Abstract
Clinical and experimental evidence has shown that myocardial ischemia activates cardiac spinal afferents that mediate sympathoexcitatory reflex responses. During myocardial ischemia, thromboxane A2 (TxA2) is released in large quantities by activated platelets in the coronary circulation of patients with coronary artery disease. We hypothesized that endogenous TxA2 contributes to sympathoexcitatory reflexes during myocardial ischemia through stimulation of TxA2/prostaglandin endoperoxide (TP) receptors. Regional myocardial ischemia was induced by occlusion of a diagonal branch of left anterior descending coronary artery of anesthetized cats. Hemodynamic parameters and renal sympathetic nerve activity were recorded after sinoaortic denervation and bilateral vagotomy. Regional myocardial ischemia evoked significant increases in mean blood pressure (122 ± 10 vs. 139 ± 12 mmHg, before vs. ischemia), aortic flow (153 ± 18 vs. 167 ± 20 ml/min), first derivative of left ventricular pressure at 40-mmHg developed pressure (2,736 ± 252 vs. 2,926 ± 281 mmHg/s), systemic vascular resistance (0.6 ± 0.1 vs. 0.9 ± 0.12 peripheral resistance units), and renal sympathetic nerve activity (by 22%). The reflex nature of the excitatory responses was confirmed by observing its disappearance after blockade of cardiac nerve transmission with intrapericardial 2% procaine treatment. Moreover, application of U-46619 (2.5–10 μg), a TxA2 mimetic, on the heart caused graded increases in mean arterial pressure and renal nerve activity, responses that were abolished 3 min after local blockade of cardiac neural transmission with intrapericardial procaine. BM 13,177 (30 mg/kg iv), a selective TP receptor antagonist, eliminated the reflex responses to U-46619 and significantly attenuated the excitatory responses during brief (5 min) regional myocardial ischemia. The sympathoexcitatory reflex responses to U-46619 were unchanged by blockade of histamine H1 receptors with pyrilamine and serotonin 5-HT3 receptors with tropisetron, indicating specificity of this TP receptor agonist. These data indicate that endogenous TxA2 participates in myocardial ischemia-mediated sympathoexcitatory reflex responses through a TP receptor mechanism.
Keywords: sympathetic nerves; U-46619; BM 13,177
clinical observations indicate that angina pectoris can be accompanied by either hypertension and tachycardia, hypotension and bradycardia, or hypotension and tachycardia (19, 20, 30). The latter response very likely is mediated by the baroreceptor-induced reflex chronotropic response to the hypertension. The former two responses more likely are caused by cardiogenic reflexes. In fact, myocardial ischemia is a powerful stimulus that can activate both vagal and sympathetic (spinal) cardiac sensory nerve fibers. Excitation of cardiac vagal afferents leads to inhibitory reflex responses, including hypotension, bradycardia, and decreased peripheral vascular resistance (33, 48, 55). Our laboratory (28) and others (29, 55) have shown that activation of cardiac sympathetic afferent elicits excitatory responses, including hypertension, tachycardia, and increased vasomotor tone. Bilateral removal of the stellate ganglia and excision of the first through fifth thoracic sympathetic ganglia in patients relieves cardiac nociception and the associated excitatory reflex responses during myocardial ischemia (56), indicating that sympathetic pathways transmit information that produce pain and excite the cardiovascular system.
Mechanisms underlying myocardial ischemia-mediated excitatory reflex responses have been investigated for many years. A number of metabolites, including reactive oxygen species, protons derived from lactic acid, bradykinin, serotonin, histamine, and cyclooxygenase products, have been reported to evoke excitatory cardiovascular reflexes and to stimulate or sensitize cardiac spinal afferents (13–15, 23, 38, 47, 49, 51). However, the role of thromboxane A2 (TxA2) in the heart with respect to its role in initiating ischemia-mediated reflex responses has not been evaluated.
TxA2, a metabolite of arachidonic acid, is produced during myocardial ischemia and functions as a vasoconstrictor agent and a platelet activator. In this respect, previous studies have documented release of TxA2 during myocardial ischemia in patients with angina and myocardial infarction, as well as in animal models following coronary arterial occlusion (11, 21, 39). Other laboratories have shown that TxA2 is a strong vasoconstrictor agent and induces platelet aggregation (2). TxA2 also may influence neuronal function, although evidence for this action is limited. In this regard, Karla and colleagues (24) observed that infusion of the stable TxA2 analog, U-46619, into the inferior vena cava causes vagally mediated, rapid, shallow breathing and pulmonary hypertension (42). Furthermore, U-46619 activates cardiac vagal afferent nerves that produce reflex bradycardia (53). U-46619 also is capable of directly stimulating hindlimb group III and IV somatic sensory nerves (25). These observations on exogenous TxA2 led us to speculate that endogenous TxA2 may activate cardiac sympathetic nerves during ischemia and thus contribute to ischemia-mediated sympathoexcitatory reflex responses. This possibility was reinforced by our laboratory's recent study showing that cardiac sympathetic afferents are activated, in part, through a mechanism involving TxA2 (16).
Previous studies have demonstrated that activated platelets, stimulated by platelet TxA2/prostaglandin endoperoxide (TP) receptor, release 5-HT and histamine from their dense granules (9, 32). Our laboratory's previous data indicate that 5-HT and histamine excite cardiac sympathetic afferents (13, 15). Thus it is likely that 5-HT and histamine may contribute to the TxA2-related excitatory reflex responses through stimulation of cardiac afferents.
The aim of the present study, therefore, was to determine whether endogenously produced TxA2 contributes to myocardial ischemia-induced sympathoexcitatory reflex responses. We hypothesized that TxA2 evokes sympathoexcitatory reflex responses through stimulation of cardiac afferent nerves, and that blockade of TP receptors would attenuate myocardial ischemia-related excitatory responses. Furthermore, we postulated that TxA2 would elicit excitatory reflexes partially through the release of 5-HT and histamine from platelets and through their actions on cardiac afferents. To achieve this goal, we used a modified model of regional myocardial ischemia-induced reflexes in baro-denervated and vagotomized cats from one that had been previously described (27). A preliminary report of a portion of this study has been published (40).
METHODS
Surgical Preparation
All experimental preparations and protocols were reviewed and approved by the Animal Care and Use Committee at the University of California, Irvine. The studies conformed to American Physiological Society's “Guiding Principles in the Care and Use of Animals.” Fifty-nine adult cats of either sex (2.1–4.8 kg) were anesthetized with intramuscular injection of ketamine (20–30 mg/kg) followed by a bolus intravenous injection of α-chloralose (40–50 mg/kg) through the femoral vein. Additional α-chloralose (5–10 mg/kg iv) was given, as needed, to maintain an adequate depth of anesthesia that was assessed by observing the absence of a conjunctival reflex. The trachea was intubated, and respiration was maintained artificially (model 661, Harvard ventilator, Ealing, South Natick, MA). A femoral vein was cannulated to administer drugs and fluids. Systemic arterial blood pressure (BP) was monitored by a pressure transducer attached to a cannula inserted into the femoral artery. Arterial blood gases and pH were measured with a blood-gas analyzer (ABL 5, Radiometer America, West Lake, OH) and maintained within physiological limits (Po2 > 100 Torr, Pco2 28–35 Torr, pH 7.35–7.45) by adjusting the respiratory rate or tidal volume or by administering NaHCO3 (1 M iv). Body temperature was monitored by a rectal thermistor and maintained at 36–38°C with a circulating water heating pad and heat lamp. At the end of the experiment, animals were euthanized by administering a solution of saturated potassium chloride into the femoral vein under deep anesthesia that was ensured by giving an additional dose of α-chloralose (50 mg/kg iv) just before injection of potassium.
After midline sternotomy, a small incision was made in the pericardium to expose the anterior portion of the heart for occlusion of the coronary arteries to induce myocardial ischemia and to allow epicardial application of drugs. The exposed portion of the heart was prevented from drying by covering the open thorax with a saline-soaked gauze pad and a plastic sheet.
In some of the cats, a small incision into the left fourth or fifth intercostal space was made to expose the pericardium. A PE 60 catheter with six perforations in its distal end was introduced into the pericardium for intrapericardial application of drugs. Care was taken to eliminate any leaks from the pericardium by tightening the small hole in the pericardium through which the catheter had been introduced with a silk suture. We observed no leakage over a 5-min period after injection of 2 ml of saline. Following this procedure, the ribs were approximated, and the chest was closed.
A ventral abdominal midline incision in some cats was used to expose the gallbladder for topical application of bradykinin. The gallbladder was kept moist by application of a saline-soaked gauze pad and a plastic sheet over the organ after exposure.
For measuring left ventricular (LV) pressure (LVP) in some of the cats, a catheter-tip (4F) pressure transducer (Millar PC-350, Millar, Houston, TX) was placed in the cavity of the LV through the apex. The first derivative of LVP (dP/dt) was obtained by processing the LVP signal with a differentiator amplifier (model 13–4615, Gould, Cleveland, OH). The LV dP/dt at 40-mmHg developed pressure (LV dP/dt40) was calculated as an index of myocardial contractility, since this index is affected minimally by alterations in preload or afterload (31).
An electromagnetic flow transducer (T106X/T206X, Transonic Systems, Ithaca, NY) was positioned around the ascending aorta to measure mean aortic flow (MAF) as an index of cardiac output (CO). Total peripheral vascular resistance was calculated as MAF divided by mean arterial pressure (MAP) and was expressed in terms of peripheral resistance units. All hemodynamic parameters were recorded continuously on a chart recorder (TA 4000B, Gould, Cleveland, OH) and were processed with a Pentium computer with Spike 2 software through an analog-to-digital converter (CED micro 1401 mkII; CED, Cambridge, UK) for subsequent off-line analysis (Spike 2, Cambridge Electronics Design, Cambridge, UK).
Sinoaortic Denervation and Cervical Vagotomy
Sinoaortic denervation and cervical vagotomy were performed to minimize the BP “buffering” action of arterial baroreceptors and to eliminate the influence of vagal cardiac afferents that could mask the reflex responses to stimulation of sympathetic afferents (23). Briefly, a midline cervical incision was made to expose the carotid arteries and cervical vagi bilaterally. Each carotid sinus nerve was identified, ligated, and cut under a dissecting microscope (Zeiss). The vagal cardiopulmonary receptors and aortic arch baroreceptors were denervated by transecting both cervical vagi. Barodenervation was verified by noting the absence of the normal decrease of heart rate (HR) in response to ∼40-mmHg increase in arterial BP induced by administration of phenylephrine (10 μg/kg iv).
Renal Sympathetic Nerve Recording
Cats were placed in the lateral decubitus position. An incision was made in the left flank, and the renal sympathetic nerve was exposed retroperitoneally. A dissecting microscope (Zeiss) was used to isolate a branch of the renal nerve from surrounding connective tissue. The nerve was covered with warm mineral oil and placed across one pole of a recording electrode. The other pole of the recording electrode was grounded with a cotton thread to the animal. The recording electrode was attached to a high-impedance probe (model HIP511, Grass-Telefactor, West Warwick, RI). The signal was amplified and processed through an audio amplifier (AM8B audiomonitor, Grass-Telefactor) with the low-frequency cutoff set at 30 Hz and high-frequency cutoff set at 3 kHz. The amplified signal was monitored on an oscilloscope (model 2201, Tektronix, Beavertown, OR) and was processed with a Pentium computer through an analog-to-digital converter (CED micro 1401 mkII, Cambridge, UK) for subsequent offline analysis. Discharge activity of the renal sympathetic nerve was analyzed with data acquisition and analysis software (Spike 2). Electrical noise level in neural recordings was determined after crushing the nerves at the end of the experiment. A window discriminator was set just above the noise level so that only renal nerve discharge signals were counted. Nerve discharge signals also were rectified and averaged over a period of 10 s with software (Spike 2) and denoted as integrated renal sympathetic nerve activity (RSNA).
Experimental Protocols
Regional myocardial ischemia model.
After completion of the surgical preparation, a minimum of 60 min was allowed for stabilization of arterial pressure. In a preliminary study, 5 min of regional myocardial ischemia were induced, as described previously (27). In brief, with the aid of a surgical microscope (Zeiss), the main left anterior descending (LAD) artery was dissected carefully from the surrounding tissue, avoiding damage to the pericoronary nerve. A snare was passed loosely around the distal third of the LAD for subsequent coronary occlusion. We determine regional ischemia by observing a regional change in the color of the myocardium, which has been shown previously to closely correlate with the production of lactic acid, as indicated by a reduction in tissue pH (38). Changes in tissue pH actually are a more direct indicator of myocardial ischemia than changes in regional cardiac function, as shown by hemodynamic parameters. We have used this method as an index of myocardial ischemia in our laboratory's previous studies (13, 15, 22, 50). We initially induced regional ischemia in three barodenervated, vagotomized cats. We observed that ischemia increased MAP by only 2 and 5 mmHg in two of the cats and decreased MAP by 2 mmHg in one cat. We, therefore, changed the model slightly to allow brief regional myocardial ischemia induced by occlusion of the second or third diagonal branch of the LAD in two barodenervated, vagotomized cats for 5 min. We observed consistent increases in MAP by 20 and 28 mmHg during regional ischemia and, therefore, chose the latter model of ischemia for the present study.
To determine the sympathoexcitatory reflex responses to myocardial ischemia, we divided 23 animals into three groups. In the first group of cats (n = 10), arterial BP, HR, aortic flow, LVP, and LV dP/dt40 were recorded during 5 min of preischemia, ischemia, and reperfusion.
In the second group of eight other cats, we evaluated the reproducibility of sympathoexcitatory reflex responses to regional myocardial ischemia. After stabilization of arterial pressure and RSNA in this group, an initial 5-min period of regional myocardial ischemia was induced, while RSNA, BP, and HR were recorded. A second 5-min period of ischemia was induced 15 min after intravenous administration of 2 ml of 2% NaHCO3 (vehicle for BM 13,177), which was 30 min after the initial ischemia.
In the third group of five separate cats, we examined the influence of cardiac afferent blockade with the local anesthetic procaine on the sympathoexcitatory reflex responses to myocardial ischemia. After the preparation had stabilized, an initial 5-min period of regional ischemia was induced while RSNA, BP, and HR were recorded. We then topically applied 0.3 ml of 2% procaine to the ischemic area around the second or third diagonal branch of the LAD using a 2-cm2 patch of filter paper. Regional myocardial ischemia was induced for a second time 3 min after application of procaine and 30 min after the initial bout of ischemia. Previous studies have demonstrated that this dose of procaine eliminates cardiac reflex responses by blocking cardiac afferent neurotransmission (3, 10), since cardiac sympathetic afferent nerve endings are located mainly in the epicardial layer of the myocardium (4). Lastly, bradykinin (3 μg, Sigma-Aldrich, St. Louis, MO) was applied to the gallbladder to verify that the animals remained reflexogenic.
Dose response.
In eight other animals, 0.1 ml of U-46619 or 2% ethanol (vehicle) was applied epicardially, either to the anterior ventricular surface or through a catheter placed in the pericardial space. Initially, we applied 0.1 ml of U-46619 to the anterior ventricular surface with a 2-cm2 filter paper in three animals and observed sympathoexcitatory reflex responses ranging from 17- to 23-mmHg increases in MAP and 16–24% increases in RSNA. In five other cats, we observed similar responses (MAP: 18–25 mmHg and RSNA: 17–27%) following injection of 0.1 ml of U-46619 into the pericardial space. Thus, in subsequent protocols, we used intrapericardial application of U-46619, 2% procaine, or vehicle rather than epicardial application. U-46619 and the vehicle were applied randomly. The heart was washed with 2 ml of warm saline (35°C) three times after each application of drug or vehicle. BP, HR, and RSNA were recorded in this protocol. Dose-response curves were generated with three doses of U-46619 (2.5, 5, and 10 μg, Sigma-Aldrich) applied at least 20 min apart to avoid tachyphylaxis. Twenty-five minutes after completion of the dose-response curve, 10 μg of U-46619 were reapplied to evaluate for consistency of the reflex response to this dose of U-46619, which served as a control group for the next U-46619-BM 13,177 protocol. Stock solution was prepared using 2 mg of U-46619 dissolved in 0.4 ml of 100% ethanol to achieve an initial concentration of 5 mg/ml that was stored in a −70°C freezer. A working solution of 100 μg/ml was made by removing 20 μl from the U-46619 stock solution and adding 980 μl of 0.9% saline to obtain the final concentration. Solutions of 50 and 10 μg/ml of U-46619 were made by further diluting the 100 μg/ml solution with 0.9% saline. Ethanol (2%) served as the vehicle.
U-46619-BM 13,177.
We examined the influence of blocking TP receptors with BM 13,177, a specific TP receptor antagonist (37), on the BP, HR, and RSNA responses following intrapericardial application of U-46619 in seven cats. In this protocol, 10 μg of a working solution of U-46619 (0.1 ml) were injected into the pericardial space, while BP, HR, and RSNA were recorded. Repeated intrapericardial application of U-46619 was conducted 15 min after intravenous administration of BM 13,177 (30 mg/kg, Hoffmann-La Roche, Nutley, NJ) and 30 min after initial stimulation with U-46619. BM 13,177 was dissolved in 1 ml of 8.4% NaHCO3 and diluted as needed with 0.9% saline to a concentration of 30 mg/ml. Previous studies have demonstrated that this dose of BM 13,177 completely inhibits platelet aggregation by blocking TP receptors (36, 37). Following each application of U-46619, the heart was washed three times with 2 ml of warm saline (35°C). Bradykinin (3 μg, Sigma-Aldrich) was applied to the gallbladder 20 min after the second application of U-46619 to verify that the animal remained reflexogenic.
U-46619 + procaine.
In six separate animals, we examined the effect of local blockade of cardiac nerve transmission with procaine on the sympathoexcitatory responses (BP, HR, and RSNA) following intrapericardial application of 10 μg of U-46619 (0.1 ml). In this protocol, 0.1 ml of the U-46619 (100 μg/ml) was injected into the pericardium, while BP, HR, and RSNA were recorded. We repeated intrapericardial application of U-46619 3 min after 0.3 ml of 2% intrapericardial procaine, which was 20 min after the initial dose of U-46619. The heart was washed three times with 2 ml of warm saline (35°C) after each application of U-46619. Bradykinin (3 μg) was applied on the gallbladder 20 min after the second application of U-46619 to verify that the animal remained responsive.
Ischemia-BM 13,177.
In 10 other animals, we examined the influence of TP receptor blockade with BM 13,177 on the sympathoexcitatory reflex responses to regional myocardial ischemia. After stabilization, an initial period of ischemia was induced, while RSNA, BP, and HR were recorded. A second period of ischemia was repeated 15 min after administration of BM 13,177 (30 mg/kg iv), which was 30 min after the initial period of ischemia. Bradykinin (3 μg) was applied to the gallbladder at the end of every experiment to demonstrate reflex responsiveness. The group noted above that was studied during repeat ischemia served as the time control for this protocol.
U-46619-pyrilamine and tropisetron.
The influence of histamine H1 and serotonin 5-HT3 receptor blockade with pyrilamine (H1 receptor antagonist) and tropisetron (5-HT3 receptor antagonist) on the BP, HR, and RSNA responses following intrapericardial application of U-46619 was evaluated in five additional cats. In this protocol, 10 μg of a working solution of U-46619 (0.1 ml) were injected into the pericardium, while BP, HR, and RSNA were recorded. Repeated intrapericardial application of U-46619 was performed 15 min after intravenous administration of pyrilamine (300 μg/kg iv) and tropisetron (300 μg/kg iv), 30 min after initial stimulation with U-46619. Our laboratory has demonstrated previously that these doses of antagonists eliminate the cardiac sympathetic afferent responses to histamine and 5-HT (13, 15).
Data Analysis
RSNA was expressed as the percent change from baseline activity to account for the variability in baseline activity that occurs with multiunit nerve recordings. Baseline activity was determined by averaging RSNA over the 1-min period immediately preceding chemical application or over the 3-min period immediately preceding ischemia. Response of RSNA to U-46619 was determined by averaging RSNA during the entire period of each response, defined as the time during which sustained activity exceeded baseline activity by 3%. The RSNA response to ischemia was determined by averaging RSNA during the 5 min of coronary occlusion.
Values are expressed as means ± SE. The Kolmogorov-Smirnov test was used to determine whether the data were distributed normally. Normally distributed data in all protocols were compared with a one-way repeated-measures ANOVA followed post hoc by Tukey's test. Nonnormally distributed data were compared with the Friedman repeated-measures ANOVA on ranks followed by Dunnett's post hoc test. All statistical calculations were performed with SigmaStat software (Jandel Scientific Software, San Rafael, CA). Values were considered to be significantly different when P < 0.05.
RESULTS
Regional Ischemia
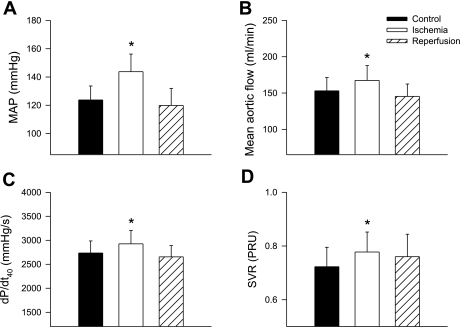
We observed that regional myocardial ischemia induced by occlusion of the second or third diagonal branch of the LAD coronary artery significantly increased the magnitude of the excitatory reflex response in the entire group of cats (n = 10). To disclose the detailed reflex responses in this group, we found that an increased or excitatory hemodynamic response to ischemia predominated in eight animals, while a slight reduction occurred in two animals. Thus ischemia evoked excitatory hemodynamic responses, including significant increases in MAP from 122 ± 10 to 139 ± 12 mmHg, MAF from 153 ± 18 to 167 ± 20 ml/min, dP/dt40 from 2,736 ± 252 to 2,926 ± 281 mmHg/s, and systemic vascular resistance from 0.6 ± 0.1 to 0.9 ± 0.12 peripheral resistance units (Fig. 1) in eight animals. HR, which was quite high at baseline (206 ± 21 beats/min), generally was unchanged. Regional myocardial ischemia also evoked increases in RSNA in eight other animals in which this parameter was measured (Figs. 2 and 3). The onset latencies of excitatory RSNA and MAP responses to myocardial ischemia averaged 33 ± 4 s (n = 8) and 39 ± 6 s (n = 8) and ranged from 29 to 44 and 32 to 49 s, respectively. The ischemia-mediated reflex responses lasted an average of 191 ± 13 s and ranged between 154 and 226 s.
Fig. 1.
Hemodynamic responses to brief (5 min) regional myocardial ischemia in eight cats following sinoaortic denervation and bilateral vagotomy. Brief ischemia reflexly increased mean arterial blood pressure (MAP; A), mean aortic flow (B), left ventricular first derivative of pressure at 40 mmHg developed pressure (LV dP/dt40; C), and systemic vascular resistance (SVR; D). PRU, peripheral resistance units. Values are means ± SE. *P < 0.05 compared with respective control.
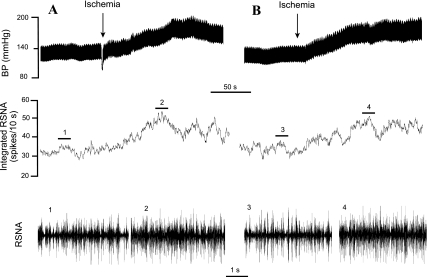
Fig. 2.
Representative tracings showing responses of arterial pressure, renal sympathetic nerve activity (RSNA), and integrated RSNA to repeated regional myocardial ischemia in a cat following sinoaortic denervation and bilateral vagotomy. A: shows an increase in MAP by 55 mmHg and integrated RSNA by 37% during the initial period of ischemia. B: demonstrated that the responses are reproducible, since MAP increased by 52 mmHg and integrated RNSA increased by 33% during the second period of ischemia after treatment with the vehicle (2% NaHCO3, 2 ml iv). Neurograms 1–4 are representative samples of RSNA at times indicated by bar above integrated RSNA recordings. BP, blood pressure.
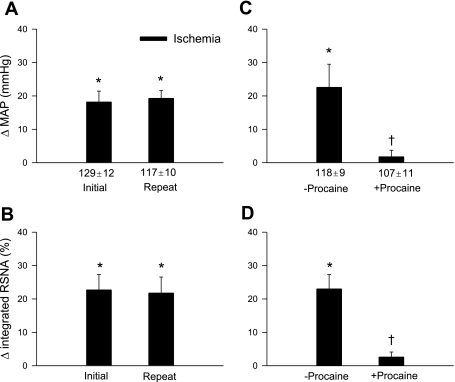
Fig. 3.
Sympathoexcitatory reflex responses to repeated regional myocardial ischemia before and after treatment with procaine in sinoaortic-denervated and bilateral-vagotomized cats. We observed consistent increases (Δ) in MAP (A) and integrated RSNA (B) evoked during repeated ischemia, before and after administration of the vehicle (2% NaHCO3, 2 ml iv) in eight cats. Conversely, epicardial application of 2% procaine abolished the increases in MAP (C) and integrated RSNA (D) evoked by ischemia in five cats. Values are means ± SE. *P < 0.05 compared with respective controls. †P < 0.05 postprocaine vs. preprocaine.
Blockade of local cardiac nerve transmission by epicardial application of procaine prevented the ischemia-evoked excitatory BP and RSNA responses (Fig. 3, C and D).
Dose Responses
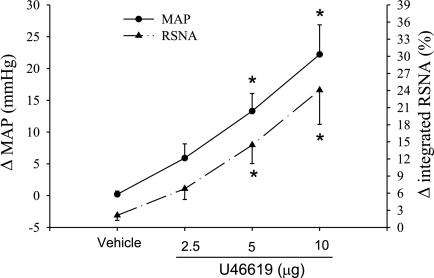
We combined the reflex response data resulting from epicardial and intrapericardial applications of U-46619 together, since the two methods of stimulation evoked a similar reflex responses. We found that application of increasing doses of U-46619 to the heart surface evoked graded sympathoexcitatory reflex responses, including increases in arterial pressure and renal nerve activity in barodenervated and vagotomized cats, while HR was unchanged (Fig. 4). In contrast, application of the vehicle (2% ethanol) did not alter MAP and RSNA.
Fig. 4.
MAP and integrated RSNA responses to application of vehicle (2% ethanol) and graded doses of U-46619 to the heart surface in eight cats. Values are means ± SE. *P < 0.05 compared with vehicle application.
U-46619-Procaine and BM 13,177
The increases in MAP and RSNA in response to intrapericardial U-46619 were eliminated by blockade of cardiac neuronal transmission with intrapericardial application of procaine and by blockade of TP receptors with BM 13,177 (30 mg/kg iv) (Fig. 5, Table 1). In contrast, application of bradykinin to the gallbladder still increased MAP (35 ± 6.4 mmHg) and integrated RSNA (53 ± 10%) after BM 13,177. The onset latency of arterial pressure and RSNA responses to the initial stimulation with U-46619 averaged from 47 ± 6 and 41 ± 4 s and ranged from 38 to 56 s and 35 to 51 s, respectively.
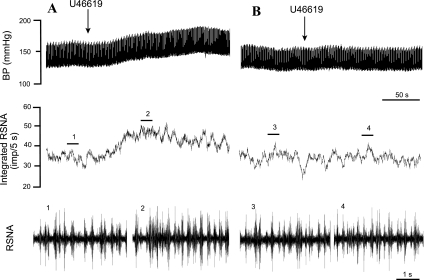
Fig. 5.
Original records of sympathoexcitatory reflex responses evoked by intrapericardial application of U-46619 before (A) and after (B) local procaine (2%). A: U-46619 (10 μg) increased MAP by 28 mmHg and peak integrated RSNA by 29%. B: the responses to U-46619 were fully inhibited by intrapericardial application of 2% procaine (0.2 ml). Neurograms 1–4 are representative of original RSNA recordings at times indicated by bar above integrated RSNAs.
Table 1.
Excitatory reflex responses to intrapericardial U-46619
| n |
ΔMAP, mmHg |
ΔRSNA, %
|
|||
|---|---|---|---|---|---|
| Initial | Repeated | Initial | Repeated | ||
| −Procaine | 8 | 21±4† | 22±5† | 20±9† | 21±10† |
| +Procaine | 6 | 22±6† | 2±1* | 21±8† | −0.3±2.0* |
| +BM 13,177 | 7 | 22±9† | 2.5±3* | 22±10† | 3.1±3.9* |
| +Tropisetron & pyrilamine | 4 | 25±8† | 27±9† | 28±11† | 26±9.8† |
Values are means ± SE; n, no. of cats. Δ, Change; MAP, mean arterial pressure; RSNA, integrated renal sympathetic nerve activity. 2% Procaine was applied in the pericardial space 3 min before repeated application of U-46619 (10 μg). BM 13,117 was injected intravenously 15 min before repeated U-46619 application.
P < 0.05 compared with initial response.
P < 0.05 compared with respective control.
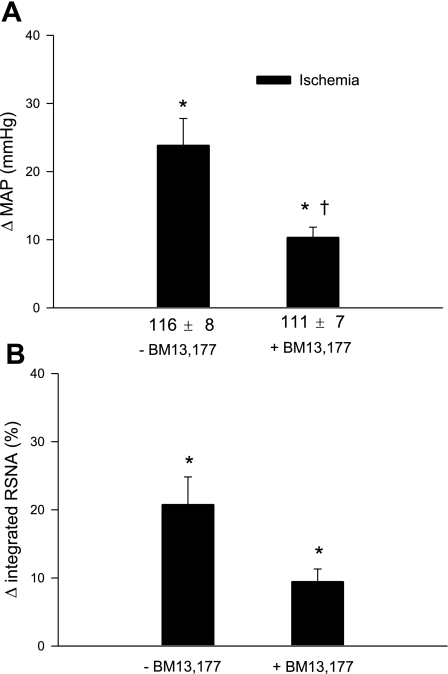
Ischemia-BM 13,177
The initial period of regional ischemia significantly increased MAP and RSNA after latencies of 43 ± 10 and 39 ± 11 s, respectively. Blockade of TP receptors with BM 13,177 (30 mg/kg iv) significantly attenuated the ischemia-mediated increases of MAP and RSNA (Figs. 6 and 7). In contrast, ischemia-mediated reflex responses, including increases in MAP and RSNA, were consistent during initial and repeat regional myocardial ischemia in the absence of blockade (Figs. 2 and 3). Application of bradykinin to the gallbladder increased MAP (31 ± 7.2 mmHg) and RSNA (48 ± 11%) after BM 13,177.
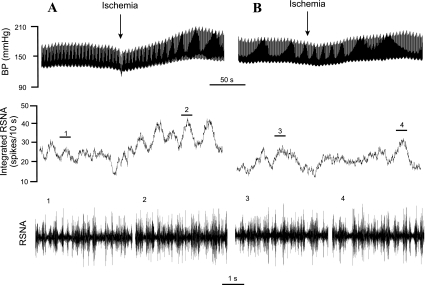
Fig. 6.
Original responses of BP, RSNA, and integrated RSNA during repeated regional myocardial ischemia, before and after treatment with BM 13,177 in a cat following sinoaortic denervation and cervical vagotomy. A: excitatory BP (MAP: 43-mmHg increase) and RSNA (peak integrated RSNA: 37% increase) responses during an initial period of ischemia. B: shows that blockade of thromboxane A2/prostaglandin endoperoxide (TP) receptors with BM 13,177 (30 mg/kg iv) attenuated the increases in MAP and integrated RSNA during ischemia. Neurograms 1–4 are representative of original RSNA recordings at times indicated by the short line above the integrated RSNA recordings.
Fig. 7.
MAP and integrated RSNA responses to repeated regional myocardial ischemia in 10 bilateral sinoaortic-denervated and vagotomized cats before and after blockade of TP receptors with BM 13,177 (30 mg/kg iv). A: responses of MAP to repeated ischemia before and after BM 13,177. B: integrated RSNA responses to repeated ischemia before and after BM 13,177. Values are means ± SE. *P < 0.05 vs. control; †P < 0.05, post-BM 13,177 vs. pre-BM 13,177.
U-46619 with Pyrilamine and Tropisetron Studies
The excitatory MAP and RSNA responses to intrapericardial application of U-46619 were unchanged by combined histamine H1 receptor blockade with pyrilamine (300 μg/kg iv) and serotonin 5-HT3 receptor blockade with tropisetron (300 μg/kg iv) (Table 1).
DISCUSSION
This is the first documentation that endogenous TxA2 contributes to sympathoexcitatory reflexes during myocardial ischemia. We observed a consistent pattern of hemodynamic responses, including increases in MAP, CO, myocardial contractility, and renal sympathetic efferent nerve activity, during coronary arterial occlusion in barodenervated and vagotomized animals. The reflex nature of the hemodynamic and renal sympathetic nerve responses during regional myocardial ischemia was documented by its disappearance after intrapericardial application of the local anesthetic procaine. Intrapericardial application of the stable TxA2 mimetic U-46619 elicited similar excitatory responses, including increases in MAP and RSNA. Like ischemia, the reflex responses to U-46619 were eliminated by blockade of cardiac nerve transmission with intrapericardial procaine. Furthermore, administration of the TP receptor antagonist BM 13,177 abolished the reflexes to U-46619 and significantly attenuated the sympathoexcitatory reflex responses to regional myocardial ischemia. In contrast, simultaneous blockade of histamine H1 receptors with pyrilamine and serotonin 5-HT3 receptors with tropisetron did not alter the sympathoexcitatory reflex responses to U-46619. Taken together, these data suggest that endogenous TxA2 contributes strongly to ischemia-related sympathoexcitatory reflex responses through direct stimulation of TP receptors.
Previous studies have suggested that myocardial ischemia in sinoaortic denervated animal evokes mixed reflex responses, including changes in sympathetic efferent activity and arterial BP. In this respect, investigators have demonstrated that myocardial ischemia in sinoaortic-denervated but vagi-intact animals evokes an increase in thoracic sympathetic efferent activity (T3 level) and no change or a decrease in BP (29). However, in a similar animal model, others have observed decreases in both renal sympathetic efferent activity and BP during myocardial ischemia, since the sympathoexcitatory reflexes were easily masked by the powerful vagal-mediated inhibitory reflex responses (33, 47, 48, 55). In acute sinoaortic-denervated and vagotomized animals, occlusion of the LAD or both the LAD and circumflex coronary arteries elicits a consistent increase in sympathetic efferent activity accompanied by a decrease or no change in arterial pressure (34, 46, 55). Lombardi and colleagues (27) consistently observed increases in both sympathetic efferent nerve activity and arterial pressure during occlusion of the distal one-third of the LAD artery in cats subjected to chronic sinoaortic denervation and acute vagotomy. However, we were unable to repeat this regional ischemia-mediated pressor reflex. In our pilot study, we observed that occlusion of the distal one-third of the LAD artery induced variable arterial pressure responses, including small increases or decreases in arterial pressure in cats that had been subjected to acute sinoaortic denervation and vagotomy. These observations led us to speculate that reflex responses during myocardial ischemia are dependent on the extent of myocardial ischemia and the associated limitation of cardiac pump function vs. the extent of stimulation of cardiac sympathetic pathways, which engenders the excitatory reflex responses. We then occluded the second or third diagonal branch of the LAD to induce more limited regional ischemia and observed that this modified model caused a smaller region of ischemia than occlusion of the distal one-third of the LAD artery based on our observation of change in regional color during ischemia. We also found that this modified model more consistently produced increases in RSNA and reflex increases in arterial pressure, CO, peripheral vascular resistance, and myocardial contractility, measured as a change in dP/dt40. Furthermore, the reflex nature of these responses during ischemia was confirmed by elimination of the responses with epicardial procaine. Thus this more constrained model of regional myocardial ischemia displayed consistent and repeatable excitatory hemodynamic and renal sympathetic nerve reflex responses.
TxA2, a biologically potent arachidonic acid metabolite, is formed by the action of thromboxane synthase on prostaglandin endoperoxide, mainly in platelets (2, 17). A number of studies have demonstrated that TxA2 is produced in large quantities in the coronary circulation of patients with coronary artery disease (43) and in animals with myocardial ischemia (21, 39). Large increases in TxA2 coincide with episodes of myocardial ischemia occurring in patients with unstable angina and myocardial infarction (11). Experimental studies have documented increases in transcardiac TxA2 during myocardial ischemia in animals with coronary artery stenosis (12, 21, 26). Parratt and Cokerm (39), for example, reported increases in TxA2 concentration in coronary venous plasma draining the ischemic region as early as 3 min after occlusion of a coronary artery branch. Thus TxA2 is rapidly available as a chemical stimulus of cardiac afferent endings during ischemia, which, when stimulated, lead to sympathoexcitatory reflexes.
TxA2's role as a vasoconstrictor and platelet activator has been studied extensively (8, 17). In contrast, only a few recent studies have reported that TxA2 excites peripheral sensory nerves. For example, exogenous TxA2 stimulates hindlimb group III and IV afferent nerves (25). Also, the TxA2 mimetic U-46619 inhibits the knee-jerk reflex through a vagal pathway originating in the lung (41). U-46619 also evokes tachypnea and a depressor reflex response, including bradycardia and hypotension through stimulation of the vagus nerve (7, 41, 53). In addition, infusion of U-46619 into the inferior vena cava of cats causes vagally mediated, rapid, shallow breathing and airway hyperresponsiveness (1, 24, 42). Intrapericardial application of exogenous TxA2 also stimulates cardiac vagal chemosensitive afferents in rats (45). However, because these studies focused on exogenous TxA2 or a TxA2 analog, it is unclear whether endogenous TxA2 stimulates afferent nerves to evoke excitatory cardiovascular reflexes. It is possible that the concentrations of TxA2 released during pathophysiological conditions like myocardial ischemia are insufficient to activate afferent nerves sufficiently to achieve reflex responses. This question is particularly relevant, since our model of ischemia is relatively brief (only 5 min). However, since TxA2 is produced mostly from activated platelets, an event that occurs rapidly during ischemia (13), we hypothesized that even brief (5 min) regional myocardial ischemia would be sufficient to activate platelets to produce TxA2, which would be sufficient to stimulate cardiac afferent nerves to a degree that would elicit reflex cardiovascular responses. This possibility recently was tested in our laboratory when we demonstrated using single-unit recording techniques to show that ischemically sensitive cardiac spinal afferents were activated by TxA2, and that TP receptor blockade with BM 13,177 attenuated the response to ischemia (16). The present study extends our observations by confirming our current working hypothesis that blockade of TP receptors with BM 13,177 also significantly attenuates sympathoexcitatory reflex responses during brief, regional myocardial ischemia by an average of 45%. Because BM 13,177 is a specific TP receptor antagonist, which selectively blocks TP receptors and eliminates the actions of TxA2 on platelets and smooth muscle at doses ranging from 5 to 30 mg/kg iv, but not the cyclooxygenase system, including both prostacyclin and thromboxane synthases, prostaglandin E, and prostacyclin receptors (36, 37, 44, 57), we feel certain that the actions of this antagonist were limited to TxA2. The present study, therefore, provides first evidence that endogenous TxA2 participates in ischemia-mediated sympathoexcitatory reflexes through stimulation of TP receptors.
Our observation that intrapericardial application of U-46619 evokes sympathoexcitatory reflex responses, including increases in arterial pressure and RSNA in sinoaortic denervated and vagotomized animal, contrasts with the results of earlier studies (41, 53), which have suggested that intrapericardial U-46619 does not alter peak systolic BP of anesthetized cats, either before or after vagotomy. Although the discrepancies between these earlier studies and our current observation are unclear, it is possible that baroreflex activation may have limited the excitatory cardiovascular response to epicardial U-46619, since these previous investigations did not perform sinoaortic denervation. Additionally, in support of the present study, our laboratory recently demonstrated that U-46619 is capable of stimulating cardiac sympathetic afferents (16), which is likely leading to excitatory cardiovascular reflex responses.
In addition to classical identification of TP receptor on platelets and smooth muscle cells, recent evidence suggests the existence of TP receptors in glial cells in the central nervous system. In this respect, immunohistochemical studies have revealed the presence of TP receptors in oligodendrocytes and astrocytes associated with myelinated fiber tracts, most notably in the striatum, spinal cord, and optic tract (5, 6). Stimulation of TP receptors in the brain stem of rats by intracerebroventricular injection of U-46619 elevates arterial BP (18). Furthermore, TP receptors are located on Schwann cells in rat sciatic nerves and nodose ganglion neurons (35, 54). Recently, we also observed that TP receptors are located in cell bodies of cardiac spinal afferent neurons in the nodose ganglia (16). It is most likely that U-46619 evoked sympathoexcitatory reflex responses through direct stimulation of cardiac sympathetic afferent nerves, since blockade of TP receptors in the heart with BM 13,177 and blockade of cardiac sympathetic afferent transmission with procaine abolished the reflex responses to U-46619.
Intravenous administration of BM 13,177 blocks TP receptors in platelets, smooth muscle cells, and peripheral nervous system (16, 36, 37). Previous studies have demonstrated that stimulation of platelet TP receptors leads to release of a number of platelet-derived mediators, including 5-HT and histamine during ischemia (13, 15). It is possible that these mediators might be responsible for the TxA2-related sympathoexcitatory reflex responses during ischemia, since our laboratory's previous studies (13, 15) have shown that both mediators are capable of stimulating cardiac sympathetic afferents during ischemia and thus might elicit the associated excitatory reflex responses. We evaluated this possibility by instituting histamine H1 receptor and 5-HT3 receptor blockade before administration of U-46619. Since this blockade did not alter the sympathoexcitatory reflex responses to the TxA2 mimetic, we suggest that TxA2-stimulated 5-HT and histamine release from activated platelets does not play a role in reflex response to this cyclooxygenase mediator. These data also indicate that U-46619 is specific for TP receptors.
Another concern related to intravenous administration of BM 13,177 is that this antagonist may enter the central nervous system to exert its effects. We administered BM 13,177 intravenously, since direct application of the bicarbonate solution, in which the antagonist had been dissolved, to the surface of the heart could limit the change in pH during myocardial ischemia, which we have shown previously can buffer protons derived from lactic acid that contribute to activation of cardiac sympathetic afferents (38). Furthermore, we do not believe that the intravenous administration of BM 13,177 influences the reflex response to myocardial ischemia by acting on the central nervous system, since BM 13,177 is a water, but not a small lipid soluble molecule that would be able to cross the blood-brain barrier and exert an action in the central nervous system. Additionally, we have documented that TP receptors are located in dorsal root ganglion, and BM 13,177 administered intravenously blocks the direct effect of U-46619 on cardiac afferent endings (16). Taken together, it is most likely that intravenous BM 13,177 acts on the cardiac sympathetic afferent endings rather than in the central nervous system to attenuate ischemia-mediated sympathoexcitatory reflex responses.
Our present observation that blockade of TP receptor with BM 13,177 only attenuated the ischemia-mediated sympathoexcitatory reflex responses by average of 45% suggests that multiple factors underlie activation of cardiac sympathetic afferents and the associated excitatory reflex responses. Besides TxA2, previous studies from our laboratory and by others have documented that several ischemic mediators, including reactive oxygen species, protons, bradykinin, and cyclooxygenase products, are capable of stimulating/sensitizing cardiac sympathetic afferents and hence have the potential to evoke the sympathoexcitatory reflexes (13–15, 23, 38, 47, 49, 52). Other metabolic products like histamine and serotonin also appear to be released during ischemia in sufficient quantities to stimulate cardiac sympathetic afferents. However, the present study suggests that the extent of afferent activation by these latter metabolites does not result in cardiac reflex responses. More studies are needed to further explore these important mechanisms, which are closely related to the clinical situation, such as angina pectoris and myocardial infarction.
In summary, regional myocardial ischemia evokes excitatory reflex cardiovascular responses in barodenervated and vagotomized cats, suggesting that cardiac sympathetic (spinal) afferents are responsible for this reflex. Application of U-46619 to the surface of the heart elicits sympathoexcitatory reflex responses that are eliminated by blockade of cardiac sympathetic nerve transmission with local procaine. The present study also provides compelling evidence indicating that TP receptors that have been shown to be located on sympathetic afferent endings in the heart are responsible, in part, for the sympathoexcitatory reflex responses occurring during myocardial ischemia. The excitatory response to stimulation of TP receptors is independent of afferent stimulation by histamine and serotonin. In a recent study, we observed that endogenous TxA2 rapidly stimulates cardiac spinal afferents during ischemia through activation of TP receptors (16). These two studies reinforce the conclusion that this arachidonic acid metabolite plays an important role in regulating cardiac spinal afferent activity and the associated reflex responses during ischemia. This new information extends our understanding of the role of endogenous TxA2 in pathophysiological responses to myocardial ischemia.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-66217.
Acknowledgments
We gratefully acknowledge the technical assistance of Kin Hang, Vu Thai Nguyen, and Ali Moazzami. We also thank undergraduate students, Ada Cheng and Ivan Aguilera, for help with experimental procedures. Hoffmann-La Roche donated a gift of BM 13,177 used in this study. J. C. Longhurst is the Larry K. Dodge and Susan Samueli Endowed Chairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aizawa H, Hirose T. A possible mechanism of airway hyperresponsiveness induced by prostaglandin F2 alpha and thromboane A2. Prostaglandins Leukot Essent Fatty Acids 33: 185–189, 1993. [PubMed] [Google Scholar]
- 2.Arita H, Nakano T, Hanasaki K. Thromboxane A2: its generation and role in platelet activation. Prog Lipid Res 28: 273–301, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Arndt JO, Pasch U, Samodelov LF, Wiebe H. Reversible blockade of myelinated and non-myelinated cardiac afferents in cats by instillation of procaine into the pericardium. Cardiovasc Res 15: 61–67, 1981. [DOI] [PubMed] [Google Scholar]
- 4.Barber MJ, Mueller TM, Davies BG, Zipes DP. Phenol topically applied to canine left ventricular epicardium interrupts sympathetic but not vagal afferents. Circ Res 55: 532–544, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Blackman SC, Dawson G, Antonakis K, Le Breton GC. The identification and characterization of oligodendrocyte thromboxane A2 receptors. J Biol Chem 273: 475–483, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Borg C, Lim CT, Yeomans DC, Dieter JP, Komiotis D, Anderson EG, Le Breton GC. Purification of rat brain, rabbit aorta, and human platelet thromboxane A2/prostaglandin H2 receptors by immunoaffinity chromatography employing anti-peptide and anti-receptor antibodies. J Biol Chem 269: 6109–6116, 1994. [PubMed] [Google Scholar]
- 7.Carrithers JA, Liu F, Shirer HW, Orr JA. Mechanisms for techypneic response to the thromboxane A2 memetic U-46,619 in rabbits. Am J Physiol Regul Integr Comp Physiol 266: R321–R327, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RA, Smith WL, Narumiya SVIII. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229, 1994. [PubMed] [Google Scholar]
- 9.Coller BS Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J Clin Invest 100: S57–S60, 1997. [PubMed] [Google Scholar]
- 10.Dorward PK, Flaim M, Ludbrook J. Blockade of cardiac nerves by intrapericardial local anaesthetics in the conscious rabbit. Aust J Exp Biol Med Sci 61: 219–230, 1983. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald DJ, Roy L, Catella F, Fitzgerald GA. Platelet activation in unstable coronary disease. N Engl J Med 315: 983–989, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Folts JD, Crowell EB Jr, Rowe GG. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation 54: 365–370, 1976. [DOI] [PubMed] [Google Scholar]
- 13.Fu LW, Longhurst JC. Activated platelets contribute to stimulation of cardiac afferents during ischaemia in cats: role of 5-HT3 receptors. J Physiol 544: 897–912, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LW, Longhurst JC. Interactions between histamine and bradykinin in stimulation of ischaemically sensitive cardiac afferents in felines. J Physiol 565: 1007–1017, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu LW, Schunack W, Longhurst JC. Histamine contributes to ischemia-related activation of cardiac spinal afferents: role of H1 receptors and PKC. J Neurophysiol 93: 713–722, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Fu LW, Guo ZL, Longhurst JC. Undiscovered role of endogenous TxA2 in activation of cardiac sympathetic afferents during ischemia. J Physiol 586: 3287–3300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furci L, Fitzgerald DJ, Fitzgerald GA. Heterogeneity of prostaglandin H2/thromboxane A2 receptors: distinct subtypes mediate vascular smooth muscle contraction and platelet aggregation. J Pharmacol Exp Ther 258: 74–81, 1991. [PubMed] [Google Scholar]
- 18.Gao H, Welch WJ, DiBona GF, Wilcox CS. Sympathetic nervous system and hypertension during prolonged TxA2/PGH2 receptor activation in rats. Am J Physiol Heart Circ Physiol 273: H734–H739, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Polese A, Fiorentini C, Magrini F, Olivari M, Bartorelli C. Comparison between angina with ST-segment depression and angina with ST-segment elevation. Br Heart J 37: 401–413, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guazzi M, Polese A, Fiorentini C, Magrini F, Bartorelli C. Left ventricular performance and related haemodynamic changes in Prinzmetal's variant angina pectoris. Br Heart J 33: 84–94, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsh P, Hillis L, Campbell W, Firth B, Willerson J. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N Engl J Med 304: 685–691, 1981. [DOI] [PubMed] [Google Scholar]
- 22.Huang HS, Pan HL, Stahl G, Longhurst J. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol Heart Circ Physiol 269: H888–H901, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Huang HS, Stahl G, Longhurst J. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. Am J Physiol Heart Circ Physiol 268: H2114–H2124, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol 87: 383–396, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res 744: 175–178, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Lewy RI, Wiener L, Walinsky P, Lefer AM, Silver MJ, Smith JB. Thromboxane release during pacing-induced angina pectoris: possible vasoconstrictor influence on the coronary vasculature. Circulation 61: 1165–1171, 1980. [DOI] [PubMed] [Google Scholar]
- 27.Lombardi F, Casalone C, Bella PD, Malfatto G, Pagani M, Malliani A. Global versus regional myocardial ischaemia: differences in cardiovascular and sympathetic responses in cats. Cardiovasc Res 18: 14–23, 1984. [DOI] [PubMed] [Google Scholar]
- 28.Longhurst J, Tjen-ALooi S, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion: mechanisms and reflexes. Ann N Y Acad Sci 940: 74–95, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol 217: 703–709, 1969. [DOI] [PubMed] [Google Scholar]
- 30.Maseri A, Severi S, De Nes M, L'Abbate A, Chierchia S, Marzilli MBAM, Parodi O, Biagini A, Distante A. “Variant” angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Am J Cardiol 42: 1019–1035, 1978. [DOI] [PubMed] [Google Scholar]
- 31.Mason DT, Braunwald E, Covell JW, Sonnenblick EH, Ross J Jr. Assessment of cardiac contractility. The relation between the rate of pressure rise and ventricular pressure during isovolumic systole. Circulation 44: 47–58, 1971. [DOI] [PubMed] [Google Scholar]
- 32.Meyers KM, Holsmen H, Seachord CL. Comparative study of platelet dense granule constituents. Am J Physiol Regul Integr Comp Physiol 243: R454–R461, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Minisi AJ, Thames MD. Reflexes from ventricular receptors with vagal afferents. In: Reflex Control of the Circulation, edited by Zucker IH and Gilmore JP. Boston, MA: CRC, 1991, p. 359–405.
- 34.Minisi AJ, Thames MD. Distribution of left ventricular sympathetic afferents demonstrated by reflex responses to transmural myocardial ischemia and to intracoronary and epicardial bradykinin. Circulation 87: 240–246, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Muja N, Blackman SC, Le Breton GC, DeVries GH. Identification and functional characterization of thromboxane A2 receptors in Schwann cells. J Neurochem 78: 446–456, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nossaman BD, McMahon TJ, Ragheb MS, Ibrahim IN, Babycos CR, Hood JS, Kadowitz PJ. Blockade of thromboxane/endoperoxide receptor-mediated responses in the pulmonary vascular bed of the cat by sulotroban. Eur J Pharmacol 213: 1–7, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Oude Egbrink MG, Tangelder GJ, Slaaf DW, Reneman RS. Different roles of prostaglandins in thromboembolic processes in arterioles and venules in vivo. Thromb Haemost 70: 826–833, 1993. [PubMed] [Google Scholar]
- 38.Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fiber afferents during ischemia. J Physiol 518: 857–866, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parratt JR, Cokerm S. The significance of prostaglandin and thromboxane release in acute myocardial ischemia. In: Prostaglandins and Thromboxanes: Proceedings of the Third International Symposium on Prostaglandins and Thromboxanes in the Cardiovascular System, edited by Forster W. New York: Pergamon, 1981, p. 21–25.
- 40.Phan A, Fu LW, Longhurst J. Thromboxane A2 contributes to cardiac-cardiovascular presser reflexes during myocardial ischemia (Abstract). FASEB J 16: A833, 2002. [Google Scholar]
- 41.Pickar JG The thromboxane A2 mimetic U-46619 inhibits somatomotor activity via a vagal reflex from the lung. Am J Physiol Regul Integr Comp Physiol 275: R706–R712, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Shams H, Scheid P. Effects of thromboxane on respiration and pulmonary circulation in the cat: role of vagus nerve. J Appl Physiol 68: 2042–2046, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Smith EF, Lefer AM, Smith JB. Influence of thromboxane inhibition on the severity of myocardial ischemia in cats. Can J Physiol Pharmacol 58: 294–300, 1980. [DOI] [PubMed] [Google Scholar]
- 44.Stegmeier K, Pill J, Muller-Beckmann B, Schmidt FH, Witte EC, Wolff HP, Patscheke H. The pharmacological profile of the thromboxane A2 antagonist BM 13.177. A new anti-platelet and anti-thrombotic drug. Thromb Res 35: 379–395, 1984. [DOI] [PubMed] [Google Scholar]
- 45.Sun SY, Wang W, Schultz HD. Activation of cardiac afferents by arachidonic acid: relative contributions of metabolic pathways. Am J Physiol Heart Circ Physiol 281: H93–H104, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Thames MD, Kinugawa T, Dibner-Dunlap ME. Reflex sympathoexcitation by cardiac sympathetic afferents during myocardial ischemia: role of adenosine. Circulation 87: 1698–1704, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Thames M, Minisi A. Reflex responses to myocardial ischemia and reperfusion. Role of prostaglandins. Circulation 80: 1878–1885, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Thoren P Evidence for a depressor reflex elicited from left ventricular receptors during occlusion of one coronary artery in the cat. Acta Physiol Scand 88: 23–34, 1973. [DOI] [PubMed] [Google Scholar]
- 49.Tjen-ALooi S, Fu LW, Longhurst JC. Xanthine oxidase, but not neutrophils, contribute to activation of cardiac sympathetic afferents during myocardial ischaemia in cats. J Physiol 543: 327–336, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjen-ALooi S, Pan HL, Longhurst JC. Endogenous bradykinin activates ischaemically sensitive cardiac visceral afferents through kinin B2 receptors in cats. J Physiol 510: 633–641, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veelken R, Glabasnia A, Stetter A, Hilgers KF, Mann JF, Schmieder RE. Epicardial bradykinin B2 receptors elicit a sympathoexcitatory reflex in rats. Hypertension 28: 615–621, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Veelken R, Glabasnia A, Stetter A, Hilgers KF, Mann JF, Schmieder RE. Epicardial bradykinin B2 receptors elicit a sympathoexcitatory reflex in rats. Hypertension 28: 615–621, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Wacker MJ, Tehrani RN, Smoot RL, Orr JA. Thromboxane A2 mimetic evokes a bradycardia mediated by stimulation of cardiac vagal afferent nerves. Am J Physiol Heart Circ Physiol 282: H482–H490, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Wacker MJ, Tyburski JB, Ammar CP, Adams MC, Orr JA. Detection of thromboxane A(2) receptor mRNA in rabbit nodose ganglion neurons. Neurosci Lett 386: 121–126, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Weaver LC, Danos LM, Oehl RS, Meckler RL. Contrasting reflex influences of cardiac afferent nerves during coronary occlusion. Am J Physiol Heart Circ Physiol 240: H620–H629, 1981. [DOI] [PubMed] [Google Scholar]
- 56.White JC Cardiac pain: anatomic pathway and physiologic mechanisms. Circulation 16: 644–655, 1957. [DOI] [PubMed] [Google Scholar]
- 57.Witt W, Sturzebecher S, Muller B. Synergistic antiplatelet and antithrombotic effects of a prostacyclin analog (iloprost) combined with a thromboxane antagonist (sulotroban) in guinea pigs and rats. Thromb Res 51: 607–616, 1988. [DOI] [PubMed] [Google Scholar]