertensive model.
Keywords: 20-hydroxyeicosatetraenoic acid, middle cerebral artery occlusion, cytochrome P-450A, N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine
hypertension is a major risk factor for stroke, but the factors that contribute to the increased incidence and severity of ischemic stroke in hypertension remain to be determined (46). Previous studies have indicated that arachidonic acid (AA) is released into cerebrospinal fluid following cerebral ischemia (1, 31, 42, 56). AA is converted by cytochrome P-450 (CYP) enzymes in cerebral arteries to a potent vasoconstrictor, 20-hydroxyeicosatetraenoic acid (20-HETE) (15, 16, 32). 20-HETE has been reported to play an important role in the regulation of cerebral vascular tone by regulating the open state probability of calcium-activated potassium channels (32). It also promotes the formation of oxygen radicals (15, 16, 20, 33, 36, 59) and contributes to endothelial dysfunction (35, 42, 45, 49, 62). More recently, inhibitors of the synthesis of 20-HETE have been reported to reverse the fall in cerebral blood flow (CBF) following subarachnoid hemorrhage (SAH) (3, 21, 28, 35, 55) and reduce infarct size following transient cerebral ischemia (35, 42, 45, 57). These findings suggest that 20-HETE contributes to vasospasm following SAH and ischemia-reperfusion injury in the brain.
The spontaneously hypertensive stroke-prone rat (SHRSP) is a genetic model of spontaneous hypertension, stroke, and endothelial dysfunction (2, 6, 9, 22, 29, 38, 50, 53). Although the SHRSP was bred as a model system of spontaneous hemorrhagic stroke (43, 66), SHRSP, like spontaneously hypertensive rat (SHR), exhibit an increased sensitivity to cerebral ischemia (5, 9), and infarct volume following transient middle cerebral artery (MCA) occlusion (MCAO) is much larger than that seen in normotensive strains. This increased sensitivity to cerebral ischemia has been suggested to be linked to a reduced dilator capacity of the cerebral circulation due to hypertension-induced vascular remodeling and endothelial dysfunction (7, 8, 22, 24). However, the mechanisms leading to cerebral vascular dysfunction in the cerebral circulation of SHR or SHRSP have not been thoroughly explored.
A 1997 genetic linkage analysis in SHRSP and Wistar-Kyoto (WKY) rats indicated that infarct size following MCAO cosegregates with a region on rat chromosome 5 that encompasses the four CYP genes of the 4A family that produce 20-HETE (26). Other studies have indicated that the formation of 20-HETE in the kidney (25, 41, 51, 52, 64) and in mesenteric arteries (68) is greater in SHR than in WKY rats. These findings suggest that the synthesis of 20-HETE may be elevated in the vasculature of hypertensive rats, but there has been no previous attempt to measure and compare eicosanoid production in the cerebral circulation of SHR or SHRSP with that in normotensive strains. Thus the present study compared the expression of CYP4A isoforms and the formation of eicosanoids in the cerebral vasculature of SHR, SHRSP, and WKY rats. We also examined the contribution of cerebral vascular 20-HETE formation to the increased infarct size following transient cerebral ischemia and the cerebral vascular oxidative stress and endothelial dysfunction previously reported in SHR.
MATERIALS AND METHODS
Animals.
Experiments were performed in 9- to 10-wk-old inbred male SHR, SHRSP, and WKY rats obtained from Charles River Laboratories (Wilmington, MA). The present studies were performed in these relatively young animals since this is the time that SHR and SHRSP begin to enter the established phase of hypertension, and the previous work on the genetics of ischemic stroke was performed in this age range to minimize the influence of hypertension-induced vascular injury and remodeling on infarct size (19, 26). This is also an age at which previous studies have indicated that the renal production of 20-HETE is markedly elevated in SHR and that inhibitors of the formation of 20-HETE blunt the development of hypertension (12, 47).
The rats were maintained in the Animal Care Facility at the Medical College of Wisconsin that is approved by the American Association for the Accreditation of Laboratory Animal Care. The rats were maintained on a 12-h:12-h light-dark cycle and had free access to standard rat chow (Cat. No. 5010; Purina Mills, St. Louis, MO) and water. All protocols were approved by the Animal Care and Use Committee of the Medical College of Wisconsin.
Measurement of systolic blood pressures.
Systolic blood pressures were measured in conscious SHR, SHRSP, and WKY rats using a tail-cuff blood pressure system (Hatteras Instruments, Cary, NC).
DOCA salt hypertension in WKY rats.
Normotensive WKY rats were treated with DOCA salt to induce hypertension. Six-week-old male WKY rats were uninephrectomized, and a 21-day timed-release DOCA salt pellet (200 mg; Innovative Research of America, Sarasota, FL) was implanted subcutaneously. After a 4-day recovery period, the rats were given a 1% solution of sodium chloride to drink. After 3 wk, systolic blood pressure was measured by tail cuff and cerebral arteries were collected for measurement of eicosanoids.
Measurement of eicosanoid production in cerebral vasculature.
Cerebral vessels were isolated from the brains of SHR, SHRSP, and WKY rats, and DOCA hypertensive WKY rats using an Evans blue sieving procedure. The rats were anesthetized with 2.0% isoflurane. Following bilateral cannulation of the carotid arteries, the cerebral circulation was flushed with 10 ml ice-cold Tyrode solution containing (in mmol/l) 145 NaCl, 5 KCl, 4.2 NaHCO3, 1 MgCl2, 0.05 CaCl2, 10 HEPES, and 10 glucose. The cerebral circulation was then filled with a Tyrode solution containing 6.0% albumin and 0.2% Evans blue dye to visualize the cerebral circulation. The brain was quickly removed and pushed through a 150-μm metal screen to separate the cerebral vasculature from brain parenchymal tissue. Blood vessels trapped on the top of the screen were collected and placed in a cold physiological salt solution (PSS) containing (in mmol/l) 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.03 EDTA, 5.5 dextrose, and 5 HEPES. The cerebral vessels were incubated at 37°C in 1 ml of PSS containing 1 mmol/l NADPH, 2 μmol/l indomethacin, and 40 μmol/l AA. The reactions were gently swirled for 90 min under an atmosphere of 100% O2, which is sufficient to maintain PO2 levels in the incubation media in the range of 100–150 Torr throughout the experiment. The reactions were terminated by acidification to pH 3.5 with formic acid, and the vessels were homogenized in the reaction buffer. The homogenate was extracted with ethyl acetate, and the organic layer was dried under nitrogen. Samples were reconstituted in methanol, and the metabolites formed were separated on a C18 column (150 × 2.1 mm, 3 μm Betabasic; Thermo Hypersil-Keystone) at a flow rate of 0.2 ml/min using an isocratic elution with a 51:9:40:0.01 mixture of acetonitrile:methanol:water:acetic acid for 30 min followed by a step gradient to 68:13:19:01 acetonitrile:methanol:water:acetic acid for 15 min. The effluent was ionized using a negative ion electrospray source, and the peaks eluting with a mass/charge ratio (m/z) of 319 > 301 [HETEs and epoxyeicosatrienoic acid (EETs)], 337 > 319 (DiHETEs), or 323 > 270 (internal standard) were monitored using an Applied Biosystems triple quadrupole mass spectrometer (API 3000; Foster City, CA).
Surrounding cortical brain tissue devoid of vessels was also collected and homogenized in a 10 mmol/l potassium phosphate buffer (pH 7.7) containing (in mmol/l) 250 sucrose, 1 EDTA, and 0.1 PMSF. The homogenates were centrifuged for 5 min at 3,000 g, and the supernatant was then centrifuged for 30 min at 11,000 g. The supernatants were centrifuged for 1 h at 100,000 g to obtain a microsomal fraction. The microsomal pellet was resuspended in 100 mmol/l potassium phosphate buffer (pH 7.25) containing 1 mmol/l EDTA, 1 mmol/l DTT, 0.1 mmol/l PMSF, and 30% glycerol. The microsomes (0.5 mg) were incubated in 0.1 M potassium phosphate buffer (pH 7.4) containing 1 mmol/l NADPH and 40 μmol/l AA at 37°C for 60 min under an atmosphere of 100% O2. The products formed were measured by liquid chromatography-tandem mass spectrometry (LC/MS) as described above.
Expression of CYP4A mRNA in cerebral arteries.
Cerebral vessels were obtained as described in Measurement of eicosanoid production in cerebral vasculature and placed in Trizol (Invitrogen, Carlsbad, CA). The vessels were homogenized and extracted with chloroform, and total RNA was then precipitated with isopropanol. The RNA pellets were resuspended in 50 μl diethyl pyrocarbonate-treated water, and the concentration of RNA was determined by measuring the 260-to-280 nm absorbance ratio. One microgram of total RNA was reverse transcribed using a SuperScript III first-strand cDNA synthesis kit (Invitrogen). Quantitative real-time PCR was performed on 100 ng cDNA using Power SYBR Green Master Mix (Applied Biosystems) and a Mx3000p real-time PCR system (Stratagene, La Jolla, CA).
The Cyp4A1, -2, -3, and -8 isoforms were amplified using the primer pairs presented in Table 1, and 18S was amplified as the reference gene. The Cyp4A1 and -4A8 isoforms were amplified for 40 cycles at 95°C for 30 s, 60° for 1 min, and 72°C for 30 s. A previously described touchdown PCR approach was used to achieve specific priming of 4A2 and 4A3 isoforms in which an annealing temperature of 70°C was used for the first 10 cycles, followed by 30 cycles at 60° (11). The results were normalized and quantified by using the ΔCt method (61).
Table 1.
Primers used for real-time PCR analysis of the expression of CYP4A isoforms in the cerebral vasculature
| Primers |
Sequence |
|
|---|---|---|
| Forward | Reverse | |
| CYP4A1 | CTCTTACTTGCCAGAATGGAGAA | GACTTGGATACCCTTGGGTAAAG |
| CYP4A2 | AGATCCAAAGCCTTATCAAT | CAGCCTTGGTGTAGGACCT |
| CYP4A3 | CAAAGCCTTCTGGAATTTATC | CAGCCTTGGTGTAGGACCT |
| CYP4A8 | ATCCAGAGGTGTTTGACCCTTAT | AATGAGATGTGAGCAGATGGAGT |
| 18S | CGGCTAACCACATCCAAGGAA | GCTGGAATTACCGCGGCT |
CYP, cytochrome P-450.
Expression of CYP4A protein in cerebral arteries.
Cerebral arteries were microdissected from freshly excised brains of WKY rats and SHRSP. The samples were homogenized in potassium phosphate buffer (pH 7.7) containing (in mmol/l) 250 sucrose, 1 EDTA, and 0.1 PMSF. The homogenates were centrifuged for 5 min at 3,000 g, and the supernatant was then centrifuged for 30 min at 11,000 g. Aliquots of the samples (30 μg protein) were loaded onto a 10% Tris·HCl SDS-PAGE gel, separated by electrophoresis, and transferred to a nitrocellulose membrane. Membranes were blocked overnight in Odyssey blocking buffer (Cat. No. 927-40000; LI-COR Biosciences, Lincoln, NE) and then probed simultaneously with a 1:2,000 dilution of goat anti-rat CYP4A1 antibody (Daiichi Pure Chemicals, Tokyo, Japan) and a 1:10,000 dilution of mouse anti-rat β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h. The primary antibodies were washed with PBS containing 0.1% Tween, and the membranes were probed with a 1:10,000 dilution of IRDye800 donkey anti-goat and a 1:20,000 dilution of IRDye700 donkey anti-mouse fluorescent secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA). The membranes were scanned, bands were quantified using the Odyssey infrared imaging system (LI-COR Biosciences), and CYP4A expression was normalized against the β-actin bands.
MCAO model of cerebral ischemia.
Experiments were performed on SHR, SHRSP, and WKY rats treated with vehicle or N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016) to determine whether there are strain differences in regional CBF (rCBF) and infarct volumes following transient cerebral ischemia and reperfusion and to determine the contribution of 20-HETE to these differences. The control animals (8 SHR, 8 SHRSP, and 6 WKY rats) received an intravenous infusion of the vehicle used for HET0016 (11% sulfobutylether-7-β-cyclodextrin in an isotonic mannitol solution) at a rate of 0.3 ml/h throughout the experiment. Additional experiments were performed on six SHR and six SHRSP that received a 1 mg/kg iv bolus injection of HET0016 5 min before the initiation of ischemia followed by a continuous infusion at a dose of 1 mg·kg−1·h−1.
The rats were subjected to a modified transient MCAO model of cerebral ischemia and reperfusion as previously described (37). Anesthesia was induced using 4% isoflurane in 70% N2-30% O2 gas mixture, and a surgical plane of anesthesia was maintained throughout the experiments using 2% isoflurane. Cannulas were placed in the femoral artery and vein for measurement of mean arterial pressure (MAP) and intravenous infusions. The skull was exposed, and the bone was thinned bilaterally, 6 mm lateral and 2 mm posterior to bregma, for measurement of rCBF using laser Doppler flowmetry. The external carotid artery was ligated, and the common carotid was tied off to force reperfusion to occur through the collateral circulation. An 18-mm segment of nylon suture (4-0; Ethicon) coated at the tip with silicon (Xantopren VL plus; Heraeus Kulzer Dental Products Division, South Bend, IN) was inserted into the left internal carotid artery and advanced to the origin of the MCA until rCBF fell by 80%. After 60 min of MCAO, the suture was removed to allow reperfusion. rCBF and MAP were recorded continuously for 60 min ischemia and 90 min reperfusion, after which the femoral catheters were removed and the animal was allowed to recover from anesthesia. Twenty-four hours following reperfusion, animals were reanesthetized with 4% isoflurane. The brain was removed and cut into six 2-mm-thick slices. The slices were immersed in a 2% solution of triphenyltetrazolium chloride for 10 min at 37°C. The slices were then fixed in 4% (vol/vol) buffered paraformaldehyde solution. Infarct size expressed as the percentage of the volume measured in the ipsilateral hemisphere was measured using Metamorph Image Analysis software (Molecular Devices, Downington, PA).
O2•− measurement in cerebral arteries.
Superoxide production was measured by incubation of cerebral arteries with dihydroethidium (DHE) with subsequent fluorescent detection of the production of 2-hydroxyethidium (EOH) by HPLC as previously described (10, 13). The rats were anesthetized using 4% isoflurane in 70% N2-30% O2 gas mixture. Catheters (PE-50) were inserted bilaterally into the common carotid arteries, and the blood was flushed from the cerebral circulation with 10 ml ice-cold Tyrode buffer (pH 7.4) with the following composition (in mmol/l) of 145 NaCl, 6.0 KCl, 0.05 CaCl2, 4.2 NaHCO3, 1.0 MgCl2, 10 HEPES, and 10 glucose. The cerebral circulation was then filled with 10 ml of Tyrode buffer containing 6% albumin and 0.2% Evans blue. The rats were euthanized by decapitation, and brains were excised and forced through a mesh screen (106 μm) to isolate cerebral arteries (Circle of Willis and pial arteries) from the surface of the brain as well as the penetrating pial arterioles. The isolated cerebral arteries were collected from the screen, and adherent parenchymal tissue was removed by microdissection. Cerebral arteries isolated from one rat were divided among four aliquots and placed in 1 ml Krebs-HEPES buffer containing (in mmol/l) 99.0 NaCl, 4.7 KCl, 1.7 CaCl2, 1.2 MgSO4, 1.03 NaH2PO4, 25.0 NaHCO3, 20.0 HEPES, and 11.0 glucose. The incubations were treated with vehicle, 1 μmol/l HET0016 to inhibit the formation of 20-HETE, 100 U/ml polyethylene glycol-superoxide dismutase (PEG-SOD) as a negative control, or 20 μmol/l of the redox cycling agent menadione as positive control for O2•− formation. The vessels were preincubated for 1 h with the various compounds under an atmosphere of 95% O2-5% CO2 at 37°C. DHE (50 μmol/l) was then added to each sample for 15 min. After 15 min incubation, the vessels were washed of DHE with 1 ml fresh Krebs-HEPES buffer six times for 5 min each and then homogenized in 0.6 ml Krebs-HEPES buffer containing 0.1% Triton X. An aliquot of the homogenate was removed to measure protein concentration, the samples were extracted with 0.8 ml water-saturated butanol, and the supernatant was collected and dried under nitrogen. The samples were reconstituted with 0.1 ml methanol. DHE, EOH, and ethidium were separated by HPLC on a C18 reverse phase column (250 × 4.6 mm; Altec) using a linear gradient ranging from 37–46% acetonitrile in water and 0.1% trifluoroacetic acid over a period of 30 min at a flow rate of 0.5 ml/min. The ethidium and EOH peaks were monitored with a fluorescent detector using an excitation wavelength of 480 nm and emission wavelength of 580 nm. DHE was detected spectrophotometrically by monitoring absorbance at 355 nm.
Contribution of 20-HETE to endothelial dysfunction in MCA of SHRSP.
Isolated MCAs were mounted onto glass micropipettes in a pressure myograph chamber as previously described (44). Briefly, animals were anesthetized with 4% isoflurane in 70% N2-30% O2 gas mixture. The brain was rapidly excised and placed in a Petri dish containing cold PSS with the following ionic composition (in mmol/l) of 119 NaCl, 4.7 KCl, 1.6 CaCl2, 1.17 MgSO4, 1.18 NaH2PO4, 24 NaHCO3, 5.5 dextrose, 5.0 HEPES, and 0.03 EDTA. MCAs were microdissected under a stereomicroscope (Leica, Bannockburn, IL) and quickly immersed in PSS at 37°C bubbled with 21% O2-5% CO2-74% N2 gas mixture in a superfusion chamber. MCAs were cannulated with glass micropipettes (100–150 μm; FHC, Brunswick, ME) and pressurized with PSS to 40 mmHg. The cannulated vessels were visualized using a videomicroscopy system (Zeiss, Thornwood, NY; and COHU Electronics, San Diego, CA), and vascular diameter was measured using a videomicrometer (Boeckeler Instruments, Tucson, AZ). The superfusate in the chamber was maintained at pH 7.4 for the duration of the experiment. After a 60-min equilibration period, the MCAs were preconstricted with serotonin (100 μmol/l) and then vascular reactivity to 10−9-10−5 mol/l ACh in the presence of indomethacin (5 μmol/l) was assessed. Experiments were performed using MCAs isolated from WKY rats and SHRSP and in SHRSP pretreated for 2 days with HET0016 (1 mg/kg ip twice daily) to chronically reduce the synthesis of 20-HETE. The concentration response curve to ACh in MCAs from HET0016-treated SHRSP was also reassessed following the addition of 1 mmol/l NG-nitro-l-arginine-methyl ester (l-NAME) to the bath to verify that the restored dilation responses to ACh were nitric oxide (NO) dependent.
Statistical analysis.
Mean values ± SE are presented. The significance of differences in mean values between and within groups was determined using ANOVA followed by Holm-Sidak post hoc test. P < 0.05 was considered significant.
RESULTS
Blood pressure.
Systolic pressures measured in conscious SHR (187 ± 7 mmHg; n = 8), SHRSP (198 ± 6; n = 8), and WKY rats treated with DOCA and salt (197 ± 7 mmHg; n = 8) were significantly greater than those measured in WKY rats (121 ± 8 mmHg; n = 8).
Cerebral vascular eicosanoid formation.
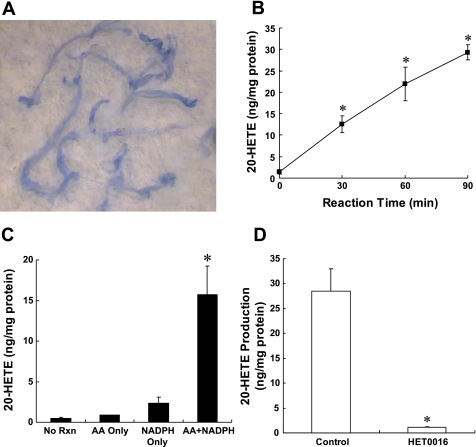
A representative example of the appearance of the vessels isolated by an Evans blue sieving procedure is presented in Fig. 1A. The procedure typically yields a relatively pure preparation of cerebral vessels free of brain parenchymal tissue, but there are some adherent perivascular neural tissue that can be microdissected from the vessels. The results of experiments to establish the reaction conditions for the isolated vessels, and to ensure that the eicosanoids were enzymatically produced, are presented in Fig. 1, B–D. Baseline levels of 20-HETE in vessels that were placed in cold PSS but not incubated averaged <1 ng/mg vessel protein. When the vessels were incubated with AA and NADPH, 20-HETE levels increased linearly for up to 90 min by more than 30-fold (Fig. 1B). Incubation of the vessels for 90 min with substrate (AA) alone in the absence of exogenous NADPH had little effect on the production of 20-HETE. Incubation of the vessels with NADPH for 90 min in the absence of added substrate increased the levels of 20-HETE by two- to threefold over control. The incubation of aliquots of these same vessels with both AA and NADPH markedly increased 20-HETE levels. Moreover, the production of 20-HETE by the vessels was reduced by more than 90% by the addition of 1 μM HET0016 to the incubations (Fig. 1D).
Fig. 1.
A: typical appearance of the cerebral arteries isolated by an Evans blue sieving procedure. The vessels are displayed on the background of a filter paper and are intact and relatively free of brain parachymal tissue. B: levels of 20-hydroxyeicosatetraenoic acid (20-HETE) measured in cerebral arteries incubated with 40 uM arachidonic acid (AA) and 1 mM NADPH at 37°C for 0, 30, 60, and 90 min. Effect of addition of AA and NAPDH on the production of 20-HETE production in cerebral arteries is presented in C. Vessels were incubated for 90 min at 37°C in the presence of 40 μM AA alone, 1 mM NADPH in the absence of added substrate, or both NADPH and substrate. No reaction (RXN) corresponds to control levels of 20-HETE that were measured in vessels that were placed in cold incubation solution and then homogenized and extracted with being incubated. D: effect of 1 μmol/l N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016) on 20-HETE production in cerebral arteries. Mean values ± SE from at least 3 separate incubations of vessels prepared from different animals are presented per time point. *Significant difference from the corresponding control value.
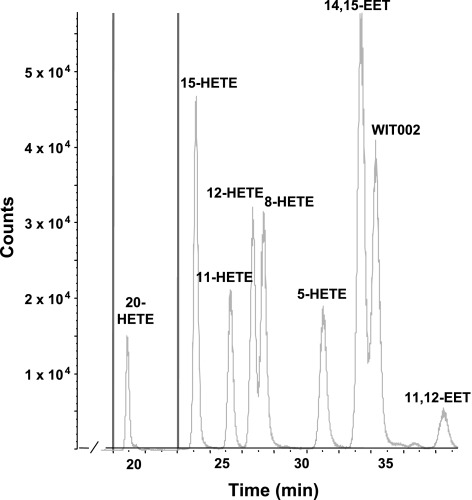
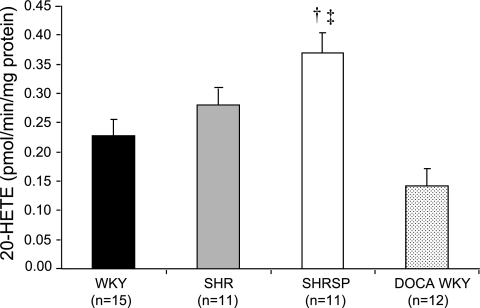
Representative LC/MS chromatograms showing the typical profile of HETEs and EETs produced by cerebral vessels obtained from SHR, SHRSP, and WKY rats are presented in Fig. 2. Cerebral arteries avidly produced 5-, 12-, 15-, and 20-HETE; 14,15-, 11,12-, 8,9-, and 5,6-DiHETEs; and 14,15-EET with lesser quantities of 11- and 8-HETE and 11,12-EET. The formation of 5-, 8-, 11-, 12-, and 15-HETE in the cerebral vasculature did not differ between the strains. In contrast, the production of 20-HETE in the cerebral vasculature of SHRSP was 62% greater compared with that seen in WKY rats and 22% greater than that seen in SHR (Fig. 3). The production of 20-HETE by cerebral cortical tissue was much lower than that seen in cerebral arteries. 20-HETE production by microsomes prepared from cerebral cortex was not significantly different in SHR, SHRSP, and WKY rats and averaged 0.11 ± 0.01, 0.14 ± 0.01, and 0.14 ± 0.03 pmol·min−1·mg−1 protein, respectively.
Fig. 2.
Representative liquid chromatography-tandem mass spectrometry chromatograms illustrating the profile of epoxyeicosatrienoic acid (EETs) and HETEs formed by rat cerebral arteries. WIT002, 20-5(Z),14(Z)-hydroxyeicosadienoic acid.
Fig. 3.
Comparison of the production of 20-HETE in the cerebral vasculature of normotensive and DOCA hypertensive Wistar-Kyoto (WKY) rats, spontaneously hypertensive rat (SHR), and spontaneously hypertensive stroke-prone rats (SHRSP). Mean values ± SE are presented; n, number of animals studied per group. †Significant difference from the corresponding value in WKY rats; ‡significant difference from DOCA hypertensive WKY rats.
Additional studies were performed to determine whether the difference in the production of 20-HETE in the cerebral vasculature of SHRSP and WKY rats might be secondary to the development of hypertension. We compared 20-HETE production in cerebral vasculature of normotensive WKY rats with that seen in WKY rats treated with DOCA salt to induce the same level of hypertension as that seen in the SHRSP. The production of 20-HETE in the cerebral vasculature of DOCA hypertensive WKY rats was not different from the levels seen in the normotensive WKY despite elevated blood pressures (0.142 ± 0.029 and 0.228 ± 0.028 pmol·min−1·mg−1 protein, respectively).
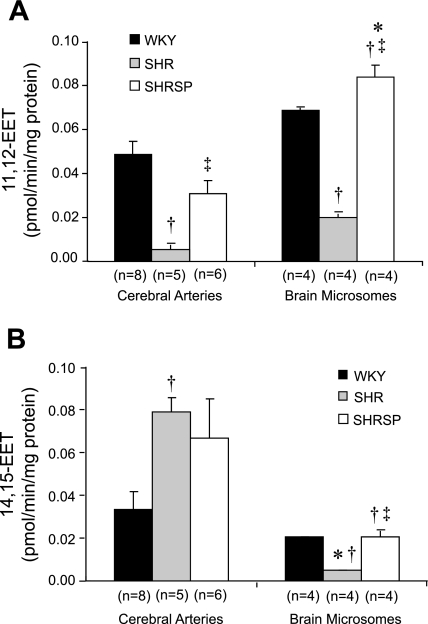
A comparison of the production of EETs in cerebral arteries and microsomes prepared from the brain of SHR, SHRSP, and WKY rats is presented in Fig. 4. The production of 11,12-EET measured in microsomes prepared from brain tissue was greater than that measured in cerebral arteries, while the production of 14,15-EET was greater in cerebral arteries than in the brain. The production of 11,12-EET measured in cerebral arteries and 11,12- and 14,15-EETs in brain microsomes was lower in SHR than in SHRSP and WKY rats. In contrast, the production of 14,15-EET measured in cerebral arteries was greater in SHR than in WKY rats. No significant differences were seen in the production of EETs in the cerebral vasculature or brain tissue of SHRSP and WKY rats.
Fig. 4.
Comparison of the formation of 11,12-EET (A) and 14,15-EET (B) in cerebral arteries and microsomes prepared cerebral cortex of SHR, SHRSP, and WKY rats. Mean values ± SE are presented; n, number of animals studied per group. *Significant difference from the corresponding value in cerebral arteries; †significant difference from the corresponding value in WKY rats; ‡significant difference from SHR.
Expression of CYP4A in cerebral arteries.
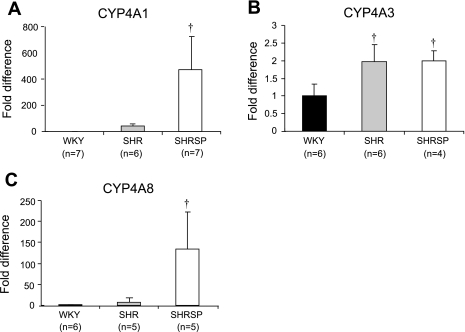
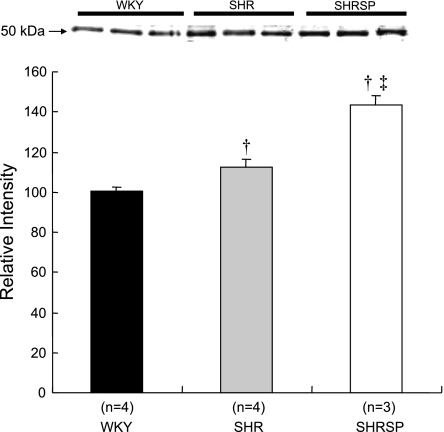
A comparison of mRNA expression of CYP4A isoforms in the cerebral vasculature of SHR, SHRSP, and WKY rats is presented in Fig. 5. The expression of CYP4A1 and CYP4A8 mRNA in the cerebral arteries of WKY rats was very low (Ct > 45 cycles). The expression of CYP4A1 and -4A8 isoforms in the cerebral arteries of SHRSP is markedly higher than in WKY rats. The expression of CYP4A3 is significantly greater in both hypertensive strains than in WKY rats. We could not detect the expression of CYP4A2 mRNA in the cerebral vasculature of SHR, SHRSP, and WKY rats. The expression of CYP4A protein in the cerebral vasculature is slightly elevated in SHR compared with that in WKY rats and is significantly greater in SHRSP compared with that seen in WKY rats and SHR (Fig. 6).
Fig. 5.
Comparison of the relative expression of cytochrome P-450 (CYP)4A1 (A), CYP4A3 (B), and CYP4A8 (C) mRNA in cerebral arteries obtained from SHR, SHRSP, and WKY rats. Mean values ± SE are presented; n, number of animals studied per group. †Significant difference from the corresponding value in WKY rats.
Fig. 6.
Comparison of the expression of CYP4A protein in cerebral arteries obtained from SHR, SHRSP, and WKY rats. Mean values ± SE are presented; n, number of animals studied per group. †Significant difference from the corresponding value in WKY rats; ‡significant difference from the corresponding value in SHR.
The effect of an inhibitor of the synthesis of 20-HETE on infarct volume in SHR and SHRSP.
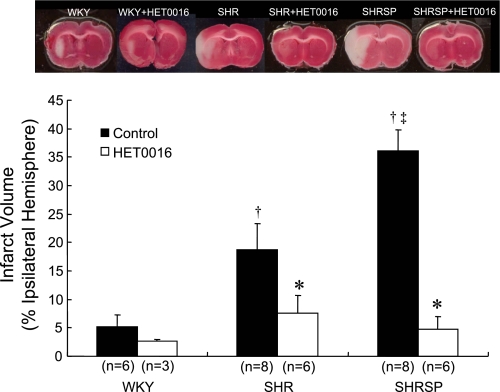
Infarct volume following 60 min ischemia and 24 h reperfusion was minimal in WKY rats, much larger in SHR, and very large in SHRSP (Fig. 7). The administration of the 20-HETE synthesis inhibitor HET0016 markedly reduced infarct volume in both SHR and SHRSP, but it did not significantly reduce or prevent the small subcortical infarct observed in WKY rats.
Fig. 7.
Effect of 1 μmol/l HET0016, an inhibitor of the synthesis of 20-HETE, on infarct size following 1 h of transient occlusion of the middle cerebral artery and 24 h of reperfusion in SHR, SHRSP, and WKY rats. Mean values ± SE are presented; n, number of animals studied per group. †Significant difference from the corresponding value in WKY rats; ‡significant difference from SHR; *significant difference from the corresponding control value within a group.
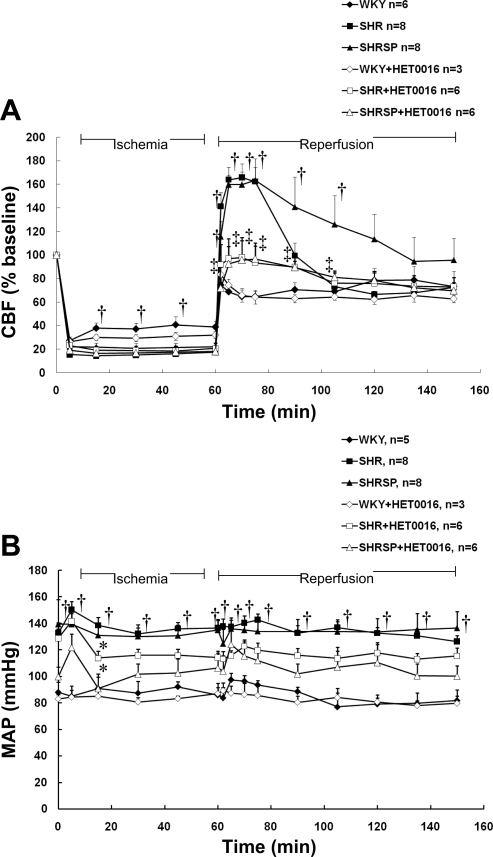
The effect of a 20-HETE inhibitor on rCBF during transient cerebral ischemia and reperfusion in SHR and SHRSP.
Baseline rCBF was similar in the ipsilateral and contralateral hemispheres and did not differ between SHR, SHRSP, and WKY rats, averaging 350 ± 25, 360 ± 10, and 325 ± 10 perfusion units, respectively. The pattern of rCBF during the transient MCAO experiments is significantly different in SHR, SHRSP, and WKY rats (Fig. 8A). In WKY rats, rCBF fell to 27 ± 3% of baseline, 5 min following MCAO, and then recovered to 38 ± 5% after 15 min of occlusion. It remained at this level throughout the ischemic period. The decline in rCBF with MCAO was similar in SHR and SHRSP, falling to 15 ± 2% and 22 ± 4% of baseline, respectively. However, in contrast with that of WKY rats, rCBF did not increase during MCAO in either SHR or SHRSP, suggesting a reduced ability to recruit collateral flow to the region of focal ischemia in both these hypertensive strains.
Fig. 8.
Effect of 1 μmol/l HET0016, an inhibitor of the synthesis of 20-HETE, on regional cerebral blood flow (CBF; A) and mean arterial pressure (MAP; B) in SHR, SHRSP, and WKY rats. Mean values ± SE are presented; n, number of animals studied per group. *Significant difference from the baseline value (time 0) within a group; †significant difference from the corresponding value in WKY rats; ‡significant difference from the corresponding control (untreated) value.
In WKY, rCBF returned to 75% of the baseline value during the reperfusion period. In contrast, there was a marked and prolonged hyperperfusion of the region of focal ischemia in both SHR and SHRSP, reaching a maximum of 166 ± 12% in SHR and 163 ± 18% in SHRSP. rCBF remained elevated for 30 min following reperfusion in SHR and 45 min in SHRSP. The administration of HET0016 had no effect on the fall in rCBF during MCAO in SHR or SHRSP (Fig. 8A). However, inhibition of 20-HETE synthesis with HET0016 prevented the hyperperfusion following the withdrawal of the occlusion in both SHR and SHRSP. The administration of HET0016 during cerebral ischemia and reperfusion did not alter the pattern of rCBF in WKY rats.
The effect of a 20-HETE inhibitor on MAP.
Baseline MAP under anesthesia averaged about 140 mmHg in the SHR and SHRSP (Fig. 8B) versus 95 mmHg in WKY rats treated with vehicle. MAP fell by 10–15 mmHg in SHR and SHRSP during the first 30 min following the administration of HET0016 and remained at this level for the remainder of the experiment. The administration of HET0016 did not change MAP in WKY rats.
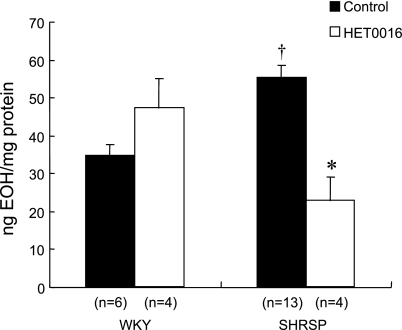
The contribution of 20-HETE to O2•− formation in cerebral arteries from SHRSP.
We used HPLC measurement of EOH to estimate intracellular O2•− formation in cerebral arteries of WKY and SHRSP (Fig. 9). The formation of EOH was 1.6-fold greater in SHRSP than in WKY rats. Incubation with HET0016 (1 μM) significantly attenuated the formation of O2•− in cerebral arteries obtained from SHRSP but had no effect on cerebral vascular O2•− formation in WKY rats. In all samples, PEG-SOD attenuated O2•− formation and menadione robustly stimulated O2•− formation (data not shown).
Fig. 9.
Effect of the 20-HETE synthesis inhibitor HET0016 (1 μmol/l) on the production of 2-hydroxyethidium (EOH; in ng/mg protein) in intact cerebral arteries of WKY and SHRSP. n, Number of samples in each group. †Significant difference from the corresponding value in WKY rats; *significant difference from the corresponding control value within a group.
Contribution of 20-HETE to endothelial dysfunction in MCA of SHRSP.
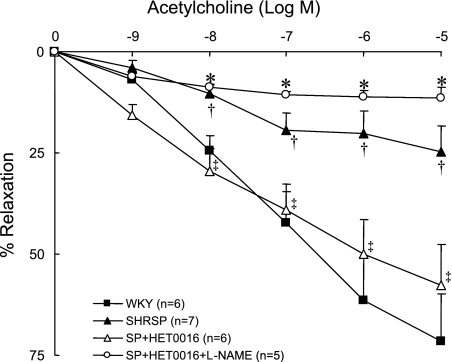
Vascular reactivity to the endothelium-dependent agonist ACh in MCAs isolated from SHRSP and WKY rats is presented in Fig. 10. Maximal dilation of serotonin-preconstricted MCAs to 10−5 M ACh is 71.4 ± 11.6% in WKY rats compared with 24.8 ± 6.5% in SHRSP. Chronic inhibition of 20-HETE synthesis with HET0016 restores endothelium-dependent dilation in MCAs of SHRSP to 58 ± 10%. The response to ACh in MCAs from HET0016-treated SHRSP is blocked by l-NAME, indicating the response is NO dependent.
Fig. 10.
Effect of chronic inhibition of the synthesis of 20-HETE with HET0016 (10 mg·kg−1·day−1 sc for 2 days) on vascular reactivity of serotonin-preconstricted middle cerebral arteries to ACh in SHRSP. †Significant difference from the corresponding value in WKY rats; ‡significant difference from corresponding control value within a strain; *significant difference from corresponding values in HET0016-treated SHRSP. l-NAME, NG-nitro-l-arginine-methyl ester.
DISCUSSION
Previous studies have indicated that the SHR and SHRSP strains are useful models of hypertension and vascular dysfunction (7–9, 22, 53, 65). These rats also exhibit a marked increase in sensitivity to cerebral ischemia and develop larger cerebral infarcts following MCAO than normotensive strains (9). The increased sensitivity of SHR and SHRSP to cerebral ischemic injury has been attributed to an impaired ability to recruit collateral flow to the ischemic penumbra, perhaps due to hypertension-induced vascular remodeling and vascular oxidative stress (7, 8, 22, 24). However, the factors that contribute to cerebral vascular dysfunction in SHR remain poorly understood.
Previous studies have shown that the formation of 20-HETE in the kidney (25, 41, 51, 52, 64) and in mesenteric arteries (68) is markedly elevated during the development of hypertension in SHR. Since 20-HETE is a potent vasoconstrictor, several investigators have postulated that elevated vascular production of 20-HETE may contribute to the development of hypertension in this strain. The finding that inhibitors of the synthesis of 20-HETE lower blood pressure by 10–15 mmHg in SHR is consistent with this hypothesis (52, 64). 20-HETE is also a potent constrictor of cerebral arteries, and the blockade of this pathway reduces infarct size following transient cerebral ischemia in normotensive rats (16, 32, 35, 40, 42, 45). However, the effect of 20-HETE inhibitors on ischemic stroke in hypertensive rats has not been determined. The present study examined whether the expression of CYP4A and the production of 20-HETE are elevated in the cerebral vasculature of SHR compared with normotensive rats and whether 20-HETE contributes to the increased sensitivity to cerebral ischemia and vascular oxidative stress previously reported in SHR and SHRSP.
Our results indicate that the production of 20-HETE in the cerebral vasculature of SHRSP is about twice that seen in WKY rats, and this is associated with a marked overexpression of CYP4A1 and -4A8 mRNA in the cerebral vasculature of SHRSP relative to the almost undetectable levels observed in WKY rats. CYP4A3 was the major isoform expressed in the cerebral vasculature of WKY rats, and the expression of this isoform was about twofold higher in SHRSP than in WKY rats. Overexpression of CYP4A was observed at the protein level as well, with a 12% and 40% increase in the expression in SHR and SHRSP, respectively, compared with that of WKY rats.
The elevated production of 20-HETE in SHRSP was specific to the cerebral vasculature since we found that there was no difference in the production of 20-HETE in microsomes prepared from the cortical brain tissue of WKY and SHRSP. This finding is of particular interest given that microsomes are enriched in CYP protein and suggests that the capacity for 20-HETE synthesis is much greater in vascular tissue than in neuronal tissue. There were no significant differences in the production of other HETEs and EETs by the cerebral vasculature of SHRSP and WKY rats, whereas cerebral vascular 11,12-EET formation was decreased and 14,15-EET formation was increased in SHR compared with WKY rats. The formation of both 11,12- and 14,15-EET was reduced in cortical microsomes of SHR compared with WKY rats. These results indicate a possible impairment in EET synthesis in SHR, although the physiological significance of this is not known. Interestingly, the production of 20-HETE was five- to 10-fold greater than the production of 11,12- or 14,15-EET in the cerebral vasculature in all strains.
We also found that the production of 20-HETE in the cerebral vasculature was similar in control WKY rats and a group treated with DOCA salt to raise blood pressure to the same level as that seen in the spontaneously hypertensive strains. This finding suggests that the difference in the production of 20-HETE in the cerebral vasculature of SHRSP and WKY rats cannot be explained by the difference in blood pressure alone in these two strains.
Additional studies were performed to determine whether the elevated production of 20-HETE in the cerebral vasculature of the SHRSP contributes to differences in rCBF and infarct size relative to normotensive WKY rats following transient MCAO. The present results confirm previous findings that infarct volume following transient MCAO is much larger in SHRSP than in WKY rats (9, 26). Infarct size in SHR was intermediate between these two strains.
The increased infarct size following transient MCAO was associated with a greater reduction in rCBF in the hypertensive strains than in WKY rats during the ischemic period (78% vs. 62% of baseline, respectively), as well as with a marked and sustained period of hyperperfusion (160% of baseline) following withdrawal of the occlusion.
Administration of HET0016, a selective inhibitor of the synthesis of 20-HETE, markedly reduced infarct volume in both SHR and SHRSP. Previous studies have examined the therapeutic potential of HET0016 in treating ischemic stroke by the administration of HET0016 at various time points following the initiation of cerebral ischemia. In the present study, animals were pretreated with HET0016 for 5 min before MCAO to determine the contribution of 20-HETE to ischemic stroke outcomes in normotensive and hypertensive rats. Administration of HET0016 also reduced MAP in SHR and SHRSP by about 30 mmHg, which is consistent with previous suggestions that elevated vascular production of 20-HETE may contribute to increased peripheral vascular tone and the maintenance of hypertension in the SHR (17, 30, 52, 63, 64, 67). This reduction in MAP in SHR and SHRSP may also contribute to the reduction in infarct size with HET0016 treatment by reducing the driving force for edema formation.
Surprisingly, inhibition of 20-HETE synthesis had no effect on baseline rCBF or the fall in rCBF during MCAO. However, it completely prevented the hyperperfusion in the hypertensive strains following removal of the MCA occlusion. Postischemic cerebral hyperperfusion has been previously reported in cats, rats, and rabbits and has been correlated with the duration or severity of ischemia (14, 23, 27, 34, 48, 54, 58). This phenomenon has been associated with vasoparalysis indicated by a loss of CBF autoregulation and vascular responsiveness to CO2 (18). There is evidence that hyperperfusion following transient cerebral ischemia may contribute to cerebral ischemia-reperfusion injury through the disruption of the blood-brain barrier and edema formation (27, 34) as well as by promoting infiltration of macrophages, inflammation, and increased reactive oxygen species (ROS) formation (27, 34, 58, 60). The mechanism by which the inhibition of 20-HETE synthesis prevents the observed hyperperfusion following cerebral ischemia in SHR and SHRSP cannot be determined by the present results. However, 20-HETE has recently been shown to stimulate formation of ROS by interactions with NADPH oxidase and uncoupling of NO synthase (NOS) (4, 39, 62), and there is evidence that the phenomenon of postischemic hyperperfusion may be associated with impairment of vascular reactivity by the elevated production of ROS in cerebral ischemia and reperfusion (27, 34, 48, 58). This suggests that the effect of the 20-HETE inhibitor on postischemic cerebral hyperperfusion and on infarct size in SHR and SHRSP may be due to a reduction in cerebral ROS production, which may in itself be neuroprotective, and also may reduce secondary neural damage associated with vascular leakage and cerebral edema and inflammation.
In support of this hypothesis, we found that basal cerebral vascular O2•− formation is elevated in SHRSP, and that inhibition of 20-HETE synthesis reduced O2•− formation by 60% in SHRSP to a level similar to that observed in WKY. In addition, chronic 20-HETE synthesis inhibition restored endothelium-dependent dilation in cerebral arteries of SHRSP, suggesting that 20-HETE plays a role in generating oxidative stress and vascular dysfunction in SHRSP.
In summary, the results of the present study indicate that CYP4A expression and 20-HETE production are elevated in the cerebral vasculature of SHRSP and that blockade of the synthesis of 20-HETE markedly reduces infarct size, postischemic cerebral hyperperfusion, and cerebral vascular ROS formation and endothelial dysfunction seen in this strain. These findings are consistent with the view that that elevated vascular production of 20-HETE contributes to the increased sensitivity to cerebral ischemia-reperfusion injury in SHRSP.
GRANTS
This work was partially supported by National Heart, Lung, and Blood Institute Grants HL-59996 and HL-29547.
Acknowledgments
We thank Kate Friedrich for technical assistance with the RT-PCR measurements of the expression of CYP4A isoforms.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe K, Kogure K, Yamamoto H, Imazawa M, Miyamoto K. Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J Neurochem 48: 503–509, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res 29: 813–820, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke 34: 1269–1275, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol 294: H1018–H1026, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Coyle P Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke 17: 520–525, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Coyle P Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY). Anat Rec 218: 40–44, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Coyle P, Heistad DD. Blood flow through cerebral collateral vessels in hypertensive and normotensive rats. Hypertension 8: II67–II71, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Coyle P, Heistad DD. Blood flow through cerebral collateral vessels one month after middle cerebral artery occlusion. Stroke 18: 407–411, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Coyle P, Jokelainen PT. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke 14: 605–611, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19: 4008, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escalante B, Sacerdoti D, Davidian MM, Laniado-Schwartzman M, McGiff JC. Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension 17: 776–779, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Fischer EG, Ames A 3rd, Lorenzo AV. Cerebral blood flow immediately following brief circulatory stasis. Stroke 10: 423–427, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol 507: 771–781, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebremedhin D, Ma YH, Imig JD, Harder DR, Roman RJ. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res 30: 53–60, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gratton JA, Sauter A, Rudin M, Lees KR, McColl J, Reid JL, Dominiczak AF, Macrae IM. Susceptibility to cerebral infarction in the stroke-prone spontaneously hypertensive rat is inherited as a dominant trait. Stroke 29: 690–694, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther 321: 18–27, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Hacein-Bey L, Harder DR, Meier HT, Varelas PN, Miyata N, Lauer KK, Cusick JF, Roman RJ. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol 27: 1350–1354, 2006. [PMC free article] [PubMed] [Google Scholar]
- 22.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol Heart Circ Physiol 266: H1027–H1033, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Heiss WD, Graf R, Lottgen J, Ohta K, Fujita T, Wagner R, Grond M, Weinhard K. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J Cereb Blood Flow Metab 17: 388–400, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Heistad DD, Mayhan WG, Coyle P, Baumbach GL. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels 27: 258–262, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Ishizuka T, Ito O, Omata K, Ito S. Role of androgens in the renal production of 20-hydroxyeicosatetraenoic acid in spontaneously hypertensive rats. [In Japanese.] Nippon Jinzo Gakkai Shi 46: 685–692, 2004. [PubMed] [Google Scholar]
- 26.Jeffs B, Clark JS, Anderson NH, Gratton J, Brosnan MJ, Gauguier D, Reid JL, Macrae IM, Dominiczak AF. Sensitivity to cerebral ischaemic insult in a rat model of stroke is determined by a single genetic locus. Nat Genet 16: 364–367, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Kastrup A, Engelhorn T, Beaulieu C, de Crespigny A, Moseley ME. Dynamics of cerebral injury, perfusion, and blood-brain barrier changes after temporary and permanent middle cerebral artery occlusion in the rat. J Neurol Sci 166: 91–99, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol 282: H1556–H1565, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 33: 1353–1358, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kroetz DL, Huse LM, Thuresson A, Grillo MP. Developmentally regulated expression of the CYP4A genes in the spontaneously hypertensive rat kidney. Mol Pharmacol 52: 362–372, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Kunievsky B, Bazan NG, Yavin E. Generation of arachidonic acid and diacylglycerol second messengers from polyphosphoinositides in ischemic fetal brain. J Neurochem 59: 1812–1819, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 272: 27345–27352, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Lin F, Rios A, Falck JR, Belosludtsev Y, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 269: F806–F816, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab 19: 467–482, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, Cambj-Sapunar L, Harder DR, Roman RJ. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 314: 77–85, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA 95: 12701–12706, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke 20: 1037–1043, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke 38: 3016–3022, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol Regul Integr Comp Physiol 276: R1691–R1700, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol 137: 1362–1370, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omata K, Abraham NG, Schwartzman ML. Renal cytochrome P-450-arachidonic acid metabolism: localization and hormonal regulation in SHR. Am J Physiol Renal Fluid Electrolyte Physiol 262: F591–F599, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, Roman RJ. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke 37: 1307–1313, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Ooshima A, Yamori Y, Okamoto K. Cardiovascular lesions in the selectively-bred group of spontaneously hypertensive rats with severe hypertension. Jpn Circ J 36: 797–812, 1972. [DOI] [PubMed] [Google Scholar]
- 44.Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J Appl Physiol 100: 1117–1123, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 26: 1551–1561, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O′Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: 25–146, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Sacerdoti D, Abraham NG, McGiff JC, Schwartzman ML. Renal cytochrome P-450-dependent metabolism of arachidonic acid in spontaneously hypertensive rats. Biochem Pharmacol 37: 521–527, 1988. [DOI] [PubMed] [Google Scholar]
- 48.Shiroyama Y, Nagamitsu T, Yamashita K, Yamashita T, Abiko S, Ito H. Changes in brain stem blood flow under various grades of brain stem ischemia. Tohoku J Exp Med 164: 237–246, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50: 123–129, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Smeda JS, VanVliet BN, King SR. Stroke-prone spontaneously hypertensive rats lose their ability to auto-regulate cerebral blood flow prior to stroke. J Hypertens 17: 1697–1705, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Stec DE, Trolliet MR, Krieger JE, Jacob HJ, Roman RJ. Renal cytochrome P4504A activity and salt sensitivity in spontaneously hypertensive rats. Hypertension 27: 1329–1336, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid ω-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regul Integr Comp Physiol 275: R426–R438, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Suno M, Kakihana M, Shibota M, Nagaoka A. Mechanism of increased sensitivity to cerebral ischemia following carotid artery occlusion in stroke-prone spontaneously hypertensive rats: importance of genetic factors. Stroke 12: 246–250, 1981. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi A, Park HK, Melgar MA, Alcocer L, Pinto J, Lenzi T, Diaz FG, Rafols JA. Cerebral cortex blood flow and vascular smooth muscle contractility in a rat model of ischemia: a correlative laser Doppler flowmetric and scanning electron microscopic study. Acta Neuropathol (Berl) 93: 354–368, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi K, Renic M, Bohman QC, Harder DR, Miyata N, Roman RJ. Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am J Physiol Heart Circ Physiol 289: H2203–H2211, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka E, Niiyama S, Sato S, Yamada A, Higashi H. Arachidonic acid metabolites contribute to the irreversible depolarization induced by in vitro ischemia. J Neurophysiol 90: 3213–3223, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka Y, Omura T, Fukasawa M, Horiuchi N, Miyata N, Minagawa T, Yoshida S, Nakaike S. Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats. Neurosci Res 59: 475–480, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Traupe H, Kruse E, Heiss WD. Reperfusion of focal ischemia of varying duration: postischemic hyper- and hypo-perfusion. Stroke 13: 615–622, 1982. [DOI] [PubMed] [Google Scholar]
- 59.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 31: 242–247, 1998. [DOI] [PubMed] [Google Scholar]
- 60.van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW. Cerebral hyperperfusion syndrome. Lancet Neurol 4: 877–888, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Wang MH, Zhang F, Marji J, Zand BA, Nasjletti A, Laniado-Schwartzman M. CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am J Physiol Regul Integr Comp Physiol 280: R255–R261, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Xu F, Straub WO, Pak W, Su P, Maier KG, Yu M, Roman RJ, Ortiz De Montellano PR, Kroetz DL. Antihypertensive effect of mechanism-based inhibition of renal arachidonic acid omega-hydroxylase activity. Am J Physiol Regul Integr Comp Physiol 283: R710–R720, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Yamori Y Importance of genetic factors in stroke: an evidence obtained by selective breeding of stroke-prone and -resistant SHR. Jpn Circ J 38: 1095–1100, 1974. [DOI] [PubMed] [Google Scholar]
- 66.Yamori Y, Tomimoto K, Ooshima A, Hazama F, Okamoto K. Proceedings: developmental course of hypertension in the SHR-substrains susceptible to hypertensive cerebrovascular lesions. Jpn Heart J 15: 209–210, 1974. [DOI] [PubMed] [Google Scholar]
- 67.Zhang F, Chen CL, Qian JQ, Yan JT, Cianflone K, Xiao X, Wang DW. Long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats by gene delivery of rAAV-mediated cytochrome P450 arachidonic acid hydroxylase. Cell Res 15: 717–724, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F, Wang MH, Krishna UM, Falck JR, Laniado-Schwartzman M, Nasjletti A. Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 38: 1311–1315, 2001. [DOI] [PubMed] [Google Scholar]