Abstract
Postnatal decreases in vascular reactivity involve decreases in the thick filament component of myofilament calcium sensitivity, which is measured as the relationship between cytosolic calcium concentration and myosin light chain (MLC20) phosphorylation. The present study tests the hypothesis that downregulation of thick filament reactivity is due to downregulation of myosin light chain kinase (MLCK) activity in adult compared with fetal arteries. Total MLCK activity, calculated as %MLC20 phosphorylated per second in intact arteries during optimal inhibition of myosin light chain phosphatase activity, was significantly less in adult (6.56 ± 0.29%) than in fetal preparations (7.39 ± 0.53%). In situ MLC20 concentrations (μM) in adult (198 ± 28) and fetal arteries (236 ± 44) did not differ significantly. In situ MLCK concentrations (μM), however, were significantly greater in adult (8.21 ± 0.59) than in fetal arteries (1.83 ± 0.13). In situ MLCK activities (ng MLC20 phosphorylated·s−1·ng MLCK−1) were significantly less in adult (0.26 ± 0.01) than in fetal arteries (1.52 ± 0.11). In contrast, MLCK activities in adult (15.8 ± 1.5) and fetal artery homogenates (17.3 ± 1.3) were not significantly different. When in situ fractional activation was calculated, adult values (1.72 ± 0.17%) were significantly less than fetal values (9.08 ± 0.83%). Together, these results indicate that decreased thick filament reactivity in adult compared with fetal ovine carotid arteries is due at least in part to greater MLCK activity in fetal arteries, which in turn cannot be explained by differences in MLCK, MLC20, or calmodulin concentrations. Instead, this difference appears to involve age-related differences in fractional activation of the MLCK enzyme.
Keywords: myofilament calcium sensitivity, postnatal maturation, regulatory myosin light chain, thick filament reactivity
cardiovascular instability is a common feature of many infants in neonatal intensive care facilities. Systematic investigation of this symptomology has revealed that regulation of vascular contractility varies markedly between immature and mature arteries (4, 46). Previous studies from our laboratory have shown that these age-related differences in contractility involve major differences in intracellular calcium regulation. For equivalent contractile tensions, immature carotid arteries require greater calcium uptake than mature arteries, indicating a greater contractile dependence of immature arteries on extracellular calcium (67). Other studies have further demonstrated that the relative size of intracellular calcium stores changes significantly during postnatal maturation (41). Despite these important differences in calcium regulation, however, intracellular calcium dynamics alone cannot completely explain the age-related differences in regulation of vascular contractility. As shown in multiple recent studies, differences in regulation of myofilament calcium sensitivity also contribute heavily to age-related changes in vascular contractility (2).
By definition, myofilament calcium sensitivity is the increment in force generated for a given increment in intracellular calcium concentration (55). Changes in myofilament calcium sensitivity influence contractile function of vascular smooth muscle during hypertension, in myometrium during parturition, and in airway smooth muscle during asthma (52). Regulation of calcium sensitivity, in turn, is mediated through both thick filament regulatory pathways, which establish the relation between intracellular calcium concentration and myosin light chain phosphorylation, and thin filament regulatory pathways, which determine the relation between myosin light chain phosphorylation and contractile force (40, 51). When examined from a developmental perspective, G protein-coupled receptor stimulation enhanced myofilament calcium sensitivity to a much greater extent in immature than in mature cerebral arteries (2). Additional detailed studies have further revealed that the extent of myosin light chain phosphorylation was greater for a given increment of cytosolic calcium in fetal than in adult arteries, suggesting that thick filament regulation was upregulated in the immature arteries (51). In contrast, myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP), which are the two main enzymes that dominate thick filament reactivity, were less abundant in fetal than in adult arteries (51). Together, these findings indicate that thick filament reactivity, and the enzymes that govern it, change dramatically during postnatal maturation.
To date, most studies of stimulation-induced changes in myosin light chain phosphorylation have focused on regulation of MLCP (55). Whereas this enzyme is clearly regulated by numerous pathways, MLCK also plays a critically important role in regulation of contraction in both smooth muscle and nonmuscle cells. Through a sequence of reactions that are particularly prominent in smooth muscle, calcium-dependent MLCK activation results in myosin light chain (MLC20) phosphorylation, activation of myosin II actomyosin ATPase activity, initiation of cross-bridge cycling, and force development. As the main high-speed kinase responsible for phosphorylating regulatory myosin light chain, MLCK is both necessary and sufficient to initiate smooth muscle contraction (24). Correspondingly, MLCK-dependent regulation of contractile function has been carefully detailed under both physiological and pathophysiological conditions in many preparations, including saphenous vein (33), pulmonary arteries (5), and myometrium (65). However, the role of MLCK activity in regulation of fetal vascular reactivity has not been widely investigated. In fetuses, low arterial pressure and oxygen tensions rapidly increase during and following birth, and vascular resistance of all organs must quickly rise in parallel to prevent peripheral microcirculatory rupture. Failure of these adjustments to occur contributes to many perinatal disorders, including neonatal stroke, intracranial hemorrhage, and hypoxic-ischemic encephalopathy (61, 66). Although the reasons why such disorders are associated with greater morbidity in neonates remain poorly understood, they have been attributed to functional immaturity of the vasculature. In light of this rich background, we designed the present studies of MLCK activity to better understand the mechanisms responsible for upregulation of myofilament calcium sensitivity in fetal compared with adult arteries. To maximize relevance to contractile function, the experimental approach relied on rapid measurements of MLC20 phosphorylation in whole arteries. Because of the relatively large amounts of tissue required for multiple sequential measurements of MLC20 phosphorylation from a single artery sample from a single animal, the experimental design was focused on common carotid artery segments from term fetal and nonpregnant adult sheep. As shown in multiple previous studies, the vascular characteristics of arteries from term fetal lambs have many similarities, both functional and compositional, to corresponding arteries from term human neonates (47).
MATERIALS AND METHODS
General preparation.
Common carotid arteries were harvested from young adult nulliparous female sheep (18–24 mo old) following intravenous injection of 100 mg/kg pentobarbital. Fetal carotid arteries were collected after delivery of each full-term fetus (∼140 days gestation) from its pregnant ewe by a midline vertical laparotomy, after which the fetus was rapidly killed by cardiac excision. The arteries were cleaned of extraneous connective and adipose tissue, and the endothelium was removed by passing a roughened needle through the lumen, as described in multiple previous studies (42). All procedures used in these studies were approved by the Animal Research Committee of Loma Linda University and adhered to all policies and practices outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Determination of in situ MLCK concentration.
Common carotid arteries from fetal and adult sheep were pulverized in liquid nitrogen using a custom-made stainless steel mortar and pestle and then homogenized with glass-on-glass in 4 volumes of buffer containing 0.5 M NaCl, 50 mM Tris·HCl at pH 7.5, 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM PMSF, 0.5% protease inhibitor cocktail (Sigma P8340), and 0.1% β-mercaptoethanol. After centrifugation at 15,000 rpm for 15 min at 4°C, the supernatant was assayed for total protein content (Bio-Rad no. 500-0006). For each determination, 20 μg of total protein were loaded on a 15-lane 8% SDS-PAGE gel, run at 15 mA for 90 min, and then transferred onto nitrocellulose membrane. The membrane was blocked overnight at 4°C with 5% nonfat dry milk in 20 mM Tris·HCl at pH 7.5, 500 mM NaCl, and 0.1% Tween 20 and then probed with mouse anti-MLCK primary antibody (Sigma M7905) at 1:7,000 dilution for 2 h. Primary antibody-antigen complexes were detected with a goat anti-mouse secondary antibody (Pierce no. 1858413) and visualized using direct photon capture with a charge-coupled device camera (Chemi-Imager; Alpha Innotech). Adjacent lanes of each gel were loaded with six different quantities of a reference MLCK standard prepared from ovine carotids to enable relative quantification. The reference MLCK standard was further calibrated against known masses of MLCK purified from chicken gizzard, which was a gift from Dr. Christine R. Cremo. The mass of MLCK (in μg/mg protein) was converted to intracellular concentration (in μM) by dividing by the age-appropriate ratio of cell water to protein (in μl/mg), which we have previously measured in ovine carotid arteries (10).
Determination of in situ MLC20 concentration.
Common carotid arteries from fetal and adult sheep were pulverized in liquid nitrogen using a custom-made stainless steel mortar and pestle. The pulverized tissue was extracted for 2 h by using a buffer containing 8 M urea, 20 mM Tris, 23 mM glycine, 10 mM EGTA, 10 mM DTT, 5 mM NaF, and 10% glycerol at pH 8.6 with a tissue-to-buffer ratio of 1:300. After 2 h of extraction on an orbital shaker, the homogenates were centrifuged at 12,000 g for 20 min, after which the supernatants were removed and the pellets were extracted a second time. The supernatants were combined, and their absolute contents of MLC20 were assayed by separating 10-μl sample aliquots on 15% SDS gels at a constant voltage (100 V) for 2 h. Varying known quantities of purified MLC20 were run on multiple adjacent lanes to enable construction of a standard curve. The purified MLC20 used as an absolute standard was prepared from chicken gizzard and was a gift from Dr. Christine R. Cremo. The gels were transferred onto nitrocellulose membranes at constant current (50 mA) for 3 h, after which immunoblotting was performed as previously described (51). Briefly, membranes were blocked with 5% milk in TBS (20 mM Tris·HCl and 500 mM NaCl, pH 7.5) for 90 min. The blocked membranes were incubated in primary anti-MLC antibody (Sigma M4401) at 1:300 dilution with 5% milk in Tween-TBS for 3 h, followed by incubation for 90 min at a 1:1,000 dilution of a goat anti-mouse secondary antibody (Pierce no. 1858413) conjugated with horseradish peroxidase. All washes were carried out with Tween-TBS containing 5% milk. Antibody-antigen complexes were detected by chemiluminescence using a mixture of equal volumes of enhanced luminol reagent and oxidizing reagent (Pierce no. 34096). Membranes were then scanned to determine the integrated optical density values of MLC20 bands using direct photon capture (Chemi-Imager), and these values were converted into MLC20 masses using the standard curve on each membrane. To enable comparisons between age groups, the values of MLC20 mass were converted to MLC20 concentration (in μM) by multiplying MLC20 mass (in μg/mg protein) by the age-appropriate ratio of cell water to protein (in μl/mg), which we have previously measured in ovine carotid arteries (10).
Determination of in situ calmodulin concentration.
Common carotid arteries were extracted for calmodulin by using the buffer used to extract MLC20 as described in Determination of MLC20 abundance, with the exception that EGTA was omitted. After extraction, 20 μl of supernatant from each sample were loaded on 15% SDS gels and run at a constant voltage of 100 V for 2 h. Varied amounts of purified calmodulin from bovine testes (Sigma P1431) were loaded as a standard curve on the same gels to measure absolute masses of calmodulin present in tissues. The gels were wet-transferred onto nitrocellulose membranes, followed by immunoblotting as described by Hulen et al. (20). The primary anti-calmodulin antibody (Sigma C3545) at 1:1,000 dilution and goat anti-mouse secondary antibody at 1:1,000 dilution (Pierce no. 1858413) were used to detect and quantify calmodulin in the samples. To enable comparisons between age groups, the values of calmodulin mass were converted (to μM) by multiplying calmodulin masses (in μg/mg protein) by the age-appropriate ratio of μl cell water to mg protein, which we have previously measured in ovine carotid arteries (10).
Determination of optimal length for contractile response in intact carotid arteries.
Given that maximal MLCK activity is prerequisite for maximal rates of contraction, which in turn develop only at optimal artery stretch, the optimal stretch ratios for carotid arteries from each age group were determined. Common carotid arteries obtained from adult sheep and near-term fetuses were cleaned of adhering tissues, cut into 2-mm lengths, and placed in Krebs solution containing 122 mM NaCl, 25.6 mM NaHCO3, 5.56 mM dextrose, 5.17 mM KCl, 2.49 mM MgSO4, 1.60 mM CaCl2, 0.114 mM ascorbic acid, and 0.027 mM EGTA that was continuously bubbled with 95% O2 and 5% CO2. Each segment was mounted within a warmed tissue bath on wires and suspended between a force transducer and a post attached to a micrometer. After equilibration in Krebs solution at 38.5°C (normal ovine core temperature) for at least 30 min, micrometer readings were obtained at 0.05-g tension, which enabled measurements of unstressed diameter (D0) for each segment. The artery segments were then stretched in regular multiples of unstressed baseline diameter to yield stretch ratios (D/D0) ranging from 1.0 to 3.0 times unstressed diameter. At each stretch ratio, the arteries were briefly contracted with 122 mM K+ until a peak response was obtained, after which the segments were returned to normal Krebs and equilibrated for 30 min before the next increment in stretch was applied. When contractile responses to K+ had been recorded at all stretch ratios, active tensions were calculated and plotted against their corresponding stretch ratios for both fetal and adult arteries, as previously described (46).
Determination of optimal concentration of MLCP inhibitors.
Both adult and fetal carotid artery segments were cleaned, wire mounted, and then stretched to their optimal length. After equilibration and initial contraction with 122 mM K+ Krebs, the arteries were washed three times with Na+ Krebs buffer and incubated with four different concentrations (0, 10, 30, and 100 nM) of calyculin-A for an hour. Next, the segments were instantly frozen after exactly 9 s of contraction with 122 mM K+ by using an acetone-TCA freezing solution held on dry ice at −70°C. Arteries were similarly frozen following incubation in four different concentrations (0, 3, 10, and 30 μl/ml) of phosphatase inhibitor cocktail (PIC; Sigma P2850). Frozen segments were extracted and analyzed on 10% urea glycerol gels to quantify the extent of myosin light chain phosphorylation as previously described (51). Briefly, the gels were loaded with equal amounts of total protein, run for 2.5 h at constant voltage (200 V), and then transferred onto nitrocellulose membrane at constant current (50 mA) for 3 h. Membranes were then blocked as described in Determination of MLC20 abundance and analyzed for MLC20 phosphorylation.
Determination of concentration- and time-dependent effects of the MLCK inhibitor ML-7 on MLCK activity.
Artery segments from both fetal and adult animals were prepared as described in Determination of optimal concentration of MLCP inhibitors. After equilibration and contraction with 122 mM K+ Krebs, the arteries were washed three times with Na+ Krebs and incubated with five different concentrations (0, 10, 30, 100, and 300 nM) of ML-7 [1-(5-iodonaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride, a MLCK inhibitor] for 30 min, followed by incubation with PIC (10 μl/ml) for an hour. In preliminary studies, the maximum values of MLC20 phosphorylation were observed after 9 s of contraction. Thus the segments used to assay for ML-7 efficacy were instantly frozen after exactly 9 s of contraction with 122 mM K+ by using an acetone-TCA buffer on dry ice at −70°C. Frozen segments were extracted and analyzed on urea gels to quantify the extent of myosin light chain phosphorylation as previously described (51). In a companion set of experiments, the time course of MLC20 phosphorylation in response to K+ contraction was determined in the presence and absence of the optimal concentration of ML-7 to verify the efficacy and selectivity of inhibition by ML-7. Arteries were frozen at exactly 0, 3, 6, and 9 s after contraction and analyzed for MLC20 phosphorylation via urea gels.
Activation of MLCK via electrical field stimulation.
To enable measurement of MLCK activity in intact tissues, we fabricated a rapid freeze apparatus following a design described by Maass-Moreno et al. (34). Previous studies have indicated that MLCK activity is limited by the rate of diffusion of agonists through the artery wall, and thus electrical field stimulation is necessary to instantaneously activate MLCK regardless of its position in the arterial wall to accurately measure MLCK activity in situ (8, 34). Our apparatus included a specialized low-volume tissue cuvette (1.5 ml) equipped with large-surface area platinum foil electrodes arranged to deliver high current density via electrical field stimulation (EFS). Current magnitude was measured directly via oscilloscope as a voltage drop across a known resistance, as previously described (45). Artery segments were mounted in the cuvette between a stationary hook and a force transducer that continuously monitored contractile force. The force transducer was mounted on a digital micrometer that enabled precise positioning (±1 μm) and stretch of the artery segments. The time of freezing was precisely controlled using a computer-based timing circuit that operated high-speed solenoid valves and enabled very rapid (≤200 ms) exchange of Krebs buffer for −70°C acetone-TCA buffer. Arteries were stimulated with 100 mA of current applied continuously at 60 Hz and 4-ms duration, simultaneously frozen at exactly 0, 1, 2, and 3 s, and analyzed for MLC20 phosphorylation.
Determination of current-contractile responses in intact carotid arteries.
Both fetal and adult arteries were mounted in the rapid freeze apparatus and equilibrated, after which contractile responses to 122 K+ Krebs were determined. The segments were then rinsed, equilibrated in normal Na+ Krebs buffer for 1 h, and then stimulated with graded EFS currents of 40, 50, 60, 70, 80, 90, and 100 mA. Contractile responses to EFS were normalized relative to the maximum responses to K+ Krebs.
Determination of MLCK activity in intact carotid arteries.
To measure MLCK activity in situ, common carotid arteries were prepared as described in Determination of optimal length for contractile response in intact carotid arteries and then mounted in the rapid freeze apparatus. After equilibration and contraction with 122 mM K+ Krebs, the arteries were washed and incubated in 10 μl/ml PIC for an hour. Next, EFS (90 mA at 60 Hz) was applied, and segments were frozen at 0, 1, 2, and 3 s of stimulation. Frozen segments were extracted and analyzed on urea gels to quantify the extent of myosin light chain phosphorylation as described in Determination of MLC20 abundances. The slope of the relation between %MLC20 phosphorylation and time, which was an estimate of the rate of phosphorylation of myosin light chain, was calculated as a measure of MLCK activity. The apparent specific activity of MLCK was calculated by dividing the rate of MLC20 phosphorylation by absolute MLCK abundance.
Determination of MLCK velocity in artery homogenates.
The activity of MLCK was measured in crude homogenates by using methods similar to those described by Liu et al. (33). Briefly, frozen carotid arteries (∼150 mg per animal) were homogenized in ice-cold buffer containing 20 mM imidazole, 1 mM cysteine, 60 mM KCl, 1 mM MgCl2, 10 mM sodium azide, 0.25 mM PMSF, and 1 mM DTT, at pH 7.5. The homogenization buffer also contained PIC at a concentration of 10 μl/ml buffer and 0.5% protease inhibitor cocktail (Sigma P8340). Homogenization was carried out for 15 min at 4°C using a motor-driven glass-on-glass mortar and pestle and then centrifuged for 1 min at 6,000 g, after which an aliquot of supernatant was taken for protein determination. Final adjustments in protein concentration were made to ensure that the amount of MLCK in each homogenate was similar. Exogenous purified MLC20 was added to the homogenates to raise MLC20 concentration at varying concentrations up to 46 μM. After incubating for 15 min at 37°C, calcium at 3 mM was added along with 1 mM ATP to activate the MLCK. The reaction was terminated at exactly 0, 1, 2 and 3 s by adding ice-cold 10 mM EDTA and then transferring the samples to dry ice. The samples were then analyzed for MLC20 phosphorylation using 10% urea glycerol gels as described in Determination of MLC20 abundance and analyzed for MLC20 phosphorylation. The observed rates of change in MLC20 phosphorylation were normalized relative to MLCK concentration to calculate apparent MLCK specific activities.
Calculations and statistics.
Standard curves relating protein mass to optical density in Western blots were fit to the logistic equation by using least-squares error minimization routines, and sample protein masses were calculated directly using the inverse function of the best-fit standard curve. The concentrations of MLCK and MLC20 were calibrated relative to MLCK and MLC20 purified from chicken gizzard and are expressed in units of micromolar concentration. Values of %MLC20 phosphorylation were calculated as the unphosphorylated mass divided by the sum of phosphorylated and unphosphorylated masses, as previously described (51). Relations between %MLC20 phosphorylation and inhibitor concentrations or time were fit to rectangular hyperbolas, also using least-squares error minimization routines. Throughout, all values indicate the mean ± SE for the number of animals indicated; values of n refer to the numbers of animals and not the numbers of segments or experiments unless indicated otherwise. For length-tension experiments, all measurements were performed in duplicate adjacent segments from each carotid, and these were averaged first within each animal and then across different animals to calculate the group response. In the other protocols, unpaired comparisons between two variables were performed using a Behren-Fisher analysis with pooled variance. All data sets were verified to be normally distributed using SPSS version 16 software. In all cases, statistical significance implies P < 0.05.
RESULTS
A total of 164 and 187 carotid segments were taken for study from 35 ovine fetuses and 41 adult sheep, respectively.
Effect of postnatal maturation on MLCK, MLC20, and calmodulin concentrations.
Measurements of MLCK abundance in fetal and adult tissues averaged 1.78 ± 0.02 and 6.10 ± 0.01 μg MLCK/mg protein, respectively. When converted to estimates of intracellular concentration, MLCK concentrations were markedly less in fetal (1.83 ± 0.13 μM) than in adult arteries (8.21 ± 0.59 μM) (Fig. 1). None of the gels examined exhibited more than a single immunoreactive band for MLCK, and no evidence of a low molecular weight immunopositive product of MLCK (telokin) was observed in either age group, although other preparations used as positive controls verified the ability of the antibody used to detect telokin.
Fig. 1.
In situ concentrations of myosin light chain kinase (MLCK) in ovine carotid arteries. Supernatants from artery homogenates containing 20 μg of total protein were loaded on 8% SDS-PAGE gels along with varying masses of a calibrated standard for MLCK in adjacent lanes. The integrated density values of MLCK were converted to their respective masses using a standard curve and then normalized to intracellular volume using previously published ratios of intracellular water to total cellular protein. In all cases, the band densities for unknown samples were within the range defined by the standards. For the standard bands shown (top), the band density given by 5 μg of MLCK was not visible by eye but was detectable by the gel analysis software (Chemi-Imager; Alpha Innotech). Values indicate the ratio of MLCK mass to intracellular water, expressed as in situ concentration (bottom). Error bars indicate SE for 7 fetal and 7 adult animals. The average value was significantly less in fetal than in adult arteries at the P < 0.05 level.
MLC20 abundances in fetal and adult tissues averaged 30.6 ± 0.1 (n = 6) and 17.2 ± 0.2 μg MLC20/mg protein (n = 6), respectively. When converted to estimates of intracellular concentration, MLC20 concentrations were not significantly different in fetal (236 ± 44 μM) and adult arteries (198 ± 28 μM) (Fig. 2).
Fig. 2.
In situ concentrations of myosin light chain (MLC20) and calmodulin in ovine carotid arteries. Artery homogenates were loaded on 15% SDS-PAGE gels along with varying masses of calibrated standards for either MLC20 (left) or calmodulin (right) in adjacent lanes. The integrated density values of each protein were converted to their respective masses by using their respective standard curves and then normalized to intracellular volume using previously published ratios of intracellular water to total cellular protein. Values indicate in situ concentrations of calmodulin and MLC20 for both fetal and adult arteries. Error bars indicate SE for the number of animals indicated in parentheses. There was a significant difference between the fetal and adult values at the P < 0.05 level for calmodulin.
Calmodulin abundances in fetal and adult tissues averaged 1.36 ± 0.02 (n = 7) and 2.54 ± 0.01 μg/mg protein (n = 7), respectively. When converted to estimates of intracellular concentration, calmodulin concentrations were significantly less in fetal (12.4 ± 1.3 μM) than in adult arteries (30.6 ± 3.7 μM) (Fig. 2).
Length-tension relationships in ovine carotid arteries.
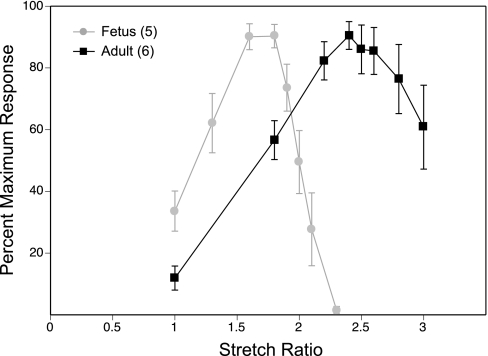
As shown in Fig. 3, the stretch ratio D/D0 associated with the maximum contractile response was significantly greater in adult (2.58 ± 0.07) than in fetal arteries (1.70 ± 0.06). These results agree with our previous findings that fetal and adult arteries have different optimum lengths and sensitivity to stretch, which has been attributed to age-related differences in artery wall thickness and compliance (46). The altered content of elastin typical of immature arteries (63) implies that a greater fraction of an imposed length change will be transferred to the intramural smooth muscle cells, and thus a smaller length increment will be required to stretch the cells to their optimal contractile length. From a practical perspective, these results also reveal the importance of determining complete length-tension relations in arteries used to study MLCK activity in situ, because MLCK velocity varies with stretch (51), suggesting that maximal MLCK activity is measurable only at optimal length.
Fig. 3.
Length-tension relations in ovine carotid arteries. Carotid artery segments of fetal and adult sheep were wire mounted and stretched to regular multiples of unstressed baseline diameter (D/D0) ranging from 1.0 to 3.0. At each stretch ratio, contractile responses to 122 mM K+ were recorded and then normalized relative to maximum response. Error bars indicate SE for a total of 10 artery segments from 5 fetuses and 12 artery segments from 6 adult sheep.
Optimum concentration of myosin light chain phosphatase inhibitors.
In both fetal and adult arteries, calyculin-A increased %MLC20 phosphorylation associated with contraction, and the maximum effect was observed at 30 nM (Table 1). The maximum %MLC20 phosphorylation averaged 47.9 ± 3.4% in fetal arteries and 27.6 ± 1.5% in adult arteries. Maximal increases in %MLC20 phosphorylation produced by PIC were observed at 10 μl/ml in both age groups, and maximum %MLC20 phosphorylation averaged 50.9 ± 2.6% in fetal arteries and 55.6 ± 3.1% in adult arteries. Because PIC at 10 μl/ml yielded greater maximal %MLC20 phosphorylation than calyculin-A, all further studies used 10 μl/ml PIC to inhibit MLCP. Because PIC was designed to inhibit more types of phosphatase than calyculin-A (30), and the difference between the maximum %MLC20 produced by PIC and calyculin A was much greater for adult than for fetal arteries, PIC was used in all subsequent experiments. In all measurements of %MLC phosphorylation, the urea gels consistently exhibited one unphosphorylated and one phosphorylated band, indicating MLC20 monophosphorylation.
Table 1.
Optimum concentrations of calyculin-A and phosphatase inhibitor cocktail
| Fetus | Adult | |
|---|---|---|
| Calyculin-A | ||
| 0 nM | 22.7±2.8 | 14.9±1.1 |
| 10 nM | 37.8±5.4* | 27.3±1.7* |
| 30 nM | 43.3±3.8* | 48.5±4.9* |
| 100 nM | 27.6±1.5* | 25.5±1.5* |
| Phosphatase inhibitor cocktail | ||
| 0 μl/ml | 37.5±2.7 | 32.7±1.0 |
| 3 μl/ml | 50.9±3.2* | 43.4±3.2* |
| 10 μl/ml | 49.4±1.2* | 52.5±1.7* |
| 30 μl/ml | 48.3±4.2* | 56.5±2.5* |
Calyculin-A and phosphatase inhibitor cocktail (PIC) were added to inhibit myosin light chain phosphatase (MLCP) activity. Artery segments from both age groups were incubated in 0, 10, 30, or 100 nM calyculin-A for an hour, depolarized with 122 mM K+ for 9 s, and then immediately frozen and analyzed for MLC20 phosphorylation using urea glycerol gels. Totals of 20 artery segments from each group were used from 5 fetuses and 5 adult sheep. Similar measurements were conducted with PIC at 0, 3, 10, or 30 μl/ml concentrations in 20 artery segments each from 5 fetus and 5 adults, respectively. Values indicate averages ± SE.
P < 0.05, significantly different from initial control values within each age group.
Concentration- and time-dependent effects of ML-7 on MLCK activity.
ML-7 at a concentration of 100 μM maximally inhibited both fetal and adult MLCK activity (Fig. 4, top). ML-7 also produced a dose-dependent decrease in contractile response of the artery segments in response to 122 mM K+ (data not shown). Using this optimal concentration of ML-7, we examined the effects of ML-7 on the time courses of MLC20 phosphorylation. As shown in Fig. 4, bottom, 100 μM ML-7 completely inhibited any time-dependent increase in MLC20 phosphorylation following 122 mM K+ stimulation. These results indicate that MLCK is most probably the only kinase involved in MLC20 phosphorylation during the first 9 s of contraction. The results do not exclude MLC20 phosphorylation by other kinases at later time points but largely rule out the participation of non-MLCK-mediated phosphorylation during the initial contraction.
Fig. 4.
Concentration- and time-dependent effects of ML-7 on MLCK activity. Concentration-dependent effects of ML-7 on MLCK activity (top) were assessed by treatment with 0, 10, 30, 100, or 300 μM ML-7 for 30 min, followed by incubation with 10 μl/ml phosphatase inhibitor cocktail (PIC) for an hour. The segments were then instantly frozen after 9 s of contraction with 122 mM K+ and analyzed for MLC20 phosphorylation. Time courses of MLC20 phosphorylation were then determined in the presence and absence of 100 μM ML-7 (bottom) by freezing at 0, 3, 6, and 9 s after contraction with 122 mM K+. A total of 65 segments each from 5 fetuses and 5 adult arteries were used for this study. Values indicate averages ± SE for the number of fetal and adult sheep indicated in parentheses. *P < 0.05, significant difference from initial control value within each age group.
Current-tension relationships in ovine carotid arteries.
To assure maximal activation of MLCK, we examined the contractile responses produced by graded currents of EFS in both fetal and adult arteries. Application of 90 mA or more of current at 60 Hz and 4-ms duration yielded 109 ± 2.4 and 106 ± 5.2% the response to 122 mM K+ Krebs in fetal (n = 3) and adult arteries (n = 3), respectively. Because these stimulation parameters yielded maximal contractile responses, they were used in all subsequent experiments. The ratio of the maximum response to EFS relative to the maximum response to 122 mM K+ Krebs was routinely calculated and verified to be maximal in every segment subjected to EFS; in all cases, this ratio averaged to more than 100%.
Measurement of MLCK activity in intact ovine carotids.
As shown in Fig. 5, top, both fetal and adult arteries reached peak MLC20 phosphorylation in ∼2 s. The slopes of these curves, which were taken as a measure of total MLCK activity, averaged 7.39 ± 0.53 and 6.56 ± 0.29 %MLC20 phosphorylation/s in fetal and adult arteries, respectively. To correct for age-related differences in MLC20 abundances, the %MLC20 phosphorylation values were multiplied by MLC20 abundance to calculate maximal tissue rates of MLC20 phosphorylation, which averaged 93.2 ± 4.1 and 89.4 ± 6.4 ng MLC20 phosphorylated·s−1·mg wet weight−1 in adult and fetal arteries, respectively; these values were not significantly different. To correct for age-related differences in MLCK abundances, these activity values were normalized relative to MLCK abundance, yielding average activity values of 0.26 ± 0.01 and 1.52 ± 0.11 ng MLC20 phosphorylated·s−1·ng MLCK−1 in adult and fetal arteries, respectively (Fig. 5, bottom); these values were significantly different.
Fig. 5.
MLCK velocity in intact ovine carotid arteries. Fetal and adult artery segments were wire mounted and positioned in a custom-made rapid freeze apparatus, activated via electrical field stimulation, and frozen at exactly 0, 1, 2, and 3 s using a computer-controlled apparatus. The segments were then analyzed for MLC20 phosphorylation. The initial rate of change of MLC20 phosphorylation was taken as a measure of MLCK velocity (top). To correct for age-related differences in MLC20 and MLCK abundances, the %MLC20 phosphorylation ratios were multiplied by the corresponding mass ratios of MLC20/MLCK abundance to obtain units of ng MLC20 phosphorylated/ng MLCK (bottom). The initial slopes (V) are given for each age group. A total of 20 artery segments from 5 fetuses and 20 artery segments from 5 adults were used for this protocol. Values indicate averages ± SE. *P < 0.05, significant difference between corresponding fetal and adult value. Error bars for adult values at bottom are smaller than the size of the symbols.
Age-related differences in MLCK specific activity and fractional activation.
To calculate the specific activities of MLCK under broken cell conditions, we divided the nanogram of MLC20 phosphorylated per second by the absolute MLCK mass in each assay to obtain velocity values in units of nanograms of MLC20 phosphorylated per second per nanogram of MLCK. Maximal MLCK velocities were observed with 20 μM MLC20 and averaged 17.3 ± 1.3 (n = 6) and 15.8 ± 1.5 ng MLC20 phosphorylated·s−1·ng MLCK−1 (n = 6) in fetal and adult homogenates, respectively; fetal and adult values were not significantly different (Fig. 6). Further increases in MLC20 concentration up to 46 μM did not significantly increase MLCK activity. All homogenates used to measure MLCK activity included 0.3 μM calmodulin and 3.0 mM calcium, which have been reported previously to be saturating concentrations for MLCK (54, 65); increases in calmodulin concentration up to 1 μM did not further increase MLCK activity. Given these results, the measurements of MLCK activity observed at 20 μM MLC20 in the presence of 0.3 μM calmodulin and 3.0 mM calcium were taken as maximal values for MLCK activity in these tissues.
Fig. 6.
Age-related differences in the apparent specific activity and fractional activation of MLCK. Apparent specific activity was calculated as the maximum velocity divided by MLCK content (left). Fractional activation of the in situ enzyme was calculated as the ratio of maximal velocity measured in intact arteries over the maximal velocity measured in the homogenates (right). Shown are averages for 6 animals from each group for specific activity measurements and 5 animals from each group for fractional activation. Error bars indicate SE for the number of animals indicated in parentheses. There was a significant difference between the fetal and adult values at the P < 0.05 level for fractional activation.
To calculate the extent of fractional activation of the enzyme in situ, the MLCK specific activity (in units of ng MLC20 phosphorylated s−1·ng MLCK−1) measured in situ (Fig. 5) was divided by the apparent specific activity obtained in the homogenates. These ratios indicated that maximal in situ fractional activation in fetal arteries (9.08 ± 0.83%) was significantly greater than in adult arteries (1.72 ± 0.17%).
DISCUSSION
The present study evaluates the hypothesis that postnatal downregulation of thick filament reactivity involves downregulation of MLCK activity. The experiments produced six main findings: 1) MLCK concentration, in situ, was 3.4-fold greater in adult than in fetal arteries; 2) MLC20 concentration was similar in adult and fetal arteries; 3) calmodulin concentration was 1.9-fold greater in adult than in fetal arteries; 4) MLCK activity, in situ, was 5.7-fold less in adult than in fetal arteries; 5) MLCK activity, in vitro, was not significantly different in adult and fetal arteries; and 6) maximal stimulation-induced fractional activation of MLCK, in-situ, was 5.3-fold less in adult than in fetal arteries. These results demonstrate that postnatal downregulation of thick filament reactivity involves decreases in MLCK activity and fractional activation in situ that cannot be explained by differences in MLCK intrinsic activity or in the abundances of MLCK, MLC20, or calmodulin.
Among the many contributions made by the late Andrew Somlyo, perhaps one of the most important was his identification of myofilament calcium sensitivity as a critical determinant of smooth muscle contractility (29). This discovery fueled numerous investigations into the mechanisms translating changes in cytosolic calcium concentration into increased MLC20 phosphorylation and subsequent force development (55). In contrast to extensive studies of myosin phosphatase regulation (14, 17, 28, 32, 35, 36, 59) and its influence on myofilament calcium sensitivity, little attention has been paid to a parallel role for MLCK. This inattention has arisen largely from evidence suggesting that endogenous MLCK activity is subject predominantly to inhibition and not activation (18, 19, 43, 58), which diminishes its potential to explain physiological enhancement of myofilament calcium sensitivity. However, studies of MLCK knockout mice have revealed that MLCK plays a critical and age-dependent role during embryonic development (56). Myofilament calcium sensitivity is also upregulated in immature compared with mature arteries (2), as is the relation between cytosolic calcium concentration and MLC20 phosphorylation (51). Because this upregulation persists even after inhibition of myosin phosphatase (Table 1), developmental differences in myofilament calcium sensitivity appear to involve significant differences in MLCK activity.
Multiple studies have demonstrated significant changes in MLCK activity associated with changes in MLCK abundance in varied tissue types, including airway (26), venous (33), and arterial (16) smooth muscle. Studies of rat pulmonary arteries also have demonstrated that postnatal maturation increases MLCK abundance (5). Consistent with this evidence, Western blots revealed a 3.4-fold greater concentration of MLCK in adult compared with fetal arteries (Fig. 1). Other studies also have shown that MLCK is the product of a single gene, with two splice variants sized at 130–150 kDa (short MLCK) and 208–214 kDa (long MLCK) (55). Whereas the long-isoform MLCK may be more prominent during embryogenesis (11), our Western blots did not reveal multiple isoforms of MLCK or the COOH-terminal truncation product telokin (44) in either age group. This evidence suggests that MLCK abundance increases dramatically during postnatal maturation and cannot explain greater thick filament reactivity in fetal compared with adult arteries (51).
Given that the tissue enzyme activity reflects both enzyme abundance and specific activity, our experimental approach included activity measurements made in both whole arteries and broken cell preparations. The whole artery approach offered multiple advantages, including preservation of the authentic in situ spatial organization and potential for interaction of MLCK with other proteins. For example, MLCK binds actin with high affinity (13, 57), and other unidentified protein-protein interactions might influence MLCK localization (13).Whole artery measurements also preserved the endogenous concentrations of MLCK, its substrate, and cofactors, as well as those of possible endogenous activators and inhibitors. Previous studies have suggested that the levels of many smooth muscle proteins are labile and vary in response to numerous physiological perturbations including development, pregnancy, and hormonal treatment (7, 37, 65). In light of the greater physiological relevance of in situ measurements, a few previous studies also have employed in situ measurements of MLCK activity (8, 25).
Whole artery measurements of MLCK activity required several optimizations and validations. Because maximal MLCK activity is observable only at optimal stretch (15, 49), it was essential to determine optimum lengths for both experimental groups. Consistent with previous findings of postnatal increases in large artery compliance (46), adult arteries required significantly greater stretch to attain optimum length than did fetal arteries (Fig. 3). This large age-related difference in optimum length suggests that reliable measurements of in situ MLCK activity can be made only at optimal length and that previous measurements of in situ MLCK activity not performed at optimal length may be subject to error (7, 25). Other validation experiments optimized inhibition of myosin phosphatase to enable measurement of MLCK activity in situ as the rate of change of MLC20 phosphorylation. Consistent with previous studies (22), calyculin-A optimally inhibited phosphatase activity between 30 and 100 nM (Table 1), although the efficacy of this inhibition in adult but not in fetal arteries was twofold less than observed with 10 μl/ml PIC. Because PIC contains multiple phosphatase inhibitors (cantharidin, bromotetramisole, and microcystin LR), this finding indirectly suggests that adult but not fetal arteries contain a myosin phosphatase that is more sensitive to one or more of the ingredients of PIC than to calyculin-A; this speculative hypothesis merits further investigation. From a practical perspective, all measurements of in situ MLCK activity in our studies included 10 μl/ml PIC, whereas not all previous studies of in situ MLCK activity have included phosphatase inhibitors. Variations in phosphatase activity probably augment the heterogeneity of MLCK activity as determined in some previous studies (7, 8, 25).
Whereas MLC20 is the only known substrate for MLCK (27), other kinases appear capable of phosphorylating MLC20. These include protein kinase A (62), protein kinase C (21), Rho-kinase (3), integrin-linked kinase (64), and p21-activated kinase (6). Because some kinases may phosphorylate only isolated MLC20 (62) or may phosphorylate sites other than S19 (21), the functional importance of these phosphorylation events, in situ, is uncertain. An important additional feature of these other kinases is that their rates of phosphorylation of MLC20 are much slower than for MLCK (38, 39), which suggests that measurements of MLC20 phosphorylation over short time courses should reflect MLCK activity almost exclusively. Correspondingly, measurements of MLCK activity with varying concentrations of ML-7 (Fig. 4, top), a specific inhibitor of MLCK (50), indicated that 100 μM ML-7 maximally inhibited potassium-induced MLC20 phosphorylation and completely inhibited all phosphorylation of MLC20 during a 9-s exposure to potassium (Fig. 4, bottom), indicating that MLCK was probably the only kinase phosphorylating MLC20 within 10 s. It remains remotely possible, however, that another kinase could phosphorylate MLC20, provided it was sensitive to ML-7 and was very fast acting; no evidence for such a kinase has yet been reported.
Using our rapid-freeze apparatus, maximal activation with EFS during optimal inhibition of myosin phosphatase increased MLC20 phosphorylation more quickly (as %MLC20 phosphorylated/s) in fetal (7.4%) than in adult arteries (6.6%) (Fig. 5, top). Thus both fetal and adult arteries produced near-equivalent rates of MLC20 phosphorylation, although perhaps through much different mechanisms. When in situ MLCK activity was calculated by normalizing the absolute tissue velocities of MLC20 phosphorylation by MLCK abundance, the fetal values (in ng MLC20 phosphorylated·s−1·ng MLCK−1) were almost sixfold greater than the adult values (Fig. 5, bottom). These large differences in apparent MLCK specific activity in situ appeared to be counterbalanced by opposite differences in MLCK abundance such that overall tissue velocities of MLC20 phosphorylation and generation of contractile force (46) were similar in the two age groups. In addition, basal levels of MLC20 phosphorylation (Fig. 5) were significantly greater in fetal than adult arteries. This difference raises the possibility that the resting balance between overall MLCK and MLCP activities is shifted toward greater MLC20 phosphorylation in fetal compared with adult arteries and/or that the basal level of phosphorylation may enhance subsequent MLCK activity.
To determine whether the differences in MLCK activity observed in situ were attributable to differences in enzyme specific activity, our experimental approach included broken cell measurements of MLCK activity performed in the presence of saturating concentrations of MLC20 and calcium-calmodulin. These homogenate measurements revealed no significant age-related differences in maximal MLCK activity (Fig. 6, left). When these homogenate values of MLCK velocity were divided into the values of MLCK activity measured in situ, the resulting values of maximal fractional activation were 5.8-fold greater in fetal (9.1%) than in adult arteries (1.7%) (Fig. 6, right). These values were reasonably consistent with those reported by Isotani et al. (23) in the mouse bladder and support the view that MLCK is only partially activated under in situ conditions (60). The reasons for this limited activation of MLCK under physiological conditions, however, remain uncertain.
As a whole, the present results demonstrate that MLCK activity, in situ, decreases during postnatal maturation. Increasing postnatal expression of MLCK appears closely coordinated with parallel decreases in in situ MLCK specific activity. Age-related shifts in MLCK isoform do not appear to be involved based on Western blot results, but posttranslational modifications of MLCK activity remain possible, particularly in light of evidence that MLCK can serve as a substrate for multiple kinases abundant in smooth muscle (58). MLCK also might be differentially regulated by endogenous activators or inhibitors, such as polyamines (48). Although endogenous levels of calmodulin may vary with age (9) and may be rate-limiting in some tissues (23, 60), in situ calmodulin concentrations were greater in adult than in fetal arteries and were probably saturating for MLCK in both age groups (Fig. 2); thus age-related differences in calmodulin concentration probably cannot explain the observed differences in MLCK activity in situ. Because the mass of agonist-releasable calcium is typically much smaller in fetal than in adult arteries (41), and depolarization-induced rates of change in cytosolic calcium concentration are also much slower in fetal than adult arteries (1), age-related differences in rates of change in cytosolic calcium concentration also probably cannot explain the observed differences in MLCK velocity in situ. Finally, it remains possible that the spatial organizations of MLCK and MLC20 differ in fetal and adult arteries such that activation of an MLCK population that is not colocalized near MLC20 may not produce either phosphorylation or contractile force. Given that maximal depolarization-induced active stresses (46) and MLC20 concentrations (Fig. 2) are similar in fetal and adult carotids, this hypothesis requires that a substantial fraction of adult MLCK is not localized near MLC20. The function of this noncolocalized MLCK is uncertain but may serve some non-kinase function including MLCK binding to actin (53, 57) and, possibly, myosin (12, 31). The low values of fractional activation of MLCK observed in this and other studies (23) are consistent with the possibility that a significant fraction of MLCK may not be colocalized near MLC20. Aside from the mechanisms involved, the present data clearly indicate that postnatal decreases in the apparent specific activity of MLCK contribute to corresponding decreases in thick filament reactivity and myofilament calcium sensitivity (2, 51). Given that overall vascular contractility and maximum active stress development remain relatively constant during the transition from fetal to adult life (46), these changes appear to be a critical component of normal postnatal vascular development.
GRANTS
The work reported was supported by National Institutes of Health Grants HL-54120, HD-31266, and HL-64867 and the Loma Linda University School of Medicine.
Acknowledgments
The generous gifts of purified MLCK and MLC20 provided by Dr. Christine R. Cremo, University of Nevada at Reno, were greatly appreciated.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akopov SE, Zhang L, Pearce WJ. Maturation alters the contractile role of calcium in ovine basilar arteries. Pediatr Res 44: 154–160, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Akopov SE, Zhang L, Pearce WJ. Physiological variations in ovine cerebrovascular calcium sensitivity. Am J Physiol Heart Circ Physiol 272: H2271–H2281, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Belik J, Halayko AJ, Rao K, Stephens NL. Pulmonary and systemic vascular smooth muscle mechanical characteristics in newborn sheep. Am J Physiol Heart Circ Physiol 263: H881–H886, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Belik J, Kerc E, Pato MD. Rat pulmonary arterial smooth muscle myosin light chain kinase and phosphatase activities decrease with age. Am J Physiol Lung Cell Mol Physiol 290: L509–L516, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Rac-induced increase of phosphorylation of myosin regulatory light chain in HeLa cells. Cell Motil Cytoskeleton 58: 186–199, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chitano P, Voynow JA, Pozzato V, Cantillana V, Burch LH, Wang L, Murphy TM. Ontogenesis of myosin light chain kinase mRNA and protein content in guinea pig tracheal smooth muscle. Pediatr Pulmonol 38: 456–464, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitano P, Worthington CL, Jenkin JA, Stephens NL, Gyapong S, Wang L, Murphy TM. Ontogenesis of myosin light chain phosphorylation in guinea pig tracheal smooth muscle. Pediatr Pulmonol 39: 108–116, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YH, Wheeler MA, Weiss RM. Ontogeny of cyclic AMP and cyclic GMP phosphodiesterase activities and of calmodulin levels in guinea pig ureter. J Urol 139: 1095–1098, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Elliott CF, Pearce WJ. Effects of maturation on cell water, protein, and DNA content in ovine cerebral arteries. J Appl Physiol 79: 831–837, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Fisher SA, Ikebe M. Developmental and tissue distribution of expression of nonmuscle and smooth muscle isoforms of myosin light chain kinase. Biochem Biophys Res Commun 217: 696–703, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Kawano K, Yoshiyama S, Kawamichi H, Wang X, Nakamura A, Kohama K. Myosin light chain kinase stimulates smooth muscle myosin ATPase activity by binding to the myosin heads without phosphorylating the myosin light chain. Biochem Biophys Res Commun 305: 16–21, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Ye LH, Kishi H, Okagaki T, Samizo K, Nakamura A, Kohama K. Myosin light chain kinase as a multifunctional regulatory protein of smooth muscle contraction. IUBMB Life 51: 337–344, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gong MC, Fuglsang A, Alessi D, Kobayashi S, Cohen P, Somlyo AV, Somlyo AP. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem 267: 21492–21498, 1992. [PubMed] [Google Scholar]
- 15.Hai CM Length-dependent myosin phosphorylation and contraction of arterial smooth muscle. Pflügers Arch 418: 564–571, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Han YJ, Hu WY, Chernaya O, Antic N, Gu L, Gupta M, Piano M, de Lanerolle P. Increased myosin light chain kinase expression in hypertension: regulation by serum response factor via an insertion mutation in the promoter. Mol Biol Cell 17: 4039–4050, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartshorne DJ, Siemankowski RF. Regulation of smooth muscle actomyosin. Annu Rev Physiol 43: 519–530, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Soderling TR. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+/calmodulin-dependent protein kinase II: comparative study of the phosphorylation sites. Arch Biochem Biophys 278: 41–45, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem 283: 18505–18512, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hulen D, Baron A, Salisbury J, Clarke M. Production and specificity of monoclonal antibodies against calmodulin from Dictyostelium discoideum. Cell Motil Cytoskeleton 18: 113–122, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Ikebe M, Hartshorne DJ, Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J Biol Chem 262: 9569–9573, 1987. [PubMed] [Google Scholar]
- 22.Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, Hartshorne DJ. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun 159: 871–877, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci USA 101: 6279–6284, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh T, Ikebe M, Kargacin GJ, Hartshorne DJ, Kemp BE, Fay FS. Effects of modulators of myosin light-chain kinase activity in single smooth muscle cells. Nature 338: 164–167, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, Rao K, Halayko AJ, Liu X, Stephens NL. Ragweed sensitization-induced increase of myosin light chain kinase content in canine airway smooth muscle. Am J Respir Cell Mol Biol 7: 567–573, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, Rao K, Liu X, Liu G, Stephens NL. Increased Ca2+ and myosin phosphorylation, but not calmodulin activity in sensitized airway smooth muscles. Am J Physiol Lung Cell Mol Physiol 268: L739–L746, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem 264: 5339–5342, 1989. [PubMed] [Google Scholar]
- 30.Knapp J, Aleth S, Balzer F, Schmitz W, Neumann J. Calcium-independent activation of the contractile apparatus in smooth muscle of mouse aorta by protein phosphatase inhibition. Naunyn Schmiedebergs Arch Pharmacol 366: 562–569, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kohama K, Ye LH, Hayakawa K, Okagaki T. Myosin light chain kinase: an actin-binding protein that regulates an ATP-dependent interaction with myosin. Trends Pharmacol Sci 17: 284–287, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol 508: 871–881, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Liu X, Rao K, Jiang H, Stephens NL. Increased myosin light chain kinase content in sensitized canine saphenous vein. J Appl Physiol 80: 665–669, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Maass-Moreno R, Burdyga T, Mitchell RW, Seow CY, Ragozzino J, Ford LE. Simple freezing apparatus for resolving rapid metabolic events associated with smooth muscle activation. J Appl Physiol 90: 2453–2459, 2001. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA 98: 2419–2424, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca2+-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol 104: 265–286, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsui K, Higashi K, Fukunaga K, Miyazaki K, Maeyama M, Miyamoto E. Hormone treatments and pregnancy alter myosin light chain kinase and calmodulin levels in rabbit myometrium. J Endocrinol 97: 11–19, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Miller-Hance WC, Miller JR, Wells JN, Stull JT, Kamm KE. Biochemical events associated with activation of smooth muscle contraction. J Biol Chem 263: 13979–13982, 1988. [PubMed] [Google Scholar]
- 39.Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J 364: 431–440, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy RA, Walker JS. Inhibitory mechanisms for cross-bridge cycling: the nitric oxide-cGMP signal transduction pathway in smooth muscle relaxation. Acta Physiol Scand 164: 373–380, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Nauli SM, Williams JM, Akopov SE, Zhang L, Pearce WJ. Developmental changes in ryanodine- and IP3-sensitive Ca2+ pools in ovine basilar artery. Am J Physiol Cell Physiol 281: C1785–C1796, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Nauli SM, Zhang L, Pearce WJ. Maturation depresses cGMP-mediated decreases in. Am J Physiol Heart Circ Physiol 280: H1019–H1028, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa M, Shirakawa S, Adelstein RS. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J Biol Chem 260: 8978–8983, 1985. [PubMed] [Google Scholar]
- 44.Numata T, Katoh T, Yazawa M. Functional role of the C-terminal domain of smooth muscle myosin light chain kinase on the phosphorylation of smooth muscle myosin. J Biochem (Tokyo) 129: 437–444, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Pearce WJ Cerebral blood flow during hemodilution and hypoxia in rats: role of ATP-sensitive potassium channels. Stroke 30: 1947–1948, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991. [DOI] [PubMed] [Google Scholar]
- 47.Pearce WJ, Longo LD. Developmental aspects of endothelial function. Semin Perinatol 15: 40–48, 1991. [PubMed] [Google Scholar]
- 48.Qi DF, Schatzman RC, Mazzei GJ, Turner RS, Raynor RL, Liao S, Kuo JF. Polyamines inhibit phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J 213: 281–288, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rembold CM, Meeks MK, Ripley ML, Han S. Longer muscle lengths recapitulate force suppression in swine carotid artery. Am J Physiol Heart Circ Physiol 292: H1065–H1070, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem 262: 7796–7801, 1987. [PubMed] [Google Scholar]
- 51.Sandoval RJ, Injeti ER, Gerthoffer WT, Pearce WJ. Postnatal maturation modulates relationships among cytosolic Ca2+, myosin light chain phosphorylation, and contractile tone in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 293: H2183–H2192, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Savineau JP, Marthan R. Modulation of the calcium sensitivity of the smooth muscle contractile apparatus: molecular mechanisms, pharmacological and pathophysiological implications. Fundam Clin Pharmacol 11: 289–299, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Smith L, Parizi-Robinson M, Zhu MS, Zhi G, Fukui R, Kamm KE, Stull JT. Properties of long myosin light chain kinase binding to F-actin in vitro and in vivo. J Biol Chem 277: 35597–35604, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Sobieszek A Regulation of smooth muscle myosin light chain kinase. Allosteric effects and co-operative activation by calmodulin. J Mol Biol 220: 947–957, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Somlyo AV, Wang H, Choudhury N, Khromov AS, Majesky M, Owens GK, Somlyo AP. Myosin light chain kinase knockout. J Muscle Res Cell Motil 25: 241–242, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: functional domains and structural motifs. Acta Physiol Scand 164: 471–482, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Stull JT, Tansey MG, Word RA, Kubota Y, Kamm KE. Myosin light chain kinase phosphorylation: regulation of the Ca2+ sensitivity of contractile elements. Adv Exp Med Biol 304: 129–138, 1991. [DOI] [PubMed] [Google Scholar]
- 59.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem 269: 9912–9920, 1994. [PubMed] [Google Scholar]
- 61.Volpe JJ Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 7: 56–64, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Walsh MP, Persechini A, Hinkins S, Hartshorne DJ. Is smooth muscle myosin a substrate for the cAMP-dependent protein kinase? FEBS Lett 126: 107–110, 1981. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe M, Sawai T, Nagura H, Suyama K. Age-related alteration of cross-linking amino acids of elastin in human aorta. Tohoku J Exp Med 180: 115–130, 1996. [DOI] [PubMed] [Google Scholar]
- 64.Wilson DP, Sutherland C, Borman MA, Deng JT, Macdonald JA, Walsh MP. Integrin-linked kinase is responsible for Ca2+-independent myosin diphosphorylation and contraction of vascular smooth muscle. Biochem J 392: 642–648, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest 92: 29–37, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu YW, Lynch JK, Nelson KB. Perinatal arterial stroke: understanding mechanisms and outcomes. Semin Neurol 25: 424–434, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Zurcher SD, Pearce WJ. Maturation modulates serotonin- and potassium-induced calcium-45 uptake in ovine carotid and cerebral arteries. Pediatr Res 38: 493–500, 1995. [DOI] [PubMed] [Google Scholar]