Abstract
Bone marrow mesenchymal stem cells (MSCs) may be a novel treatment modality for organ ischemia, possibly through the release of beneficial paracrine factors. However, an age threshold likely exists as to when MSCs gain their beneficial protective properties. We hypothesized that 1) VEGF would be a crucial stem cell paracrine mediator in providing postischemic myocardial protection and 2) small-interfering (si)RNA ablation of VEGF in adult MSCs (aMSCs) would equalize the differences observed between aMSC- and neonatal stem cell (nMSC)-mediated cardioprotection. Female adult Sprague-Dawley rat hearts were subjected to ischemia-reperfusion injury via Langendorff-isolated heart preparation (15 min equilibration, 25 min ischemia, and 60 min reperfusion). MSCs were harvested from adult and 2.5-wk-old neonatal mice and cultured under normal conditions. VEGF was knocked down in both cell lines by VEGF siRNA. Immediately before ischemia, one million aMSCs or nMSCs with or without VEGF knockdown were infused into the coronary circulation. The cardiac functional parameters were recorded. VEGF in cell supernatants was measured via ELISA. aMSCs produced significantly more VEGF than nMSCs and were noted to increase postischemic myocardial recovery compared with nMSCs. The knockdown of VEGF significantly decreased VEGF production in both cell lines, and the pretreatment of these cells impaired stem cell-mediated myocardial function. The knockdown of VEGF in adult stem cells equalized the myocardial functional differences observed between adult and neonatal stem cells. Therefore, VEGF is a critical paracrine mediator in facilitating postischemic myocardial recovery and likely plays a role in mediating the observed age threshold during stem cell therapy.
Keywords: ischemia-reperfusion
coronary artery disease is a source of tremendous morbidity and mortality (28). The medical management of the more severe cases of cardiac ischemia is often inadequate, thereby warranting revascularization via percutaneous stenting or arterial bypass. The subsequent reperfusion injury associated with the ischemic episode is also detrimental and results in significant aberrations to the inflammatory cascade (3, 8, 22). In this regard, bone marrow mesenchymal stem cells (MSCs) represent a novel treatment modality with increasing therapeutic potential (1, 12, 17, 23, 27).
Many studies have previously demonstrated that MSC differentiation is not absolutely required for stem cell-mediated tissue protection (18). The infusion of MSCs into myocardium subjected to acute ischemia and reperfusion injury was noted to improve functional recovery, decrease proinflammatory cytokine production, and decrease the activation of proapoptotic caspases in the absence of differentiation and incorporation into the myocardium (34, 38). This led to the important appreciation that stem cells release a variety of paracrine factors which may confer protection to injured cardiac tissue (5, 34, 41).
Although MSCs from hosts of varying ages are able to show multipotent potential, the increasing age of stem cells and their hosts has been associated with a decreased functional capacity under conditions of stress (2, 29). Specifically, older age has been associated with telomere shortening and dysfunction, mesenchymal progenitor cell dysfunction, a reduced capacity of bone marrow stromal cells to maintain functional hematopoeitic stem cells, and noted changes in cytokine production (15). Despite the proposed benefits of younger stem cells, we have observed that neonatal stem cells produce lower levels of vascular endothelial growth factor (VEGF) in cell culture and do not confer acute protection to ischemic myocardium (20, 21). This has led to the appreciation that an age threshold may exist as to when younger MSCs gain their protective properties.
In this regard, elevated levels of circulating VEGF may be an important mediator in preserving myocardial function following injury. Therefore, the lower levels of VEGF secreted by neonatal MSCs (nMSCs) may play a role in the lack of cardioprotection that they can afford. We therefore hypothesized that 1) VEGF would be a crucial stem cell paracrine mediator in providing postischemic myocardial protection and 2) small-interfering (si)RNA ablation of VEGF in adult MSCs (aMSCs) would equalize the differences observed between adult and neonatal stem cell-mediated cardioprotection.
METHODS
Animals.
Normal adult female Sprague-Dawley rats (200–250 g, Harlan, Indianapolis, IN) and adult female C57/BL6J mice (Jackson, Bar Harbor, ME) were fed a standard diet and acclimated in a quiet quarantine room for 1 wk before the experiments. Female neonatal C57/BL6J mice (2.5 wk old, Jackson) were allowed to birth naturally and were kept with their mothers until the time of death. The animal protocol was reviewed and approved by the Indiana Animal Care and Use Committee of the Indiana University. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Preparation of mouse bone marrow stem cells.
A single-step stem cell purification method using adhesion to cell culture plastic was employed as previously described (26). Briefly, nMSCs and aMSCs were collected from bilateral femurs and tibias after death by removing the epiphyses and flushing the shaft with complete media [Iscove's modified Dulbecco's medium (GIBCO Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (GIBCO Invitrogen)] using a syringe with a 26-gauge needle. Cells were disaggregated by vigorous pipetting several times and were passed through a 30-μm nylon mesh to remove the remaining clumps of tissue. Cells were washed by adding complete media and centrifuged for 5 min at 300 g at 24°C, and the supernatant was removed. The cell pellet was then resuspended and cultured in 75-cm2 culture flasks with complete media at 37°C in 5% CO2-95% room air. MSCs preferentially attached to the polystyrene surface; after 48 h, nonadherent cells in suspension were discarded. Fresh complete media was added and replaced every 3 or 4 days thereafter. When the cultures reached 90% of confluence, the stem cell culture was passaged; cells were recovered by the addition of a solution 0.25% trypsin-EDTA (GIBCO Invitrogen) and replated in flasks. Adult and neonatal stem cells maintained similar MSC markers. Both cell lines were negative for the hematopoietic markers CD34, CD45, and CD117 and were positive for the MSC marker CD44 (21). Cells were used for experimentation between passages 3–7.
VEGF knockdown via siRNA.
siRNA was designed to target the common sequence of the three mouse VEGF-A isoforms. The sense sequence of the 19 nucleotide (plus 2 amino acids overhanging at 5′) VEGF siRNA was 5′-CCG ACG AGA UAG AGU ACAU-3′. The uniqueness of the designed siRNA was examined by using NCBI BLAST. VEGF siRNA as well as scrambled-control siRNA were obtained from Dharmacon (Lafayette, CO).
MSCs were prepared for transfection by seeding 105 cells into each well of a six-well culture plate with complete media. After we allowed the cells to adhere for 24 h, the media was removed, the cells were washed with PBS, and the antibiotic free/low serum Optimem media (GIBCO Invitrogen) was added. siRNA was then complexed with Lipofectamine 2000 (Invitrogen) in Optimem media and allowed to incubate at room temperature for 20 min. Complexes were then added to culture wells to yield a final siRNA concentration of 100 nM/well. After 24 h of incubation, the media and siRNA complexes were removed, normal complete media was added, and the cells were allowed to incubate for an additional 72 h. Transfection did not appear to affect cellular viability since a similar number of cells were recovered from the wells for each experiment.
Isolated heart preparation: Langendorff.
Rats were anesthetized (pentobarbital sodium, 60 mg/kg ip) and heparinized (500 U ip), and the hearts were rapidly excised via median sternotomy and placed in 4°C Krebs-Henseleit solution containing (in mM) 119 NaCl, 20.8 NaHCO3, 11 Dextrose, 12 CaCl2·2H2O, 47 KCl, 11.7 MgSO4·7H2O, and 11.8 KH2PO4. The aorta was cannulated and the heart was perfused under constant pressure (mean, 75 mmHg) with oxygenated (95% O2-5% CO2) Krebs-Henseleit solution (37°C). A left atrial resection was performed before the insertion of a water-filled latex balloon through the atrium into the ventricle. The balloon was initially adjusted to a desired mean end-diastolic pressure (EDP) of 5 mmHg, and the hearts were allowed to equilibrate for 15 min. Pacing wires were fixed to the atrium, and the hearts were paced at ∼6 Hz, 3 V, and 2 ms (350 beats/min) during equilibration and reperfusion to ensure a standard heart rate between groups. A three-way stopcock above the aortic root was used to create warm global ischemia, during which time the heart was placed in a 37°C degassed organ bath. After 25 min of ischemia, the hearts were reperfused for 60 min. The cardiac contractility (+dP/dt), the rate of myocardial relaxation (−dP/dt), and EDPs were continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments, Milford, MA) and an Apple G4 PowerPC computer (Apple Computer, Cupertino, CA).
Experimental isolated heart groups.
Rat hearts were divided into the following groups (n = 5 per group): 1) vehicle control, 2) adult stem cell infusion, 3) neonatal stem cell infusion, 4) adult stem cell VEGF siRNA infusion, 5) adult stem cell Scramble siRNA infusion, 6) neonatal stem cell VEGF siRNA infusion, and 7) neonatal stem cell Scramble siRNA infusion. Stem cells were recovered from 10 culture wells by the addition of 0.25% trypsin-EDTA (GIBCO Invitrogen). The cells were counted with the aid of a hemocytometer and trypan blue exclusion, and one million viable cells were isolated. The cells were centrifuged at 300 g, the media was removed, and the cells were resuspended in 1 ml of Krebs-Henseleit solution (37°C). Over the course of 1 min immediately before ischemia, the MSC solution was infused into the coronary circulation.
Enzyme-linked immunosorbent assay.
VEGF in the MSC supernatant was determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available ELISA set (R&D Systems, Minneapolis, MN). ELISA was performed according to the manufacturer's instructions. All samples and standards were measured in duplicate (n = 6–26/group).
Real-time polymerase chain reaction.
Total RNA was extracted from aMSCs or nMSCs using RNA STAT-60 (TEL-TEST, Friendswood, TX). Total RNA (0.5 μg) was subjected to cDNA synthesis using cloned AMV first-strand cDNA synthesis kit (Invitrogen Life Technologies). cDNA from each sample was analyzed for 18S (assay identification number: Hs99999901_s1) and VEGF-A (assay identification number: Mm00437304_m1) by using TaqMan gene expression assay (RT-PCR) (Applied Biosystems, Foster City, CA). The experiments were repeated to confirm the results.
Presentation of data and statistical analysis.
All reported values are means ± SE, and P < 0.05 was considered statistically significant. Myocardial functional indexes are displayed at end reperfusion, and +dP/dt and −dP/dt are presented as a percentage of baseline during equilibration. Data were compared using two-way ANOVA with post hoc Bonferroni or Student's t-test when appropriate.
RESULTS
MSC VEGF production.
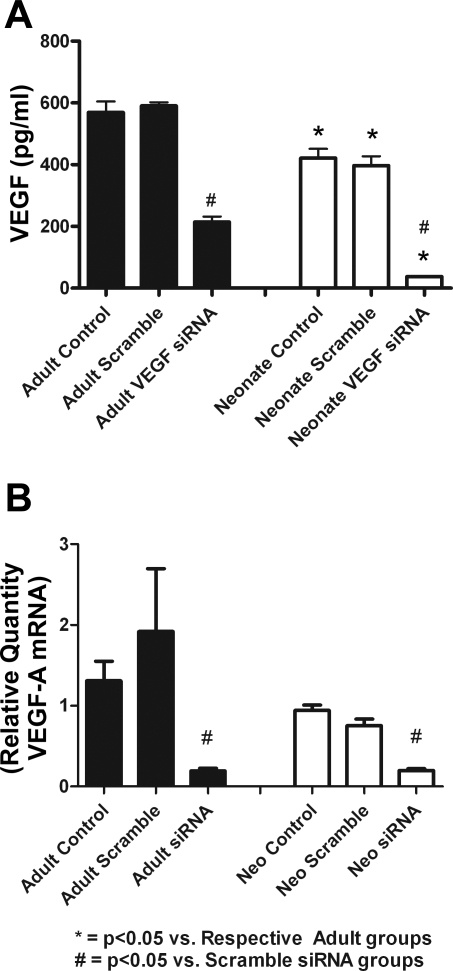
nMSCs produced lower levels of VEGF compared with aMSCs following 72 h of normal culture (Fig. 1A). The transfection of MSCs with VEGF siRNA also significantly decreased cellular VEGF RNA levels in both cell lines compared with Scramble siRNA (Fig. 1B). No significant differences were noted in VEGF production from nontransfected and Scramble siRNA-transfected groups.
Fig. 1.
Adult stem cells produce more VEGF than neonatal stem cells after 72 h of culture. Transfection of cells with VEGF small-interfering (si)RNA significantly decreased VEGF production as seen by ELISA. Transfecting mesenchymal stem cells (MSCs) with Scramble siRNA did not significantly alter VEGF production compared with controls (A). Transfection of VEGF siRNA also significantly decreased adult and neonatal stem cell VEGF RNA expression as seen by PCR (B).
Adult stem cells improve myocardial functional recovery following ischemic injury.
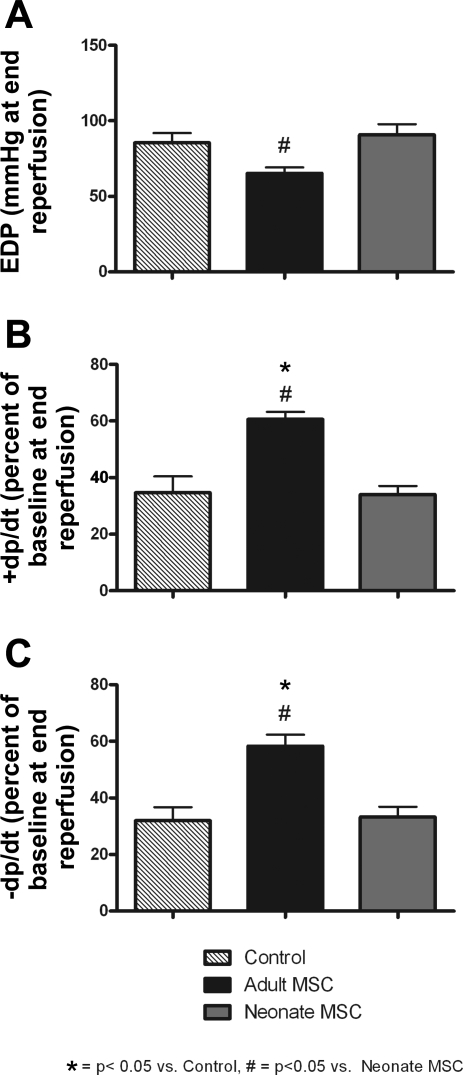
The pretreatment of the myocardium with aMSCs before ischemia resulted in significantly improved postischemic functional recovery compared with vehicle controls. At end reperfusion, +dP/dt and −dP/dt remained significantly higher in the aMSC groups. Although EDP was significantly higher throughout reperfusion in the aMSC group compared with controls (data not shown), there was no significant difference in EDP at end reperfusion (Fig. 2). The infusion of neonatal stem cells before ischemia did not improve the postischemic protection of the injured myocardium. This was denoted by similar myocardial functional indexes between vehicle control hearts and those infused with nMSCs.
Fig. 2.
Intracoronary infusion of adult MSCs (aMSCs) provided significantly better cardioprotection compared with neonatal MSCs (nMSCs). This was seen in end-diastolic pressure (EDP; A), cardiac contractility (+dP/dt; B), and rate of myocardial relaxation (−dP/dt; C) at end reperfusion. These data allowed for the important appreciation that an age threshold may exist as to when stem cells can provide beneficial tissue protection (n = 5 animals/group).
VEGF is a critical component of stem cell-mediated cardioprotection.
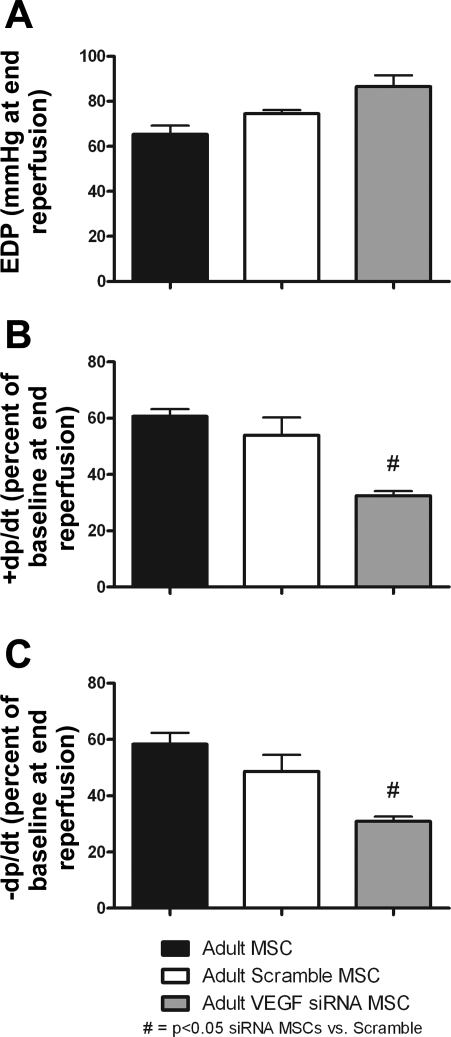
Intracoronary infusion of aMSCs transfected with VEGF siRNA significantly impaired postischemic myocardial function compared with aMSCs transfected with Scramble siRNA. This was noted by a significant decrease in the +dP/dt and −dP/dt at end reperfusion in VEGF siRNA-treated groups. There were no differences in cardiac function at end reperfusion between nontransfected aMSCs and Scramble siRNA aMSC-transfected groups (Fig. 3).
Fig. 3.
Infusion of aMSCs that were transfected with VEGF siRNA significantly impaired postischemic myocardial recovery. End-diastolic pressure (A) was higher, and +dP/dt (B) and −dP/dt (C) were lower during reperfusion after the application of MSCs treated with VEGF siRNA compared with Scramble siRNA groups. No differences in myocardial function were observed between Scramble siRNA-transfected and nontransfected adult stem cell groups at end reperfusion (n = 5 animals/group).
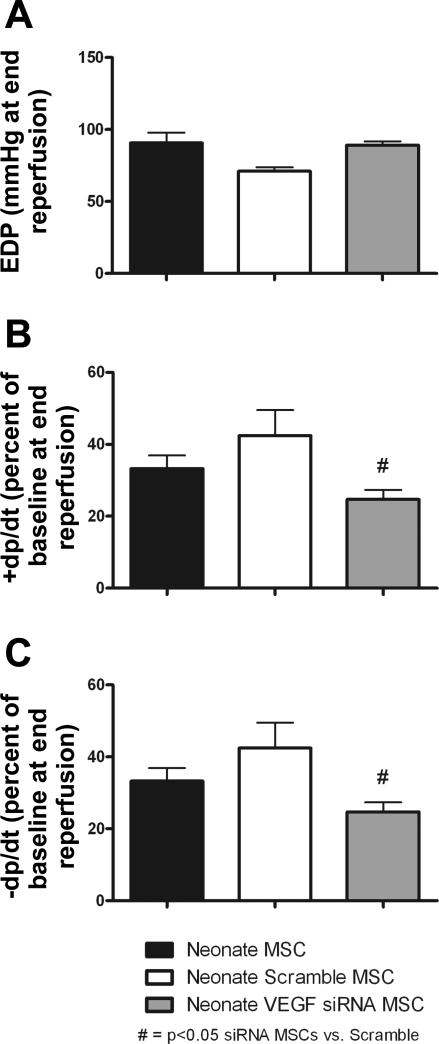
Intracoronary infusion of nMSCs transfected with VEGF siRNA further impaired postischemic myocardial function compared with the infusion of Scramble siRNA-transfected nMSCs. Myocardial impairment was noted by the decreased recovery of +dP/dt and −dP/dt after the infusion of VEGF siRNA-transfected nMSCs. No significant differences were noted in the cardiac function between nontransfected nMSCs and Scramble siRNA-transfected nMSC groups (Fig. 4).
Fig. 4.
Transfection of VEGF siRNA into nMSCs impaired stem cell-mediated myocardial function as noted by increased EDP (A) and a decreased percentage of baseline +dP/dt (B) and −dP/dt (C) during recovery. No significant differences in myocardial function were noted between Scramble siRNA-transfected and nontransfected nMSC groups (n = 5 animals/group).
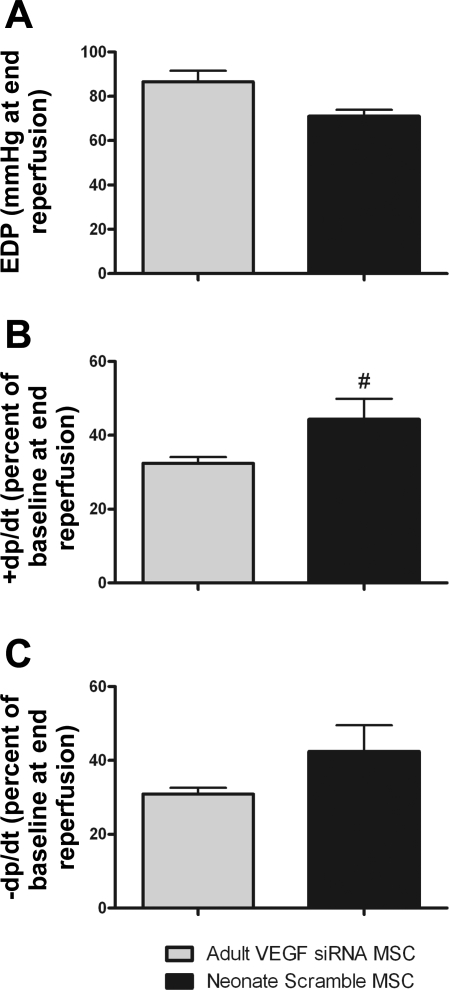
Knockdown of VEGF in adults equalizes the cardioprotective differences observed between aMSCs and nMSCs.
Not only did the application of VEGF siRNA to aMSCs impair their ability to promote postischemic myocardial recovery, but it also equalized the myocardial functional differences observed between adult and neonatal stem cell therapy. Specifically, the average EDP and −dP/dt were equivalent between hearts exposed to VEGF siRNA-transfected aMSCs and Scramble siRNA-transfected nMSCs. Moreover, the average contractility from hearts infused with VEGF siRNA-transfected aMSCs was actually lower at end reperfusion compared with Scramble siRNA-transfected nMSCs (Fig. 5).
Fig. 5.
Transfection of VEGF siRNA into aMSCs equalized the myocardial functional differences seen between adult and neonatal stem cell therapy. This was seen in EDP (A), +dP/dt (B), and −dP/dt (C). In fact, the percent recovery of +dP/dt and −dP/dt was lower in the VEGF siRNA aMSCs compared with Scramble siRNA-transfected nMSCs (n = 5 animals/group). #P < 0.05 vs. adult VEGF siRNA MSC groups.
DISCUSSION
Stem cells have previously been shown to improve myocardial functional recovery following ischemic injury. Herein we confirmed that aMSC infusion increased postischemic myocardial functional recovery, whereas nMSC infusion had no benefit above vehicle controls. In addition, we showed that 1) VEGF RNA and protein production were decreased in MSCs after VEGF knockdown with siRNA, 2) VEGF was a critical component of stem cell-mediated myocardial protection following ischemia, and 3) VEGF may be partially responsible for the age threshold seen in stem cell-mediated tissue protection.
Stem cells are thought to aid injured tissue via several different mechanisms. Some feel that stem cells differentiate into specific end-organ cells, which then become incorporated into the tissue to increase postinjury functional recovery (19). However, others have shown in cardiac, pulmonary, and renal tissue that protection from injury can be achieved in the absence of stem cell differentiation, thereby suggesting that stem cells aid native tissues via cell-cell interactions or via the release of protective paracrine substances during their transit through injured tissue (25, 33, 36, 39). Many studies have applied stem cell therapy in the postischemic setting and have seen mixed results. Our studies, however, used the preischemic application of stem cells to prevent impending myocardial infarction and heart failure. This model may be more applicable to planned ischemic episodes, such as may occur during cardiac surgery or during other operative interventions that may apply major stress to the myocardium. Preischemic stem cell therapy may therefore have the ability to prevent major cardiac morbidity in those patients who are at high risk for surgical intervention.
Stem cell paracrine properties aid the recovery of injured tissue via a variety of mechanisms. These include the production of antioxidants such as catalase, glutathione peroxidase, and manganese superoxide dismutase, which work to decrease the number of damaging oxygen-free radicals present in ischemic tissue (7). In addition, stem cells are an abundant source of growth factors, such as VEGF, hepatocyte growth factor, and insulin-like growth factor-1, which are believed to protect ischemic tissues via the promotion of angiogenesis, the inhibition of apoptosis, and the stimulation of cellular proliferation (4, 37).
VEGF has been shown to chronically inhibit leukocyte/epithelial cell adherence and the effects of chronic inflammation (30). In addition, VEGF promotes angiogenesis during acute inflammation and ischemia and may protect transplanted stem cells during therapy (10, 35, 40). Therefore, elevated local concentrations of VEGF may be beneficial to the injured myocardium. In fact, our group has recently observed that the infusion of recombinant VEGF before planned ischemia improved cardiac function following injury (11). In this regard, the elevated levels of VEGF produced by MSCs may be an important mediator in their ability to provide protection to ischemic tissues (5).
In the present study, we saw that the knockdown of VEGF by siRNA significantly impaired both MSC VEGF production as well as stem cell-mediated myocardial recovery following ischemia. Impaired function was seen by significantly decreased +dP/dt and −dP/dt after VEGF siRNA-transfected stem cell infusion. Although EDP was elevated in the VEGF siRNA groups compared with the Scramble siRNA groups, thereby indicating a higher degree of injury, there were no statistically significant differences in values. The lack of significance in measured EDPs was likely due to the decreased sensitivity of this parameter in the Langendorff model.
Interestingly, the knockdown of VEGF in the aMSCs ablated their cardioprotective properties and functionally made the aMSCs appear like the nMSCs in terms of their ability to facilitate postischemic myocardial recovery. We have shown in this study that VEGF is a critical paracrine factor in stem cell-mediated myocardial protection. Furthermore, aMSCs have been shown to produce, on average, 200 pg/ml more VEGF in culture compared with neonates (21). The noted differences in VEGF production between aMSCs and nMSCs have been associated with differential p38, ERK, and NF-κB activation among these cell lines (21, 24). Despite the differences in VEGF protein levels, VEGF mRNA levels were similar between adults and neonates, suggesting a mechanism for posttranslational modification of VEGF. Given the results of studies demonstrating that the recombinant VEGF application increased myocardial recovery following injury, we suspect that manipulating neonatal stem cells to overexpress VEGF would also facilitate a better myocardial recovery following injury. It therefore stands to reason that neonatal MSCs, due to lower VEGF levels and an activation of different intracellular signaling cascades, might not protect the injured myocardium as well as adult stem cells. This would suggest that VEGF plays an integral role in postischemic cardioprotection and may be involved, at least in part, in facilitating the age threshold seen in stem cell-mediated tissue protection.
Understanding the mechanisms of VEGF-induced tissue protection in the adult stem cell population is of particular interest, since methods to further increase VEGF in the heart after injury would likely be beneficial (11). In this regard, sex hormones have been shown to play a role in stem cell function, tissue protection, and intracellular signaling cascades (6, 14, 16, 32). This protection is thought to be due to the downregulation of proinflammatory cytokines and the upregulation of VEGF through the activation of estrogen receptor-α by endogenous estrogen (9, 13, 31). Because the female adult stem cell hosts used in this study had achieved sexual maturity, their levels of endogenous estrogen were higher than the neonatal hosts before stem cell harvest. Therefore, chronic in vivo exposure to estrogen in the adult stem cell population may have enhanced VEGF production within these cells, thereby providing them with a greater potential for therapeutic protection. It is therefore highly plausible that the activation of intracellular signaling cascades associated with adolescence has a positive effect on stem cells and may work to increase the protective properties of stem cells during therapeutic intervention. Further studies examining sex differences in neonatal stem cell cytokine production are therefore warranted to determine the role that sex hormones play in stem cell activation.
In conclusion, MSCs confer postischemic cardioprotection by a VEGF-dependent mechanism. VEGF also appears to play a role in facilitating the age threshold seen in stem cell-mediated tissue protection. It is likely, though, that VEGF is not the only paracrine factor responsible for the differences observed between adult and neonatal stem cell function. Further studies designed to explore the effects that these other mediators induce is therefore warranted. Defining the genes that initiate protective signaling mechanisms within younger stem cells may allow for the genetic amplification of these vital genes in autologous stem cells, thereby allowing for the generation of “super stem cells” that provide maximum protection to ischemic tissues.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-GM-070628, R01-HL-085595, K99/R00-HL-0876077-01, and F32-HL-085982 and an American Heart Association (AHA) grant-in-aid and an AHA Postdoctoral Fellowship 0526008Z.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol 40: 1089–1099, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ballard VL, Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res 100: 1116–1127, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, Fullerton DA, Meng X. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg 85: 1678–1685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3: e1886, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol 42: 142–149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock 26: 571–574, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104: 3591–3597, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA, Pomerantz BJ. Proinflammatory cytokines in heart disease. Blood Purif 19: 314–321, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 27: 151–156, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954–958, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Guzman MJ, Crisostomo PR, Wang Y, Wang M, Markel TA, Erwin GE, Meldrum DR. Vascular endothelial growth factor improves myocardial functional recovery following ischemia/reperfusion injury. J Surg Res. In press. [DOI] [PubMed]
- 12.Haider H, Ashraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol 38: 225–235, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh YC, Frink M, Thobe BM, Hsu JT, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. 17Beta-estradiol downregulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Mol Immunol 44: 2165–2172, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med 13: 742–747, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kan WH, Hsu JT, Ba ZF, Schwacha MG, Chen J, Choudhry MA, Bland KI, Chaudry IH. p38 MAPK-dependent eNOS upregulation is critical for 17β-estradiol-mediated cardioprotection following trauma-hemorrhage. Am J Physiol Heart Circ Physiol 294: H2627–H2636, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology 132: 944–954, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. In press. [DOI] [PubMed]
- 19.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation 116: I46–I54, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markel TA, Crisostomo PR, Manukyan MC, Al-Azzawi D, Herring CM, Lahm T, Novotny NM, Meldrum DR. Are neonatal stem cells as effective as adult stem cells in providing ischemic protection? J Surg Res. In press. [DOI] [PMC free article] [PubMed]
- 21.Markel TA, Wang M, Crisostomo PR, Manukyan MC, Poynter JA, Meldrum DR. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling that distinguish them from their adult counterparts. Am J Physiol Regul Integr Comp Physiol 294: R1491–R1497, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Meldrum DR Tumor necrosis factor in the heart. Am J Physiol Regul Integr Comp Physiol 274: R577–R595, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Nagy RD, Tsai BM, Wang M, Markel TA, Brown JW, Meldrum DR. Stem cell transplantation as a therapeutic approach to organ failure. J Surg Res 129: 152–160, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Novotny NM, Markel TA, Crisostomo PR, Meldrum DR. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NF-kB. Cytokine 43: 215–219, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Patel KM, Crisostomo P, Lahm T, Markel T, Herring C, Wang M, Meldrum KK, Lillemoe KD, Meldrum DR. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res 143: 281–285, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Raeburn CD, Zimmerman MA, Arya J, Banerjee A, Harken AH. Stem cells and myocardial repair. J Am Coll Surg 195: 686–693, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69–e171, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Scalia R, Booth G, Lefer DJ. Vascular endothelial growth factor attenuates leukocyte-endothelium interaction during acute endothelial dysfunction: essential role of endothelium-derived nitric oxide. FASEB J 13: 1039–1046, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene 23: 1052–1063, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Szalay L, Shimizu T, Suzuki T, Hsieh YC, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Androstenediol administration after trauma-hemorrhage attenuates inflammatory response, reduces organ damage, and improves survival following sepsis. Am J Physiol Gastrointest Liver Physiol 291: G260–G266, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Von Dobschuetz E, Meyer S, Thorn D, Marme D, Hopt UT, Thomusch O. Targeting vascular endothelial growth factor pathway offers new possibilities to counteract microvascular disturbances during ischemia/reperfusion of the pancreas. Transplantation 82: 543–549, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Wairiuko GM, Crisostomo PR, Wang M, Morrell ED, Meldrum KK, Lillemoe KD, Meldrum DR. Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reperfusion injury. J Surg Res 141: 241–246, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 291: R880–R884, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock 25: 454–459, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock 25: 454–459, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 42: 441–448, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]