Abstract
Ischemic tolerance decreases with aging, and the cardioprotective effect of ischemic preconditioning (IPC) is impaired in middle-aged animals. We have demonstrated that short-term caloric restriction (CR) improves myocardial ischemic tolerance in young and old animals via the activation of adiponectin-AMP-activated protein kinase (AMPK)-mediated signaling. However, it is unknown whether prolonged CR confers cardioprotection in a similar manner. Furthermore, little is known regarding the myocardial expression of silent information regulator 1 (Sirt1; which reportedly mediates various aspects of the CR response) with prolonged CR. Thus, 6-mo-old male Fischer-344 rats were randomly divided into ad libitum (AL) and CR groups. Six months later, isolated perfused hearts were subjected to 25 min of global ischemia followed by 120 min of reperfusion with or without IPC. CR improved the recovery of left ventricular function and reduced infarct size after ischemia-reperfusion and restored the IPC effect. Serum adiponectin levels increased, but myocardial levels of total and phosphorylated AMPK did not change with prolonged CR. Total levels of Sirt1 did not change with CR; however, in the nuclear fraction, CR significantly increased Sirt1 and decreased acetyl-histone H3. Eleven rats from each group were given N-nitro-l-arginine methyl ester in their drinking water for 4 wk before death. In these hearts, chronic inhibition of nitric oxide synthase prevented the increase in nuclear Sirt1 content by CR and abrogated CR-induced cardioprotection. These results demonstrate that 1) prolonged CR improves myocardial ischemic tolerance and restores the IPC effect in middle-aged rats and 2) CR-induced cardioprotection is associated with a nitric oxide-dependent increase in nuclear Sirt1 content.
Keywords: aging, myocardial ischemia, nutrition, preconditioning, reperfusion injury, silent information regulator 1
several clinical studies have demonstrated that morbidity and mortality after myocardial infarction are higher in the elderly (9, 31). Hearts from senescent animals are more susceptible to ischemia than those from young animals (7, 20, 45). Recently, a number of investigators have demonstrated that the cardioprotective effect of ischemic preconditioning (IPC) is impaired in aged animals (1, 2, 28, 40, 46). Aging also loses the cardioprotection afforded by postconditioning (8). Both the increased susceptibility to ischemia and loss of IPC/postconditioning in aged hearts might reflect a generalized deterioration in the innate adaptive response of tissues against various stresses with aging. Therefore, discovering novel interventions that improve ischemic tolerance and/or restore the cardioprotective effect of IPC/postconditioning in the aged heart would have considerable clinical implications.
Caloric restriction (CR) has been widely investigated as a powerful intervention that can prevent and reverse senescent changes (25, 32, 48). Increasing evidence has demonstrated that lifelong CR profoundly affects the physiological and pathophysiological alterations associated with aging and markedly increases lifespan in several species including mammals (25, 32, 48). We have demonstrated that short-term CR (4 wk) confers cardioprotection in both young and aged rat hearts and that, in both cases, the protection is independent of the opening of ATP-sensitive K+ channels (42). Using adiponectin antisense transgenic mice, we have shown that the increase in circulating adiponectin levels, especially in the high-molecular-weight form of adiponectin, is necessary for the cardioprotective effects of short-term CR (43). We have also found that subsequent activation of AMP-activated protein kinase (AMPK) plays an obligatory role in this cardioprotection.
However, fundamental questions pertaining to CR remain unresolved. Specifically, first, is the protective effect of CR against myocardial ischemia-reperfusion injury sustained over the long term? And, second, if so, what mechanisms are involved? Although short-term CR has been found to be protective, the effects of prolonged CR on myocardial ischemia-reperfusion injury are unknown. Furthermore, the mechanism by which life-long CR attenuates the physiological and pathophysiological alterations associated with aging and increases both the average and maximal lifespan has not been fully clarified. In the past decade, silent information regulator 2 (Sir2; a longevity gene) has been reported to mediate lifespan extension by CR in lower organisms such as Caenorhabditis elegans (16, 34, 49). In addition, increasing evidence indicates that mammalian sirtuins (the mammalian orthologs of Sir2) regulate various aspects of the CR response, namely, glucose homeostasis, insulin secretion, fat metabolism, stress resistance, and physical activity. Sirt1, one of the mammalian orthologs of Sir2, is a NAD-dependent deacetylase and prevents apoptosis in cardiac myocytes (6). Several investigations have suggested that sirtuin-activating compounds, such as resveratrol, have great potential as a novel therapeutic approach to attenuate myocardial ischemia-reperfusion injury (13, 24, 26). However, little is known regarding the myocardial expression of Sirt1 with aging and prolonged CR. Furthermore, evidence that Sirt1 plays a role in the cardioprotection afforded by prolonged CR is still lacking.
In the present study, we subjected 6-mo-old rats to 6 mo of CR (prolonged CR) to address the following questions: 1) whether prolonged CR improves myocardial ischemic tolerance and restores the development of IPC in middle-aged rats, 2) whether adiponectin-AMPK signaling is activated in middle-aged rat hearts subjected to prolonged CR, 3) whether prolonged CR affects the myocardial expression of Sirt1, and 4) whether the change in the subcellular localization of Sirt1 is associated with the cardioprotection afforded by prolonged CR. Our results demonstrate, for the first time, the cardioprotective effect of prolonged CR in ischemia-reperfusion injury.
METHODS
All procedures in the present study conformed to principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committee of Keio University School of Medicine.
CR protocols.
Male Fischer-344 rats of 24 wk old of age were obtained from Charles River Japan. Rats were housed in individual cages according to institutional protocols at the Keio University Experimental Animal Center and fed ad libitum (AL) for 2 wk with modified semipurified diet A (Oriental Yeast, Tokyo, Japan). The average caloric intake was calculated from the daily food intake over 2 wk. After being weaned, 26-wk-old rats were randomly divided into two groups: AL rats continued to be fed AL using control diet A for the next 26 wk, whereas CR rats were fed with 90% of the average value of caloric intake during the AL period for 2 wk (10% restriction) followed by 65% of that for 24 wk (35% restriction) using modified semipurified diets B and C, respectively, which were enriched in vitamins and minerals. Thus, the daily intake of vitamins and minerals was constant during the CR period. A total of 44 rats were assigned to the AL and CR groups (n = 32 rats/group).
Langendorff perfusion of hearts.
Rats of 52 wk of age were anesthetized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg). Hearts were excised quickly and perfused with modified Krebs-Henseleit buffer gassed with 95% O2-5% CO2 at 37°C according to the Langendorff procedure (42, 45, 46). The coronary perfusion pressure was maintained at 70 mmHg.
Measurements of left ventricular function.
A plastic catheter with a latex balloon was inserted into the left ventricle (LV) through the left atrium. Before the induction of ischemia, hearts were paced at 5 Hz, and the LV end-diastolic pressure (LVEDP) was adjusted to 10 mmHg by filling the balloon with water. Pacing was turned off during global ischemia and turned on 10, 20, and 30 min after reperfusion to measure the recovery of LV function. Indexes of LV function [LV systolic pressure (LVSP); LV developed pressure (LVDP; equal to LVSP − LVEDP); and LV peak positive and negative dP/dt] were recorded as previously described (42, 45, 46).
Ischemia-reperfusion injury and IPC.
All hearts underwent 10 min of washout perfusion in nonrecirculating mode followed by 10 min of initial perfusion in recirculating mode. Isolated perfused hearts from the AL or CR group were then subjected to 25 min of global ischemia followed by 30 min of reperfusion (n = 6 each) without IPC. In the second series of the experiments, IPC was induced with three cycles of 5-min global ischemia and 5-min reperfusion before 25 min of global ischemia (n = 4 each). The perfusate was collected at the end of reperfusion, and total lactate dehydrogenase (LDH) and creatine kinase (CK) activity released into the perfusate was measured by standard enzymatic methods (and expressed as IU/g wet wt of the ventricle). In the third series of the experiments, hearts were subjected to 25 min of ischemia followed by 120 min of reperfusion with or without IPC. Infarct size was identified by triphenyltetrazolium chloride staining and quantitated as previously described (43, 44).
Measurement of serum adiponectin levels.
Rats were fasted overnight, and blood samples were collected from the chest cavity when the hearts were excised. Serum adiponectin levels were measured using commercially available ELISA kits (Otsuka Pharmaceutical Laboratories, Tokyo, Japan).
Tissue sample preparation.
Four hears from each group were used for Western immunoblot analysis. Rats were anesthetised with an overdose of pentobarbital sodium (40 mg/kg). After a state of deep anesthesia had been reached, rats were intubated and ventilated with 100% O2. This procedure avoids artifactual activation of AMPK due to postanesthesia respiratory depression. The heart was then quickly excised, and the LV was stored at −140°C until use. Tissue samples were prepared as previously described (42–44). For the assessment of the subcellular distribution of Sirt1 protein and cytochrome c, cytosolic and nuclear fractions or cytosolic and mitochondrial fractions were prepared according to the manufacturer's instructions.
Western immunoblot analysis.
Standard SDS-PAGE Western immunoblot techniques were used to assess the protein levels of AMPK, phosphorylated AMPK-α at the Thr172 residue, acetyl-CoA carboxylase (ACC), phosphorylated ACC at the Ser79 residue, Sirt1, acetyl-histone H3 (diacetylated at Lys9 and Lys14), and cytochrome c as previously described (42–44). Levels of phosphorylated AMPK-α and phosphorylated ACC were normalized to the total levels of AMPK and ACC in each sample. Cytochrome c release into the cytosol was normalized to the expression levels of GAPDH in each sample. Protein levels of Sirt1 and acetyl-histone H3 were expressed as percentages of the corresponding value in the AL group. Polyclonal antibodies against AMPK, phosphorylated AMPK-α at the Thr172 residue, ACC, phosphorylated ACC at the Ser79 residue, and cytochrome c were purchased from Cell Signaling (Beverly, MA). Polyclonal antibodies against Sirt1, acetyl-histone H3, and p300 were purchased from Upstate (Lake Placid, NY). Polyclonal antibodies against GAPDH were purchased from Chemicon (Temecula, CA).
Measurement of caspase-3 activity.
Caspase-3 activity in the cytosolic fraction was measured using commercially available colorimetric assay kits (R&D Systems, Mineapolis, MN).
Effect of chronic nitric oxide synthase inhibition.
Eleven rats from each group were given N-nitro-l-arginine methyl ester (l-NAME) in their drinking water (200 mg/l) for 4 wk before being killed (AL + l-NAME and CR + l-NAME groups). During l-NAME treatment, the CR protocol was continued, and the average dosage of l-NAME was estimated to be ∼15 mg·kg−1·day−1. This dose of l-NAME was chosen according to previous reports in which near-maximal inhibition of nitric oxide (NO) synthase (NOS) in the body was achieved (27, 41). Four weeks later, isolated hearts were subjected to the same ischemia-reperfusion protocol (n = 7 each). Four hearts from each group were immediately harvested for Western immunoblot analysis to assess total and subcellular Sirt1 protein levels as described above. In some rats, systemic blood pressure was measured by the tail-cuff method before death.
Statistical analysis.
Data are reported as means ± SE. For intragroup comparisons, hemodynamic variables were analyzed by two-way ANOVA (time and group) followed by Scheffè's post hoc test. For intergroup comparisons, data were analyzed by two-way ANOVA (AL vs. CR, with or without IPC, and with or without l-NAME) followed by Scheffè's post hoc test. Western immunoblot data (except for Sirt1 with or without l-NAME) and caspase-3 activity were compared by an unpaired Student's t-test. P < 0.05 was defined as statistically significant. Statistical analyses were performed using Stat-View 5.0 software (SAS Institute, Cary, NC) for Windows.
RESULTS
Effects of CR on body weight, ventricular weight, and serum adiponectin levels.
As a result of the random assignment of rats to the two groups, there were no differences in body weight at baseline between AL and CR groups (Table 1). No rats died during the observation period. Over the course of the study, body weights increased by 40% in the AL group and decreased by 1% in the CR group. Total ventricular weights (right ventricle and LV combined) were smaller in the CR group, but the ratios of total ventricular weight to body weight did not differ between the two groups (Table 1). Serum adiponection levels increased by 82% in the CR group (Table 1).
Table 1.
Body weight, total ventricular weight, and serum adiponectin levels
| AL | CR | |
|---|---|---|
| Body weight at baseline (6 mo old), g | 320±5 | 321±5 |
| Body weight before euthanization (12 mo old), g | 449±5 | 317±7* |
| Total ventricular weight, g | 1.24±0.01 | 0.92±0.01* |
| Total ventricular weight/body weight, % | 0.28±0.01 | 0.29±0.01 |
| Serum adiponectin levels, mg/ml | 5.0±0.2 | 9.1±0.9* |
Values are means ± SE; n = 14 rats in the ad libitum (AL) group and 14 rats in the caloric restriction (CR) group except for serum adiponectin levels, where n = 8 rats in the AL group and 8 rats in the CR group.
P < 0.05 vs. the AL group.
Effect of CR on ischemia-reperfusion injury and IPC.
There were no differences in LV function at baseline between the two groups (Table 2). Compared with the corresponding AL group, CR improved the recovery of LVSP, the percent recovery of LVDP, and LV peak positive and negative dP/dt after reperfusion (Table 2). CR also attenuated the elevation of LVEDP during reperfusion, although there were no differences between the two groups in the elevation of LVEDP at the end of ischemia (data not shown). In the AL group, IPC failed to improve the recovery of LV function after ischemia-reperfusion. In the CR group, IPC tended to improve the recovery of LVSP and the percent recovery of LVDP and attenuate the elevation of LVEDP during reperfusion, but the differences did not reach statistically significance (Table 2). This may be due, at least in part, to the near-complete recovery of function in the CR group without IPC.
Table 2.
Cardiac parameters
| n |
Baseline |
10 min of Reperfusion
|
20 min of Reperfusion
|
30 min of Reperfusion
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVSP | LVEDP | LVDP | +dP/dt | −dP/dt | LVSP | LVEDP | LVSP | LVEDP | LVSP | LVEDP | LVDP | %Recovery of LVDP | %Recovery of +dP/dt | %Recovery of −dP/dt | ||
| AL | ||||||||||||||||
| Without IPC | 6 | 106±5 | 10.2±0.3 | 96±5 | 3,440±220 | 1,910±130 | 70±5 | 26.6±2.5 | 74±5 | 23.9±2.7 | 68±4 | 20.8±2.5 | 47±3 | 49±4 | 54±4 | 57±4 |
| With IPC | 4 | 105±5 | 10.1±0.3 | 95±5 | 3,420±280 | 1,890±150 | 70±5 | 28.5±2.7 | 70±5 | 25.8±2.8 | 67±4 | 23.3±2.7 | 44±4 | 46±5 | 51±5 | 55±5 |
| CR | ||||||||||||||||
| Without IPC | 6 | 106±5 | 10.2±0.3 | 96±5 | 3,410±210 | 1,850±120 | 80±5 | 21.5±2.1 | 84±4* | 14.9±2.0* | 84±4* | 11.3±1.4* | 73±4* | 76±5* | 87±4* | 82±4* |
| With IPC | 4 | 105±3 | 10.4±0.1 | 95±3 | 3,390±270 | 1,880±160 | 88±4 | 15.4±1.8 | 91±3* | 13.1±1.8* | 90±2* | 10.9±1.5* | 79±2* | 83±6* | 91±4* | 86±4* |
| With l-NAME | ||||||||||||||||
| AL | 4 | 107±3 | 10.0±0.1 | 97±3 | 3,410±280 | 1,850±150 | 71±5 | 29.8±2.5 | 70±4 | 23.9±2.3 | 66±4 | 21.5±2.5 | 44±3 | 47±5 | 51±4 | 54±5 |
| CR | 4 | 105±3 | 10.1±0.1 | 95±3 | 3,380±270 | 1,870±140 | 74±5 | 25.2±2.3 | 74±4† | 20.1±2.4 | 71±4† | 18.6±2.4† | 52±6† | 54±4† | 60±4† | 61±4† |
Values are means ± SE; n = no. of animals/group. LVSP, left ventricular (LV) systolic pressure (in mmHg); LVEDP, LV end-diastolic pressure (in mmHg); LVDP, LV developed pressure (in mmHg); +dP/dt, peak positive LV dP/dt (in mmHg/s); −dP/dt, peak negative LV dP/dt (in mmHg/s).
P < 0.05 vs. the AL group;
P < 0.05 vs. the corresponding CR group.
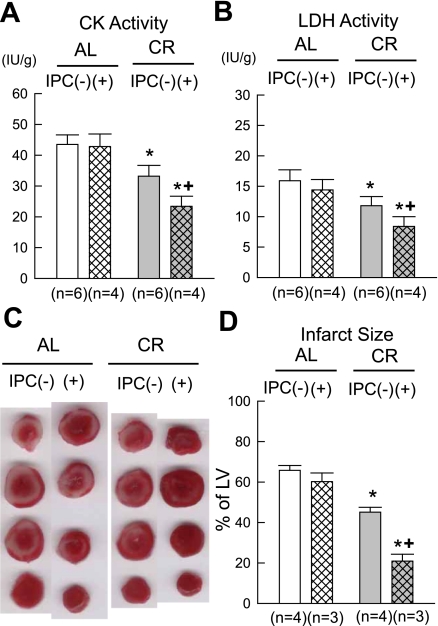
CR significantly attenuated total CK and LDH release during reperfusion compared with the corresponding AL group (Fig. 1, A and B). In the AL group, IPC failed to attenuate total CK and LDH release during reperfusion. In contrast, in the CR group, IPC further attenuated total CK and LDH release (Fig. 1, A and B). Consistent with the results of LDH and CK release, CR significantly reduced infarct size compared with the corresponding AL group (Fig. 1, C and D). In the AL group, IPC failed to reduce infarct size. In contrast, the IPC effect against myocardial infarction was restored in the CR group (Fig. 1, C and D).
Fig. 1.
Effect of prolonged caloric restriction (CR) on lactate dehydrogenase (LDH) and creatine kinase (CK) activity released into the perfusate during reperfusion as well as infarct size. A: CK activity. B: LDH activity. C: representative triphenyltetrazolium chloride staining. D: infarct size. AL group, ad libitum group; IPC, ischemic preconditioning. IPC(−) and IPC(+) groups indicate animals not subjected or subjected to IPC, respectively. Data are expressed as means ± SE. *P < 0.05 vs. the corresponding AL group; +P < 0.05 vs. the corresponding IPC(−) group.
Western immunoblot analyses for AMPK, ACC, and Sirt1.
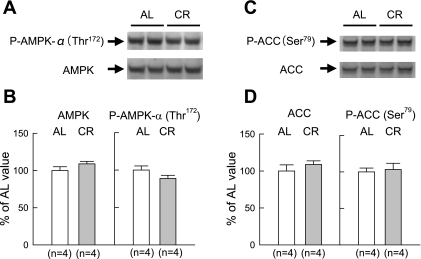
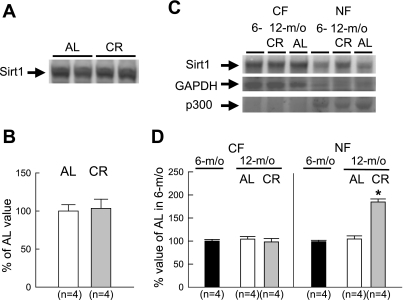
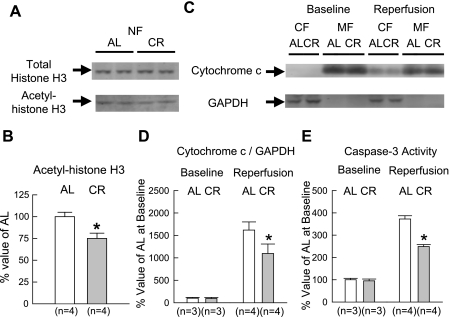
Myocardial levels of both total AMPK protein and AMPK-α phosphorylated at the Thr172 residue were similar between the two groups (Fig. 2). This result differed from our previous finding that phosphorylated AMPK was significantly increased with short-term CR (43). Similar to AMPK phosphorylation, myocardial levels of phosphorylated ACC at the Ser79 residue did not differ between the two groups (Fig. 2). Total myocardial levels of Sirt1 protein were similar between the two groups (Fig. 3, A and B). Western immunoblots showing the expression of GAPDH (cytosolic marker) and p300 (nuclear marker) indicated that the contamination of the nuclear fraction with the cytosolic fraction was minimal (Fig. 3C). Compared with 6-mo-old rats, in 12-mo-old rats, no changes in the expression levels of total Sirt1 and its subcellular distribution were observed in the AL group (Fig. 3, C and D). However, CR significantly increased Sirt1 in the nuclear fraction. Furthermore, acetyl-histone H3 was significantly decreased with CR in the nuclear fraction, suggesting that increased Sirt1 protein is enzymatically active (Fig. 4, A and B).
Fig. 2.
Western immunoblot analyses for AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC). A: representative Western immunoblots showing the expression of phosphorylated (P-)AMPK-α (Thr172) and total AMPK. B: densitometric analysis of P-AMPK-α (Thr172) and total AMPK signals. C: representative Western immunoblots showing the expression of P-ACC (Ser79) and total ACC. D: densitometric analysis of P-ACC (Ser79) and total ACC signals. Densitometric measurements of protein immunoreactivity are expressed as percentages of the average value measured in the AL group. Data are expressed as means ± SE.
Fig. 3.
Western immunoblot analyses for total silent information regulator 1 (Sirt1) protein and its subcellular distribution. A: representative Western immunoblots showing the expression of total Sirt1 protein. B: densitometric analysis of Sirt1 signals. C: representative Western immunoblots showing the expression of Sirt1, GAPDH, and p300 in each fraction. D: densitometric analysis of Sirt1 in each fraction. Densitometric measurements of protein immunoreactivity are expressed as percentages of the average value measured in the AL group. CF, cytosolic fraction; NF, nuclear fraction; m/o, months old. Data are expressed as means ± SE. *P < 0.05 vs. the corresponding AL group.
Fig. 4.
Western immunoblot analyses for histone H3 and cytochrome c as well as caspase-3 activity. A: representative Western immunoblots showing the expression of total and acetyl-histone H3 (Lys9 and Lys14) in the NF. B: densitometric analysis of acetyl-histone H3 (Lys9 and Lys14). C: representative Western immunoblots showing the expression of cytochrome c and GAPDH in each fraction. D: densitometric analysis of cytochrome c/GAPDH in the CF. E: caspase-3 activity in the CF. MF, mitochondrial fraction. Data are expressed as means ± SE. *P < 0.05 vs. the corresponding AL group.
Cytochrome c release and caspase-3 activity.
To evaluate the antiapoptotic effect of prolonged CR, we assessed cytochrome c release into the cytosol and caspase-3 activity in the cytosolic fraction. At baseline, cytochrome c was almost absent in the cytosolic fraction (Fig. 4, C and D). Compared with the AL group, CR significantly attenuated the increase in cytochrome c release into the cytosol after ischemia-reperfusion. Caspase-3 activity in the cytosolic fraction dramatically increased after ischemia-reperfusion in the AL group (Fig. 4E): CR significantly attenuated this increase to 37% of the AL group.
Effect of chronic NOS inhibition.
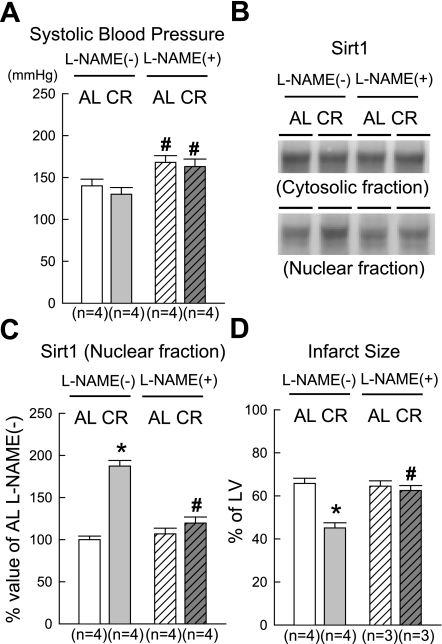
The administration of l-NAME for 4 wk did not significantly change body weights or total ventricular weights in middle-aged rats (data not shown). Chronic inhibition of NOS significantly elevated systolic blood pressure in both groups to the same extent (Fig. 5A). In the AL group, the administration of l-NAME did not affect Sirt1 in the nuclear fraction (Fig. 5, B and C); in contrast, it prevented the CR-induced increase in nuclear Sirt1 (CR + l-NAME group). There were no significant differences in LV function at baseline between groups with and without l-NAME treatment (Table 2). l-NAME did not affect the recovery of LV function or infarct size after ischemia-reperfusion in the AL group but completely abrogated CR-induced cardioprotection in the CR group (Table 2 and Fig. 5D).
Fig. 5.
Effect of chronic administration of N-nitro-l-arginine methyl ester (l-NAME) on systolic blood pressure, nuclear Sirt1, and CR-induced cardioprotection. A: systolic blood pressure measured by the tail-cuff method. B: representative Western immunoblots showing the expression of Sirt1 in each fraction. C: densitometric analysis of Sirt1 in the NF. D: infarct size. l-NAME(−) and l-NAME(+) groups indicate animals not treated or treated with l-NAME, respectively. Data are means ± SE. *P < 0.05 vs. the corresponding AL group; #P < 0.05 vs. the corresponding l-NAME(−) group.
DISCUSSION
This study provides several major findings. In middle-aged rats, 1) prolonged (6 mo) CR improves the recovery of LV function and limits infarct size after ischemia-reperfusion, 2) prolonged CR also restores the protectve effects of IPC, 3) different mechanisms are involved in the cardioprotection afforded by short-term (4 wk) vs. prolonged (6 mo) CR, 4) the cardioprotection afforded by prolonged CR is associated with changes in the subcellular localization of Sirt1, and 5) NOS activity is necessary for the increase in nuclear Sirt1 content during prolonged CR.
Effect of CR on myocardial ischemia-reperfusion injury and IPC.
Most investigations have concluded that myocardial ischemic tolerance decreases with age (7, 20, 45). In addition, several experimental and clinical investigations have addressed the issue of whether IPC occurs in aged hearts (1, 2, 28, 40, 46). The efficacy of IPC has been reported to be decreased or lost in senescent patients (2, 28) and in aged animals (1, 40). We have also found that myocardial ischemic tolerance begins to decrease and that IPC is impaired in middle-aged (12 mo old) rats (46).
Mounting evidence has indicated that life-long CR improves not only aging-dependent but also aging-independent physiological responses to various stresses (25, 32, 48). For instance, life-long CR significantly attenuates myocardial oxidative stress during ischemia-reperfusion and the postischemic inflammatory response (15). Broderick et al. (14) reported that 8 mo of CR improves the recovery of aortic flow after ischemia-reperfusion in isolated working rat hearts. Abete et al. (3) demonstrated that 12 mo of CR could restore the cardioprotective effect of IPC in 24-mo-old rat hearts; however, CR did not attenuate myocardial ischemia-reperfusion injury in itself. Long et al. (30) reported similar results in 10-mo-old rats treated with 6 mo of CR. In the present study, 6 mo of CR improved the recovery of LV function after ischemia-reperfusion and restored the IPC effect in 12-mo-old rat hearts (Table 1 and Fig. 1). Together with those of previous reports (3, 14, 30), our results indicate the potential of nutritional interventions aimed at reversing cardiovascular aging. The effects of prolonged CR on postconditioning are unclear at present and need to be evaluated in the future.
Prolonged CR abrogates the activation of AMPK and ACC.
The exact mechanism(s) by which CR counteracts the physiological deterioration in the innate adaptive response to ischemic stress has not been clarified. In the setting of short-term CR, we have established an essential role of the activation of adiponectin-AMPK signaling in CR-induced cardioprotection (43). Adiponectin is one of the most abundant adipocyte-derived hormones (adipokines) and increases significantly with CR (42, 43). AMPK plays an important role in regulating the energy balance in the myocardium; the activation of AMPK during ischemia-reperfusion can reduce ischemia-induced necrosis and apoptosis (23, 39). The increase in the cellular AMP-to-ATP ratio is a major regulator of AMPK activity (23, 38), but adiponectin has also been reported to activate AMPK (21, 38). Most of the beneficial effects of adiponectin are reportedly mediated by AMPK-associated signaling (21). CR might induce mild shortage of energy substrates. It seems reasonable to assume that short-term CR switches off ATP-consuming pathways and switches on ATP-generating pathways via the activation of the adiponectin-AMPK signaling pathway in the myocardium. In the present study, serum adiponectin levels still increased with 6 mo of CR, as seen in short-term CR (Table 1). However, the expression levels of phosphorylated AMPK or phosphorylated ACC did not increase after 6 mo of CR, suggesting that AMPK or ACC were not activated in CR hearts (Fig. 2). This is not inconsistent with the literature because it is still controversial whether the activation of AMPK contributes to the various effects of life-long CR (22). Alterations in cardiac AMPK activity are reportedly associated with cardiac hypertrophy, cardiomyopathy, and Wolf-Parkinson-White syndrome (4, 19), but CR does not induce any pathological cardiac phenotype. Our results suggest that different mechanisms are involved in the cardioprotection afforded by prolonged versus short-term CR. However, we cannot exclude the possibility that the transient activation of AMPK-associated signaling is necessary for the induction of a cardioprotected phenotype during life-long CR. To clarify this issue, pharmacological inhibition of AMPK activity or genetic modification by gene transfer with dominant negative AMPK will be necessary.
Possible involvement of Sirt1 in CR-induced cardioprotection.
The Sirt2 family and sirtuins have been shown to play an important role in various aspects of CR (16, 34, 49). Cohen et al. (18) demonstrated that Sirt1 protein levels are increased in various tissues of rats subjected to CR, including the brain, visceral fat pads, kidney, and liver.
Boily et al. (10) demonstrated that CR did not extend lifespan in Sirt1-null mice. Chen et al. (17) demonstrated that the favorable effect of CR on physical activity is Sirt1 dependent. However, evidence that Sirt1 plays a role in CR-induced cardioprotection is lacking.
Alcendor et al. (6) demonstrated that overexpression of Sirt1 prevents apoptosis induced by serum starvation in cardiac myocytes. Furthermore, they recently reported that low to moderate overexpression of Sirt1 protects the heart from the oxidative stress induced by paraquat (5). In view of the above evidence, it is plausible that CR upregulates Sirt1 protein in the myocardium and confers cardioprotection in a Sirt1-dependent manner. Unexpectedly, we found that myocardial levels of total Sirt1 protein did not change with CR (Fig. 3, A and B). However, CR significantly increased Sirt1 in the nuclear fraction (Fig. 3, C and D). Tanno et al. (47) demonstrated that nuclear shuttling of Sirt1 enhances the deacetylation of histone H3 in C2C12 cells and suppressed apoptosis of C2C12 cells induced by oxidative stress. Although we could not measure its enzymatic activity directly, we found that the increase in Sirt1 in the nuclear fraction was associated with a decrease in acetyl-histone H3 (at Lys9 and Lys14; Fig. 4, A and B). Sirt1 is known to deacetylate histone H3 (at Lys9 and Lys14) and H4 (at Lys16) (47). Accordingly, our findings strongly suggest that the activated form of Sirt1 increases in CR-treated hearts. With regard to apoptosis, the finding that the release of cytochrome c into the cytosol and activation of caspase-3 were attenuated in CR hearts (Fig. 4, C–E) suggests that apoptotic cell death is reduced by prolonged CR (12). The activation of Sirt1 exerts cytoprotective effects mainly by preventing apoptotic cell death, but the involvement of caspase-independent forms of cell death in the salubrious actions of Sirt1 has not been excluded (6). In conclusion, it is plausible that the cardioprotective effects of prolonged CR involve Sirt1. Future studies in which the rate of apoptotic cell death is measured directly and the increase in nuclear Sirt1 content is inhibited will be necessary to clarify this issue.
The increase in nuclear Sirt1 content is NO dependent and essential for CR-induced cardioprotection.
In C2C12 cells, the subcellular localization of Sirt1 is regulated by at least two nuclear localization signals and two nuclear export signals (47). In addition, Tanno et al. (47) demonstrated that the nuclear shuttling of Sirt1 is inhibited by the administration of a phosphatidylinositol 3-kinase (PI3K) inhibitor, suggesting that it requires Akt signaling. We were unable to test this concept because a single administration of a PI3K inhibitor in our in vivo chronic CR model would not achieve sustained effects. Instead, we evaluated the effects of chronic inhibition of NOS by giving CR rats l-NAME in drinking water for 4 wk. Since mounting evidence has indicated that Sirt1 directly activates endothelial NOS (eNOS) and induces eNOS protein in endothelial cells (33, 36), we expected to find that NOS is located downstream of the activation of Sirt1 and that Sirt1-regulated NOS activity contributes, at least in part, to Sirt1-mediated cardioprotection. Contrary to our expectations, the chronic inhibition of NOS completely abrogated not only CR-induced cardioprotection but also the increase in nuclear Sirt1 content (Table 2 and Fig. 5), which suggests that NOS is located upstream, not downstream, of Sirt1 activation. Our results are consistent with those of a previous report by Nisoli et al. (35). They demonstrated that NO donors upregulate the Sirt1 promoter in white adipocytes and that the induction of Sirt1 by CR is reduced in white adipocytes obtained from an eNOS knockout mouse. NOS may play a dual role (trigger and mediator) in the development of CR-induced cardioprotection, as it does in the development of late preconditioning (11). Since l-NAME is a nonselective NOS inhibitor, it is still unknown which isoform of NOS is the most important for this phenomenon. Because the chronic inhibition of NOS significantly elevated systolic blood pressure (Fig. 5A), it is difficult to completely exclude the influence of hemodynamic changes on the subcellular localization of Sirt1 and the loss of CR-induced cardioprotection. However, it seems unlikely that the hemodynamic change caused by l-NAME was the primary cause of either phenomenon. The dosage of l-NAME that we used in the present study was relatively low, such that the increase in systolic blood pressure was mild compared with previous studies (29, 37) of myocardial ischemia. Moreover, elevated blood pressure associated with chronic NOS inhibition did not exacerbate myocardial ischemia-reperfusion injury and affect Sirt1 in the nuclear fraction in the AL + l-NAME group (Fig. 5, B–D). Further investigations will be necessary to clarify the mechanism by which NOS modulates Sirt1.
Clinical implications and conclusions.
This study demonstrates that prolonged CR improves myocardial ischemic tolerance and restores the development of IPC in middle-aged animals. In addition, this study indicates, for the first time, a possible involvement of Sirt1 in CR-induced cardioprotection. The concept that dietary changes are effective in rescuing the myocardium from ischemic damage in middle-aged animals provides a rationale for the clinical application of CR to humans since coronary heart disease develops commonly in middle age. Clearly, the development of CR mimetics that can replicate the cytoprotective effects of CR would be much easier to incorporate into clinical practice than a strict CR protocol. Our study offers further insights into the usefulness of sirtuin-activating compounds as a novel therapeutic approach for treating patients with coronary heart disease.
GRANTS
This work was supported in part by the Vehicle Racing Commemorative Foundation (2005–2008), by the Nateglinide Memorial Toyoshima Research and Education Fund (2007), by the Novartis Foundation for Gerontological Research (2008) (to K. Shinmura), and by National Heart, Lung, and Blood Institute grants HL-55757, HL-70897, HL-76794, and HL-78825 (to R. Bolli).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol 27: 1777–1786, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Abete P, Napoli C, Rengo F. Loss of cardioprotective effects of preinfarction angina in elderly but not in adult patients. Am J Cardiol 88: 721, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol Heart Circ Physiol 282: H1978–H1987, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, Tian R, Goodyear LJ, Berul CI, Ingwall JS, Seidman CE, Seidman JG. Increased alpha2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation 112: 3140–3148, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res 95: 971–980, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation 86: II371–II376, 1992. [PubMed] [Google Scholar]
- 8.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102: 131–135, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 101: 2557–2567, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE 3: e1759, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli R Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bopassa JC, Vandroux D, Ovize M, Ferrera R. Controlled reperfusion after hypothermic heart preservation inhibits mitochondrial permeability transition-pore opening and enhances functional recovery. Am J Physiol Heart Circ Physiol 291: H2265–H2271, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol 465: 115–123, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Broderick TL, Driedzic WR, Gillis M, Jacob J, Belke T. Effects of chronic food restriction and exercise training on the recovery of cardiac function following ischemia. J Gerontol A Biol Sci Med Sci 56: B33–B37, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 280: H2094–H2102, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med 13: 64–71, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science 310: 1641, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol 291: H2557–H2569, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Frolkis VV, Frolkis RA, Mkhitarian LS, Fraifeld VE. Age-dependent effects of ischemia and reperfusion on cardiac function and Ca2+ transport in myocardium. Gerontology 37: 233–239, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab 89: 2563–2568, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab 287: E1032–E1037, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG AMP-activated protein kinase: the guardian of cardiac energy status. J Clin Invest 114: 465–468, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of nitric oxide. Am J Physiol Heart Circ Physiol 282: H1988–H1995, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78: 361–369, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hung LM, Su MJ, Chu WK, Chiao CW, Chan WF, Chen JK. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br J Pharmacol 135: 1627–1633, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension 46: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation 105: 334–340, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Ma JY, Kerr I, Chakravarty S, Dugar S, Schreiner G, Protter AA. Selective inhibition of p38alpha MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. Am J Physiol Heart Circ Physiol 291: H1972–H1977, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev 123: 1411–1413, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The Investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI-2). N Engl J Med 329: 1442–1448, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Masoro EJ Overview of caloric restriction and ageing. Mech Ageing Dev 126: 913–922, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43: 571–579, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Rossoni G, Manfredi B, De Gennaro Colonna V, Berti M, Guazzi M, Berti F. Sildenafil reduces l-NAME-induced severe hypertension and worsening of myocardial ischaemia-reperfusion damage in the rat. Br J Pharmacol 150: 567–576, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans 31: 202–206, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Russell RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 281: H1630–H1636, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Scrogin KE, Hatton DC, Chi Y, Luft FC. Chronic nitric oxide inhibition with l-NAME: effects on autonomic control of the cardiovascular system. Am J Physiol Regul Integr Comp Physiol 274: R367–R374, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol 39: 285–296, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 116: 2809–2817, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA 97: 10197–10202, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y, Guo X, Takayama M. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol 29: 3081–3089, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Hayashi Y, Guo X, Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Circulation 95: 2559–2566, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 116: 641–654, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab 17: 186–191, 2006. [DOI] [PubMed] [Google Scholar]