Abstract
Adenoviral gene transfer of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a to the hypertrophic heart in vivo has been consistently reported to lead to enhanced myocardial contractility. It is unknown if the faster skeletal muscle isoform, SERCA1, expressed in the whole heart in early failure, leads to similar improvements and whether metabolic requirements are maintained during an adrenergic challenge. In this study, Ad.cmv.SERCA1 was delivered in vivo to aortic banded and sham-operated Sprague-Dawley rat hearts. The total SERCA content increased 34%. At 48–72 h posttransfer, echocardiograms were acquired, hearts were excised and retrograded perfused, and hemodynamics were measured parallel to NMR measures of the phosphocreatine (PCr)-to-ATP ratio (PCr/ATP) and energy substrate selection at basal and high workloads (isoproterenol). In the Langendorff mode, the rate-pressure product was enhanced 27% with SERCA1 in hypertrophic hearts and 10% in shams. The adrenergic response to isoproterenol was significantly potentiated in both groups with SERCA1. 31P NMR analysis of PCr/ATP revealed that the ratio remained low in the hypertrophic group with SERCA1 overexpression and was not further compromised with adrenergic challenge. 13C NMR analysis revealed fat and carbohydrate oxidation were unaffected at basal with SERCA1 expression; however, there was a shift from fats to carbohydrates at higher workloads with SERCA1 in both groups. Transport of NADH-reducing equivalents into the mitochondria via the α-ketoglutamate-malate transporter was not affected by either SERCA1 overexpression or adrenergic challenge in both groups. Echocardiograms revealed an important distinction between in vivo versus ex vivo data. In contrast to previous SERCA2a studies, the echocardiogram data revealed that SERCA1 expression compromised function (fractional shortening) in the hypertrophic group. Shams were unaffected. While our ex vivo findings support much of the earlier cardiomyocyte and transgenic data, the in vivo data challenge previous reports of improved cardiac function in heart failure models after SERCA intervention.
Keywords: sarco(endo)plasmic reticulum Ca2+-ATPase, glucose oxidation, fatty acid oxidation, phosphocreatine-to-ATP ratio, myocardial energy potential
the changes in intracellular Ca2+ handling reported for the failing myocardium have been linked to sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). Calcium uptake into the sarcoplasmic reticulum (SR) by SERCA determines the rate of calcium removal for relaxation, the SR calcium content, and calcium released for contractions (33, 45). In the failing heart, it has been proposed that SERCA contributes to reduced contractility and relaxation as the activity and number of these calcium transporters are reduced and calcium transients are blunted and slow to decay (1, 3, 12). Increasing the number of active transporters by adenovirus-mediated gene transfer of SERCA2a resulted in improved cardiac function and survival in several models of heart disease (7, 8, 23, 34). Despite extensive examination as to how these changes affect cardiac function, little is known regarding the subsequent effect on cardiac metabolism (8). If contractility improves without a sustained energy supply, the benefits of SERCA overexpression could be limited, particularly under conditions of stress.
The balance between energy supply and demand is mediated, in part, by calcium. As a key regulator of mitochondrial dehydrogenase activity, calcium plays a crucial role in balancing both the rates and selection of carbohydrate and fatty acid oxidation (13, 26). Whether altering calcium homeostasis with SERCA overexpression affects energy substrate metabolism (carbohydrates versus fatty acids) in the failing heart is unknown. Interestingly, it has been reported that the expression of SERCA2 in nonfailing transgenic mice led to a shift in substrate selection from fats to glucose metabolism (2). The potential for this shift in the failing heart could be advantageous (10, 11). Comparable drug therapies (ranolazine and trimetazine) designed to shift the balance of substrate oxidation toward greater glucose oxidation in the failing myocardium led to enhanced function and an increase in the myocardial energy potential [phosphocreatine (PCr)-to-ATP ratio (PCr/ATP)] (10, 51, 58).
Indeed, a previous study (8) reported that overexpression of the SERCA2a isoform in the aortic banded rat model of cardiac failure lead to an increase in PCr/ATP at basal workloads. However, in that study, glucose was provided as a sole substrate, and the PCr/ATP ratio was, in fact, depressed in nonfailing hearts overexpressing SERCA2a. In the present study, we assessed the substrate selection and energy state (PCr/ATP) from both healthy and hypertrophic hearts [left ventricular (LV) pressure-overload hypertrophy (LVH)] and provided a more physiological selection of substrates (glucose and palmitate) at both basal and high workloads (β-adrenergic challenge). While the increase in SERCA content may enhance calcium handling and contractile force at basal workloads in the failing heart, it is not known if any improvement in PCr/ATP observed at basal workloads with SERCA overexpression could be sustained at higher workloads.
Importantly, in this study, we overexpressed the skeletal muscle isoform of SERCA (i.e., SERCA1) in healthy and failing hearts in vivo. Compared with the cardiac isoform (SERCA2a), SERCA1 is not regulated by phospholamban (44); it has a higher activity and a twofold greater calcium uptake relative to SERCA2a (5, 19), and it is also more resistant to oxidative stress (61) and acidosis (63). Overexpressing this isoform in isolated rat cardiomyocytes and transgenic mice resulted in a two- to fourfold increase in the rates of Ca2+ transport activity and increased tension development (5, 19, 25). In addition, isolated human cardiomyocytes from end-stage failing hearts overexpressing the SERCA1 isoform showed markedly improved contractile function (62). Whether these findings translate to the intact functioning heart in failure is unknown.
The aim of this study was to assess cardiac metabolism parallel to measures of cardiac function in healthy and hypertrophic hearts (aortic banded, 10–12 wk) 48–72 h after SERCA1 gene transfer. The metabolic response was assessed in the isolated heart preparation based on 31P NMR spectroscopy analysis of energy potential (PCr/ATP) and 13C NMR analysis of substrate selection, tricarboxylic acid (TCA) cycle flux, and metabolite exchange between the mitochondria and cytosol [α-ketoglutarate (α-KG)-malate transport]. Cardiac function was measured both in vivo (echocardiograms) and ex vivo (isolated heart preparation). Based on previous transgenic mice data and isolated cardiomyocyte data for SERCA1, we hypothesized that function would be enhanced parallel to a shift away from fatty acid oxidation in all hearts overexpressing SERCA1. We repeated our study under conditions of an adrenergic challenge to determine if any further increase in function could be achieved and energy supplies sustained. Indeed, the results of this study demonstrate enhanced function and sustained energy potential with SERCA1 expression. However, careful examination of the data revealed important and unexpected limitations.
METHODS
Pressure-overload hypertrophy.
Cardiac hypertrophy from LVH was induced by constricting the transverse aorta (hemoclip) of 3-wk-old male Sprague-Dawley rats as previously described (27, 42, 50). This banding procedure relies on the natural growth of the animal to produce a gradually increasing degree of aortic constriction. The rats develop a concentric hypertrophy and increased heart-to-body weight ratio, which is associated, in the short term, with improvement in the systolic function of the heart (27, 42, 46, 48, 60). At 10–12 wk postbanding, the animals enter a decompensated stage with depressed LV developed pressure (LVDP) and dP/dt. In this model of LVH, no systemic activation of the sympathetic nervous system or of the renin-angiotensin-aldosterone system occurs (46). Consequently, there are no signs of cardiac lesions, peripheral arteritis, myocardial necrosis, or extensive fibrosis. The rats progress to a dilated cardiac hypertrophy with acute end-stage heart failure at 4–6 mo postbanding. Sham (SHM) groups underwent similar surgery without the placement of the aortic band. The protocol was approved by the Animal Care Policies and Procedures Committee of the University of Illinois at Chicago (Institutional Animal Care and Use Committee accredited), and animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
SERCA1 and LacZ gene transfer in vivo.
The recombinant first generation adenovirus Ad.cmv.LacZ (Clontech) with a ΔE1/ΔE3 deletion and encoding for β-galactosidase under control of the cytomegalovirus (CMV) promoter was used to establish gene transfer efficiency. An adenovirus expressing the skeletal muscle SERCA1 gene (a generous gift from Dr. G. Inesi) under a CMV promoter and coexpressing the c-myc tag (for Western blot analysis) was used to modulate cardiac function (18, 52). As previously described, viruses were amplified (1012 viral particles/ml), purified by standard techniques, and dialyzed in PBS before being used (36, 38). Control groups underwent a similar surgical procedure with PBS injection replacing the adenovirus. The decision not to use an adenovirus base reporter gene control is discussed in the discussion in Limitations.
Adenoviral delivery in vivo.
At 10–12 wk postbanding, adenoviruses carrying cDNA for SERCA1 (or PBS control) were delivered to the heart in vivo by coronary perfusion as described in our previous reports (36, 38) with the following modifications. All vessels to/from the heart were cross-clamped simultaneously, and the heart was retrograde perfused in vivo for 7 min with calcium-free Tyrode solution through catheters position in the aortic root (delivery) and right ventricle (efflux). At the time of adenovirus injection, 0.2 ml of AdV.cmv.SERCA1 (1012 viral particles/ml) were first delivered through the catheter position in the aortic root. This allowed the adenoviruses to circulate down the coronary. Next, the efflux catheter positioned in the right ventricle was removed, and an additional 0.5 ml/kg of adenovirus (∼0.2 ml) were delivered to the aortic root at 300 ± 100 mmHg of peak pressure. After 90 s, catheters were repositioned in the right and left ventricles, and unsequestered virus was flushed from the heart with Krebs buffer containing calcium (1.5 mM). The heart rate recovered, the cross-clamp was removed, the chest was closed, and the rats recovered. After 48–72 h, hearts were excised for the analysis of SERCA expression (Western blots), metabolic experiments (31P and 13C NMR), and functional measurements (in vivo and ex vivo) according to the protocols below.
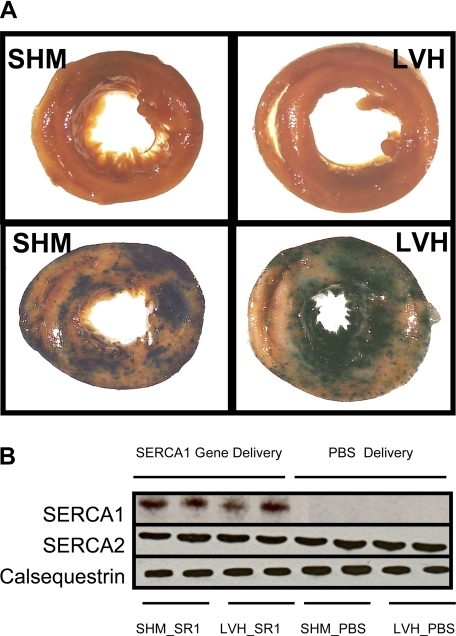
The gene delivery technique, referenced above, was reported and confirmed earlier by following the delivery and expression of Ad.cmv.LacZ in the whole intact nonfailing rat heart (36, 38). In the present study, the gene delivery technique was confirmed in a subset (n = 3 for each group) of SHM and aortic banded rat hearts receiving a similar dose of Ad.cmv.LacZ (1012 viral particles/ml) or PBS. Three days after LacZ gene transfer in vivo, hearts were excised, stained for β-galactosidase expression, and photographed (see Fig. 2) as previously described (36, 38). The transfer and expression of the exogenous SERCA1 gene were verified in the heart by Western blot analysis.
Protein expression of SERCA2, SERCA1, and calsequestrin.
Seventy-two hours after Ad.cmv.SERCA1 or PBS delivery to coronaries in vivo, SHM and aortic banded rat hearts were excised and retrograde perfused with a bolus of PBS to flush blood from the coronaries/ventricles. Hearts were then freeze clamped for Western blot analysis. Because the exogenous gene is heterogeneously expressed throughout the heart (9, 36, 38), whole ventricles were pulverized via a pestle and mortar, and 0.25 g of the sample were homogenized for 1 min (4 × 15 s) in 1.5 ml of ice-chilled RIPA buffer [0.5 mM Tris·HCl, 1.5 mM NaCl, 2.5% deoxycholic acid, 10 mM EDTA, 0.1% SDS, 1% Nonidet P-40, 10% glycerol, 1% Triton X-100, and 10 μl/ml protease inhibitor (SIGMA P8340)]. Homogenates were then rocked on ice for 1 h followed by centrifugation at 3,000 rpm for 20 min at 4°C. The pellet was discarded, and the protein concentration of the supernatant was determined (BCA protein assay). Equal sample volumes of homogenate (100 μl) and 2× Laemmli sample buffer (Bio-Rad) were combined, and 1 M DDT was added. Samples were vortexed for 3–12 h at 4°C followed by centrifugation at 14,000 rpm for 20 min at 4°C. The supernatant protein was loaded (30 μg SERCA2 and 50 μg SERCA1) onto minielectrophoresis gels (4–12% Bis-Tris NuPAGE Gel, Invitrogen) and run at 150 V for 1.5 h. Separated proteins were transferred to a polyvinylidene difluoride membrane in transfer buffer (Invitrogen NUPAGE) with 1% SDS added and methanol excluded. The transfer was run overnight at 30 V and 4°C. Membranes were blocked using washing buffer (0.5% Tween and 5% milk for 1 h) followed by an incubation with monoclonal anti-SERCA2 antibody (1:2,000 Affinity BioReagent no. MA3-919), monoclonal anti-c-Myc epitope tag (for SERCA1; 1:1,000, Affinity BioReagents no. MA1-980), and monoclonal anti-calsequestrin antibody (1:2,500, PA1-913). Calsequestrin was used as an internal protein content reference (30, 47). Membranes were then washed and incubated with secondary antibody for 1 h at room temperature [1:100,000 for c-myc (i.e., SERCA1) and 1:50,000 for SERCA2 and calsequestrin] (goat anti-mouse horseradish peroxidase-conjugated antibody, Pierce). Protein bands were visualized after enhanced chemiluminescence treatment (Pierce ThermoScientific). The total amount of SERCA protein (SERCA1 + SERCA2) was determined in triplicate using serial dilutions of proteins (40, 60, and 80 μg) loaded and run on 4–12% separating gels and Coomassie blue stained as previously described (29, 52). Relative changes in total SERCA content were assessed by densitometry analysis of band intensities. The SERCA band was identified with reference to the band positions indicated by Western blot analysis.
Echocardiography.
Echocardiographic measurements were made 48–72 h after adenoviral gene transfer of SERCA1 or PBS delivery in both SHM controls and hypertrophic rat hearts before the excision for NMR experiments. Transthoracic M-mode and two-dimensional echocardiography were performed with an Acuson 256 with a 15-MHz linear-array transducer (15L8). The M-mode cursor was positioned perpendicular to the interventricular membrane and posterior wall of the LV in the parasternal short axis at the papillary muscle level. M-mode images were obtained for measurements of wall thickness and chamber dimensions.
Isolated retrograde-perfused rat hearts.
Hearts were excised at 48–72 after gene transfer and perfused in retrograde fashion with modified Krebs-Henseleit buffer as previously described [116 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4, and 1.2 mM NaH2PO4 equilibrated with 95% O2-5% CO2 with 0.4 mM of unlabeled palmitate/albumin complex (3:1 molar ratio) and 5 mM glucose] (27, 42, 50). The hydrostatic perfusion pressure was 100 cmH2O, and a water-filled latex balloon in the LV set to a diastolic pressure of 5–10 mmHg was connected to a pressure transducer and provided hemodynamic recordings (Powerlab, ADInstruments, Colorado Springs, CO). LVDP, ±dP/dt, and HR were continuously recorded. Perfused hearts were maintained at 37°C and positioned in a NMR spectrometer for metabolic measurements.
NMR spectroscopy.
Perfused hearts were positioned in a 20-mm NMR probe within a vertical 89-mm bore, 9.4-T magnet. NMR data from isolated beating hearts were obtained with a Bruker 400 AVANCE NMR spectrometer (Bruker Daltonics, Billerica, MA). 31P and 13C NMR measurements were acquired by previously described methods (35, 42, 50). 13C NMR spectra were collected in 2-min time blocks over the 40-min protocol, and 31P NMR spectra were each collected over 2-min intervals. In vitro 13C NMR analysis was performed on spectra from heart tissue extract at 14.1 T as previously described (32, 42).
Experimental protocols.
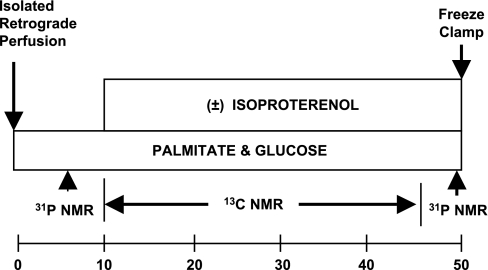
The protocol is shown in Fig. 1. Isolated hearts were initially supplied buffer containing unlabeled palmitate (complexed to albumin) and glucose to ensure metabolic equilibrium. The energetic state was then monitored by 31P NMR (PCr and ATP content). This was followed by the collection of 13C NMR background signals of naturally abundant 13C (1.1%). The perfusate was switched to buffer (0.7 liters, recirculated) containing 0.4 mM [2,4,6,8,10,12,14,16-13C8]palmitate (Isotec, Miamisburg, OH) plus unlabeled 5 mM glucose. Perfusion with 13C-enriched medium was continued for 40 min with the collection of sequential 13C NMR spectra (every 2 min). At the end of each protocol, a second 31P NMR spectrum was acquired, and hearts were then freeze clamped for biochemical analysis. 13C NMR enrichment curves (not shown) were used to assess TCA cycle and α-KG-malate transport rates as described below (35, 65, 66), and tissue extracts were used for high-resolution NMR analysis of palmitate oxidation (35, 42, 50). This protocol assessed function and metabolic rates at basal workloads in the following four groups: SHM_PBS (n = 8), SHM_SR1 (n = 6), LVH_PBS (n = 6), and LVH_SR1 (n = 6), where SR1 represents SERCA1.
Fig. 1.
Protocol. Three-week-old Sprague-Dawley rats underwent aortic banding [left ventricular (LV) hypertrophy (LVH)] or sham (SHM) operation. At 10–12 wk postbanding, adenovirus containing the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)1 gene under a cytomegolavirus promotor (Ad.cmv.SERCA1) or PBS was delivered in a retrograde fashion to the heart in vivo. At 48–72 h after gene transfer, echocardiograms were performed to assess cardiac function. Hearts were then excised, retrograde perfused, and positioned in a 9.4-T NMR spectrometer to assess metabolism parallel to function. Hearts were perfused with glucose and [2,4,6,8,10,12,14,16-13C8]palmitate for 40 min at baseline. The protocol was repeated at high workloads [isoproterenol (ISO)]. 31P NMR was used to assess energy potential [phosphocreatine (PCr)-to-ATP ratio (PCr/ATP)], and 13C NMR was used to assess palmitate oxidation, tricarboxylic acid (TCA) cycle rates, and α-ketoglutamate (α-KG)-malate transport.
A second protocol at high workloads (adrenergic challenge) repeated the first protocol to determine if any changes observed at basal workloads with SERCA1 overexpression could be sustained at high workloads. As described above, isolated hearts were first perfused with unlabeled substrates. Ten minutes before the switch to 13C-enriched substrates, a β-adrenergic challenge was induced with isoproterenol (ISO; 0.01 μM). The four ISO groups are identified as follows: SHM_PBS_ISO (n = 7), SHM_SR1_ISO (n = 7), LVH_PBS_ISO (n = 7), and LVH_SR1_ISO (n = 7).
Kinetic analysis of 13C NMR data.
Metabolic flux was determined in intact, hypertrophied and SHM rat hearts ex vivo using a previously described method for kinetic analysis of the progressive 13C enrichment of glutamate during [13C]palmitate and glucose oxidation, as detected via NMR spectroscopy (35, 37, 40, 65, 66). Our previously described kinetic models were applied for a simultaneous, three-parameter, nonlinear least-squares fit to 13C-enrichment curves of the 3- and 4-carbon positions of glutamate (MATLAB, MathWorks, Natick, MA) (35, 66). Solutions to the model gave TCA cycle flux (VTCA) and the interconversion rate between cytosolic glutamate and mitochondrial α-KG via the α-KG-malate carrier (F1).
Tissue biochemistry.
Tissue concentrations of glutamate, aspartate, citrate malate, and α-KG from perchloric acid extracts of the frozen LV were determined spectrophotometrically and fluorometrically using previously described assays (65, 66). The metabolite data were used for the kinetic analysis of 13C NMR data. The percentage of labeled acetyl-CoA entering the TCA cycle (Fc) from [2,4,6,8,10,12,14,16-13C8]palmitate was determined from high-resolution in vitro 13C NMR spectra (32, 35).
Statistical analysis.
Data are presented as means ± SE unless otherwise stated. Data set comparisons were performed with Student's unpaired two-tailed t-test. Differences in mean values were considered statistically significant at probability levels of <5% (P < 0.05).
RESULTS
LacZ, SERCA2, SERCA1, and calsequestrin expression in the heart.
Figure 2A shows cross sections from KCl-arrested SHM (left photographs) and LVH rat hearts (right photographs) stained for β-galactosidase expression 72 h after PBS (top photographs) or Ad.cmv.LacZ gene transfer (bottom photographs) in vivo. As described by Wright et al. (64), there was the potential to generate “false positives” for LacZ expression in the heart as a consequence of microinfarctions formed during the intracoronary infusion (rabbit model). In this study, the PBS control hearts from the rats stained for β-galactosidase expression (Fig. 2A, top photographs) showed no signs of false positives. In addition, β-galactosidase expression was significant and heterogeneously expressed throughout the heart (Fig. 2A, bottom photographs) after AdV.cmv.LacZ delivery, in agreement with our previous reports (36, 38). Importantly, at this stage of the disease, hypertrophic hearts showed the same relative levels of expression compared with SHM hearts.
Fig. 2.
LacZ and SERCA1 gene expression in SHM and aortic banded (LVH) rat hearts after adenoviral gene transfer in vivo. A: cross sections of K+-arrested hearts stained for β-galactosidase (β-Gal) expression 72 h after PBS delivery (top photographs) or AdV.cmv.LacZ gene delivery (bottom photos) in vivo. PBS hearts show no signs of false positives. LacZ groups showed widespread and heterogeneous β-Gal expression. The same gene delivery procedure was used to transfer SERCA1 cDNA to SHM and LVH rat hearts. B: Western blot analysis of SERCA1, endogenous SERCA2a, and calsequestrin expression after Ad.cmv.SERCA1 or PBS delivery by retrograde perfusion in vivo. Total SERCA protein (SERCA1 + SERCA2) increased 34% after gene transfer, whereas endogenous SERCA2 levels were unchanged.
Western blot analysis for SERCA2a, SERCA1, and calsequestrin are shown in Fig. 2B. Calsequestrin was used as an internal control to verify equal protein loading of the gels (30). As expected for this early stage of heart failure, there were no differences in endogenous SERCA2a expression between SHM (SHM_PBS) and LVH hearts (LVH_PBS). SERCA2a levels were not expected to drop until late-stage failure in the rat (1). Importantly, adenoviral delivery and expression of SERCA1 cDNA were detected in both SHM (SHM_SR1) and LVH (LVH_SR1) hearts. In addition, densitometry analysis of Coomassie blue staining indicated that the total SERCA content increased 34 ± 15%, and the overexpression of SERCA1 in whole hearts did not coincide with a measurable drop in endogenous SERCA2a levels. This contrasts with the earlier transgenic mouse model and our own cardiomyocyte data (5, 30). In transgenic mice expressing SERCA1, endogenous SERCA2a expression was reduced by 50% and total SERCA levels increased by 2.5-fold (17, 25, 30). In cardiomyocytes, total SERCA levels increased by three- to fourfold, whereas endogenous SERCA2a was reduced by 30–60% (5, 18). There is an important distinction between the cardiomyocyte and present in vivo models. The efficiency of gene transfer to the cardiomyocyte culture was 100%, and the multiplicity of infection (MOI) was 2–4 plaque-forming units/myocyte. In the present in vivo model, the efficiency of infection was ∼40–50% (36, 38), and the MOI to myocytes versus fibroblasts or endothelial cells is unknown. Importantly, despite the lower expression level in vivo, a contractile response was still elicited both in vivo and ex vivo with SERCA1 overexpression.
Echocardiographic measurements.
The echocardiographic measurements made 48–72 h after PBS delivery or adenoviral gene transfer of SERCA1 in both SHM controls and LVH rat hearts are listed in Table 1. There were no significant differences between SHM groups receiving either coronary perfusion with PBS or Ad.cmv.SERCA1. LV dimensions were similar, as were LV wall thickness and fractional shortening. After 10 wk of aortic banding, LVH_PBS animals showed echocardiographic signs of LVH relative to the SHM_PBS group, as expected, including a significant increase in the LV systolic dimension and a decrease in fractional shortening. These parameters were not reverse with SERCA1 treatment. Importantly, LV diastolic and systolic dimensions increased 10–15%, whereas fractional shortening was reduced by an additional 17%. The increase in LV dimensions and decrease in fractional shortening in the LVH_SR1 group relative to the LVH_PBS group indicates further LV remodeling with SERCA1 expression in failing hearts in this study.
Table 1.
Echographic results 48–72 h after adenoviral gene transfer of SERCA1 or PBS injection in SHM and aortic banded LVH rats
| Group | n | Heart Weight/Body Weight, g/kg | HR, beats/min | LVDd, mm | LVSd, mm | FS, % | Vcf, circumference/s | LVPWD, mm | LVPWS, mm |
|---|---|---|---|---|---|---|---|---|---|
| SHM_PBS | 4 | 6.20 | 335±25 | 8.2±0.9 | 4.8±0.4 | 40.6±5.7 | 0.027±0.005 | 1.3±0.4 | 2.4±0.3 |
| SHM_SR1 | 7 | 6.19 | 334±36 | 8.5±0.8 | 5.2±0.9 | 38.9±5.6 | 0.026±0.003 | 1.2±0.2 | 2.2±0.3 |
| LVH_PBS | 6 | 9.22* | 332±20 | 8.5±0.1 | 5.9±0.1* | 30.5±4.2* | 0.025±0.003 | 1.4±0.1 | 2.3±0.1 |
| LVH_SR1 | 7 | 9.25† | 320±27 | 9.1±0.1† | 6.8±0.1†‡ | 25.3±5.3† | 0.020±0.004‡ | 1.3±0.1 | 2.0±0.1 |
Values are means ± SD; n, no. of rats/group. The following groups were used: sham-operated (SHM) rats injected with PBS (SHM_PBS), SHM rats injected with adenovirus containing sarco(endo)plasmic reticulum Ca2+-ATPase 1 (SERCA1) (SHM_SR1), aortic banded {left ventricular (LV) hypertrophic (LVH)} rats injected with PBS (LVH_PBS), and LVH rats injected with adenovirus containing SERCA1 (LVH_SR1). HR, heart rate; LVDd, LV end-diastolic dimension; LVSd, LV end-systolic dimension; FS, fractional shortening; Vcf, velocity of circumferential shortening; LVPWD, LV posterior wall thickness at diastole; LVPWS, LV posterior wall thickness at systole.
P < 0.05, LVH_PBS vs. SHM_PBS or SHM_SR1 rats;
P < 0.05, LVH_SR1 vs. SHM_PBS or SHM_SR1 rats;
P < 0.05, LVH_SR1 vs. LVH_PBS rats.
Cardiac function of isolated hearts.
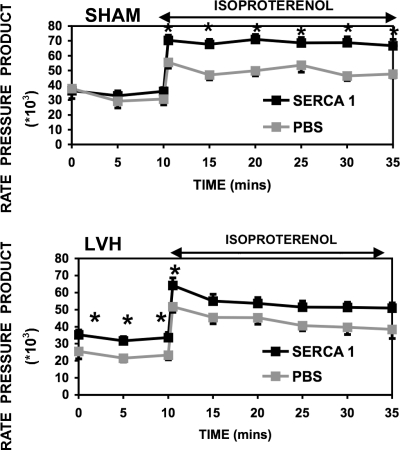
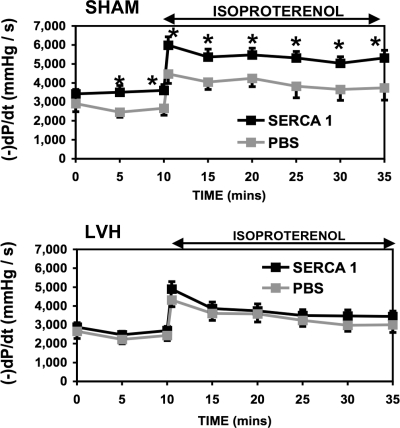
The heart-to-body weight ratio was 6.2 ± 0.5 g/kg for SHM animals and 8.3 ± 1.0 g/kg for LVH animals. Table 2 shows HR, LVDP, and +dP/dt for isolated heart preparations at both basal and high workloads (ISO), whereas Fig. 3 shows the rate-pressure product (RPP) and Fig. 4 shows −dP/dt. At basal workloads, RPP was depressed by 21% in the LVH_PBS group relative to the SHM_PBS group. This loss was expected for this early stage of heart failure (42, 50). In addition, relaxation (−dP/dt) was also slower in the LVH_PBS group compared with the SHM_PBS group (27, 42, 50). The slower relaxation of the failing heart has been suggested by some to reflect a slower sequestration of calcium into the SR. With the β-adrenergic challenge (ISO), RPP nearly doubled for both SHM_PBS_ISO and LVH_PBS_ISO groups.
Table 2.
Hemodynamic parameters of isolated retrograde-perfused SHM and LVH hearts after SERCA1 cDNA or PBS delivery to coronaries in vivo
| Group |
Basal |
ISO
|
||||
|---|---|---|---|---|---|---|
| HR, beats/min | LVDP, mmHg | +dP/dt, mmHg/s | HR, beats/min | LVDP, mmHg | +dP/dt, mmHg/s | |
| SHM_PBS | 260±18 | 111±9 | 2,770±420 | 409±15 | 115±10 | 4,300±480 |
| SHM_SR1 | 262±14 | 122±7 | 3,690±280* | 434±12 | 154±8* | 6,050±310* |
| LVH_PBS | 210±22 | 109±18 | 2,480±360 | 377±25 | 119±11 | 4,130±400 |
| LVH_SR1 | 260±10† | 120±8 | 2,850±320 | 431±17† | 121±8 | 4,620±370 |
Values are means ± SE. ISO, isoproterenol treatment.
P < 0.05, SHM_SR1 vs. SHM_PBS hearts and SHM_SR1_ISO vs. SHM_PBS_ISO hearts;
P < 0.05, LVH_SR1 vs. LVH_PBS hearts and LVH_SR1_ISO vs. LVH_PBS_ISO hearts.
Fig. 3.
Rate-pressure product [RPP (in mmHg·beats·min−1) ± SE] from isolated, retrograde-perfused rat hearts 48–72 h after Ad.cmv.SERCA1 or PBS delivery in vivo. The rat model includes 10-wk aortic banded LVH rats (bottom) and SHM healthy rats (top). Data are presented for RPP at basal workloads and during an adrenergic challenge. *Significant difference (P < 0.05). RPP was significantly enhanced with Ad.cmv.SERCA1 gene transfer.
Fig. 4.
Relaxation (−dP/dt) from isolated retrograde-perfused rat hearts after Ad.cmv.SERCA1 or PBS delivery to coronaries in aortic banded LVH rats (bottom) and SHM healthy rats (top). Data are presented for −dP/dt at basal workloads and during an adrenergic challenge. *Significant difference (P < 0.05).
Hemodynamic parameters for the SHM group overexpressing SERCA1 (i.e., the SHM_SR1 group) are also shown in Table 2, Fig. 3, and Fig. 4. There was a modest (10%, not significant) increase in the RPP with SERCA1 expression in the SHM_SR1 group relative to the SHM_PBS group. However, −dP/dt was enhanced significantly (36% faster). This faster relaxation of the heart after SERCA1 overexpression is consistent with a faster sequestration of calcium by the SR. With the β-adrenergic challenge, relaxation (−dP/dt) was again enhanced significantly (36% faster) with SERCA1 expression in the SHM_SR1_ISO group compared with the SHM_PBS_ISO group. The SHM_SR1_ISO group also revealed a significantly greater LVDP (25%) relative to the SHM_PBS_ISO group. Therefore, these results demonstrate that the overexpression of SERCA1 in healthy SHM animals results in enhanced function in the isolated, retrograde-perfused rat heart. These findings are consistent with the hypothesis that calcium uptake by the SR is accelerated with the overexpression of SERCA1.
Importantly, the hemodynamic response of the LVH group overexpressing SERCA1 was also enhanced. In LVH_SR1 isolated hearts, the basal RPP was 27% greater than the LVH_PBS group. In fact, the RPP was normalized in LVH hearts (LVH_SR1) relative to the SHM_PBS group. Interestingly, the improvement was accounted for by a significant increase in HR, whereas LVDP was not significantly different, and the relaxation rate (−dP/dt) was only modestly enhanced (14%, not significant). With ISO treatment, the RPP was again enhanced in the LVH_SR1_ISO group relative to the LVH_PBS_ISO group (Fig. 3). Accordingly, function in the LVH_SR1_ISO group was again normalized to the SHM_PBS_ISO group during adrenergic challenge.
31P NMR spectroscopy (PCr/ATP).
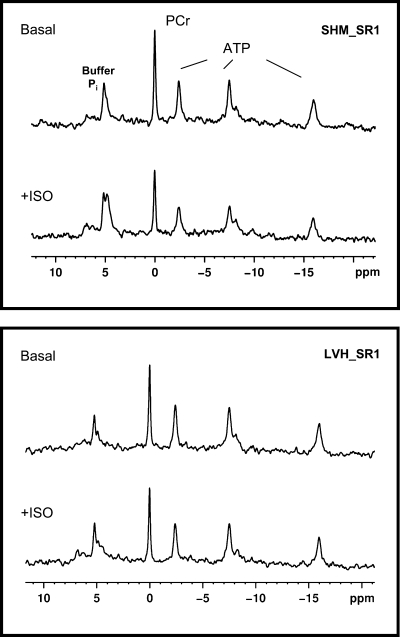
Representative 31P NMR spectra are shown in Fig. 5. Ratios for PCr/β-ATP peak areas for all eight groups are shown in Table 3. PCr/β-ATP for the SHM_PBS group (1.7) was low compared with the well-established ratio of 1.8–2 reported by our group and others for the isolated retrograde perfused rat heart (41, 56). This loss is attributed to the invasiveness of the open-chest surgical procedure for gene (or PBS) delivery and agrees with the 31P NMR data previously reported by other groups using a similar open-chest protocol for gene transfer (8).
Fig. 5.
Representative 31P NMR spectra from SHM_SR1 (top) and LVH_SR1 (bottom) hearts at basal and high workloads (ISO) 48–72 h after Ad.cmv.SERCA1 gene transfer. The overexpression of SERCA1 in SHM and LVH hearts did not compromise, or enhance, the energy potential (PCr/ATP) relative to the energy potential of PBS groups.
Table 3.
Metabolic analysis
| Group | n | VTCA, μmol·min−1·g dry wt−1 | F1, μmol·min−1·g dry wt−1 | PCr/ATP |
|---|---|---|---|---|
| SHM_PBS | 8 | 10.5±2.1 | 4.3±0.6 | 1.67±0.32 |
| SHM_SR1 | 6 | 9.7±1.7 | 5.6±1.4 | 1.69±0.28 |
| SHM_PBS_ISO | 7 | 13.2±1.0† | 5.7±0.4 | 1.77±0.38 |
| SHM_SR1_ISO | 7 | 18.2±2.5*† | 4.1±0.3 | 1.69±0.18 |
| LVH_PBS | 6 | 8.8±0.3 | 3.5±0.7 | 1.43±0.38 |
| LVH_SR1 | 6 | 9.3±1.4 | 3.0±0.3 | 1.24±0.14§ |
| LVH_PBS_ISO | 7 | 15.1±2.6‡ | 3.9±0.5 | 1.31±0.28§ |
| LVH_SR1_ISO | 7 | 15.3±2.5‡ | 3.3±0.3 | 1.23±0.17§ |
Values are means ± SD. 13C NMR kinetic analysis provided the tricarboxylic acid cycle flux (VTCA) and metabolite exchange between the mitochondria and cytosol via the α-ketoglutamate-malate transporter (F1). 31P NMR spectroscopy was used to assess energy potential {phosphocreatine (PCr)/ATP}.
P < 0.05, SHM_SR1_ISO vs. SHM_PBS_ISO groups;
P < 0.05, SHM_PBS_ISO and SHM_SR1_ISO groups vs. SHM_PBS and SHM_PBS_ISO groups;
P < 0.05, LVH_PBS_ISO and LVH_SR1_ISO groups vs. LVH_PBS and LVH_PBS_ISO groups;
P < 0.05, LVH vs. SHM groups.
PCr/ATP in SHM animals was sustained during the β-adrenergic challenge and with SERCA1 overexpression. This finding shows that even with enhanced contractility, energy demands could still be met in the healthy SHM group overexpressing SERCA. In agreement with previous reports by our group and others (41, 56), PCr/ATP was lower (20%) in the failing PBS heart relative to SHM_PBS hearts (Table 3). The ratio was sustained in the failing-PBS group during the β-adrenergic challenge. Failing hearts overexpressing SERCA1 showed a slight, nonsignificant drop in the ratio at baseline despite the enhanced function compared with the failing-PBS group. This energy potential did not change significantly in the LVH_SR1 group during the adrenergic challenge. These findings demonstrate that the overexpression of SERCA1 in the failing heart did not compromise, or improve, the low energy potential despite enhanced function.
13C NMR analysis of palmitate oxidation.
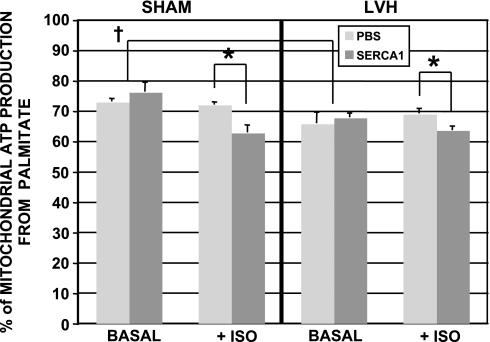
In vitro 13C NMR analysis of tissue extracts provided the fractional contribution of palmitate to mitochondrial ATP production based on the incorporation of 13C from labeled palmitate to endogenous acetyl-CoA pools (Fc; Fig. 6) (32, 42). In this model, palmitate contributed significantly less to mitochondrial ATP production in the LVH_PBS group (66% contribution) compared with the SHM_PBS group (73% contribution) at baseline. These contributions (Fc) were not altered with the overexpression of SERCA1 at baseline in either failing or SHM hearts. Fc values were also unchanged during the β-adrenergic challenge in SHM_PBS and LVH_PBS groups, again. However, the fractional contribution from palmitate was significantly reduced during the adrenergic challenge in both LVH_SR1_ISO and SHM_SR1_ISO groups (see Fig. 6).
Fig. 6.
Percent contribution of palmitate oxidation to mitochondrial ATP production at basal and high workloads (ISO) from SHM or LVH hearts after Ad.cmv.SERCA1 or PBS delivery in vivo. Both SHM and LVH hearts overexpressing SERCA1 showed a decrease in palmitate contribution during the adrenergic challenge (ISO). *Significant difference (P < 0.05); †significant difference (P < 0.05).
13C NMR analysis of mitochondrial metabolite transport and TCA cycle flux.
We also determined whether SERCA1 expression influenced metabolite exchange between the cytosol and mitochondria via the α-KG-malate transporter (F1) (i.e., as part of the malate-aspartate shuttle) parallel to changes in the TCA cycle rate (VTCA). We have previously reported that this balance, which is regulated by calcium-sensitive α-KG dehydrogenase, can be measured by 13C NMR (27, 40). Sequential 13C NMR spectra were collected from SHM and LVH hearts to follow the incorporation of the 13C label from [2,4,6,8,10,12,14,16-13C8]palmitate into endogenous glutamate pools. A nonlinear least-square fit of our established kinetic models of cardiac metabolism to 13C NMR data for glutamate gave VTCA and F1 (i.e., as part of the malate-aspartate shuttle). The results are shown in Table 3. In agreement with our recent report (50), VTCA rates were lower in the LVH_PBS group relative to the SHM_PBS group. These oxidative rates closely followed changes in workload, as expected. With workloads normalized in the LVH_SR1 group relative to the SHM_PBS group, VTCA values were also matched. The α-KG-malate transport rates (F1) were nearly similar (nonsignificant difference, P > 0.05) between LVH_PBS and SHM_PBS groups. This finding supports our earlier study (27) on hypertrophic hearts. In addition, the rate (F1) was not significantly influenced by either the overexpression of SERCA or adrenergic challenge.
DISCUSSION
There were several important findings in this study. First, adenoviral gene transfer of SERCA1 in SHM and aortic banded rat hearts resulted in 1) a 34% increase in total SERCA content. In the isolated heart preparation, this lead to 2) a significant increase in cardiac function in SHM and LVH hearts, 3) without affecting either PCr/ATP at basal and high workloads or 4) the transport of glycolytic reducing equivalents into the mitochondria via the α-KG-malate transporter. In addition, 5) the mitochondrial ATP production shifted from fats to carbohydrates at the higher workloads with SERCA1 expression in both SHM and LVH hearts. Consistent with the hypothesis that SERCA1 overexpression leads to accelerated calcium uptake by the SR, the SHM group displayed enhanced relaxation (−dP/dt) at both workloads, and LVDP was also significantly potentiated, particularly during the adrenergic challenge. Unexpectedly, enhanced function in the LVH group was linked to increased HR rather than enhanced relaxation or LVDP. Importantly, 6) the improvements observed in the isolated rat heart preparations did not translate to in vivo conditions. Echocardiographic measures of fractional shortening in vivo revealed no changes in function in the SHM group overexpressing SERCA1, whereas function for the LVH group was further depressed. As discussed below, this disconnect between isolated and in vivo heart data is not entirely unexpected, and it highlights the importance and value of these separate models as we elucidate the role of SERCA and move closer toward a strategy for the genetic treatment of heart disease.
Nonfailing sham hearts overexpressing SERCA1 showed enhanced contractility.
We found (Table 2, Fig. 3, and Fig. 4) that the overexpression of SERCA1 had a modest impact on the RPP, yet a profound impact on contractility rates (+dP/dt and −dP/dt), in the isolated heart preparation from SHM rats. HR was unaffected. The accelerated relaxation (−dP/dt) is consistent with a faster removal of calcium from the cytosol as the number of SERCA pumps are overexpressed. These results compared favorably with functional data reported for isolated, work-performing hearts from SERCA1 transgenic mice (17). Huke et al. (17) also reported a 20% increase in LVDP, whereas +dP/dt and −dP/dt nearly doubled, and HR was unchanged. However, much like our own in vivo data, they did not find dramatic changes in vivo (17). Relaxation parameters were unchanged, and contractile parameters were enhanced to a far lesser degree than in ex vivo experiments. They suggested that the differences between ex vivo and in vivo data reflect the presence of in vivo compensatory mechanisms that “normalize” cardiac function in transgenic mice.
This discordance between ex vivo and in vivo function may be specific to the SERCA1 isoform. In a similar finding, Logeart and colleagues (29) overexpressed the SERCA1 isoform in the healthy rabbit heart via adenovirus-mediated delivery to LV coronaries. Three days after gene transfer in vivo, SERCA protein levels were up 23% and the maximal rate of Ca2+ uptake had increased by 37%. However, baseline hemodynamics and conventional echocardiographic measurements of global LV function, in vivo, were poorly affected. Modest increases in baseline maximal wall velocities and strain rates were only detected by tissue Doppler imaging. In contrast, in mice with moderate increases in SERCA2a, hemodynamic measurements in vivo revealed significant increases in maximal +dP/dt and −dP/dt, similar to what was observed in isolated myocytes and isolated papillary muscles (14).
RPP recovered to normal levels at baseline in the LVH group overexpressing SERCA1.
The recovery supports the potential therapeutic value of overexpressing the SERCA1 isoform in humans with LVH heart disease. However, the relaxation rate (−dP/dt) and LVDP did not improve with treatment. Instead, the improvement in the RPP was linked to a 20% increase in HR. These hearts were not paced. This finding was unexpected and suggests a physiological link between SERCA function and HR regulation. It is unclear if SERCA influences the electrical excitability and repolarization of cardiomyocytes or potentially the neurons that innervate the heart. Yet, a similar connection has been described for a conditionally mutant SERCA form in Drosophila (49). Sanyal et al (49) found HR strikingly reduced when SERCA activity was inactivated. In addition, Rubio et al. (47) also demonstrated the involvement of augmented Ca2+ cycling in arrhythmogenesis after overexpression of SERCA1 in a L-type voltage-dependent calcium channel-overexpressing transgenic mouse model.
It was also unexpected that the overexpression of SERCA1 in the LVH group did not lead to enhanced LVDP or relaxation rates in isolated hearts. We originally hypothesized that the overexpression of SERCA1 would lead to a faster calcium uptake by the SR, thereby accelerating relaxation and potentiating LVDP. However, our finding was more consistent with the hypothesis that calcium sequestration by the SR may not be the limiting factor affecting LVDP and relaxation in the failing heart (22, 43). In brief, if reuptake of Ca2+ becomes too fast, the cytoplasmic calcium concentration near the myofilaments will decline rapidly, preventing the appropriate activation of myofilaments and hindering adequate force development (22, 54). Thus, the SR competes with troponin C for calcium binding (16, 22, 30, 54). In heart failure, the calcium sensitivity of myofilaments is increased in both human and rodent models (for a review, see Ref. 15). A potential adverse effect of increasing calcium sensitivity is slowed relaxation and diastolic dysfunction (31). If myofilament properties are limiting the relaxation rate and force development in heart failure, the overexpression of SERCA would be competitive and deleterious.
In addition, the majority of studies that concluded that calcium sequestration limited the relaxation rate were performed in isolated cardiomyocytes. Under such unloaded conditions, cross-bridges cycle much faster than loaded cross-bridges, and the sequestration rate by the SR may indeed limit cytosolic calcium decline (21). However, under loaded conditions, relaxation of the myocardium may be more closely linked to myofilment properties (21, 22). Indeed, Teucher and colleagues (54) also reported that moderate SERCA1 gene transfer and expression improved contractility and Ca2+ cycling in cardiomyocytes. However, higher SERCA1 expression levels impaired myocyte shortening because of higher SERCA activity and Ca2+ buffering (54). Vangheluwe et al. (59) also demonstrated that replacement of the SERCA2a isoform with SERCA2b, an isoform with increased Ca2+ affinity, led to severe cardiac hypertrophy, stress intolerance, and a reduced life span in transgenic mice.
SERCA1 overexpression potentiated the functional response of healthy and hypertrophic hearts to ISO.
An important finding in this study was that the β-adrenergic response (LVDP, ±dP/dt, and HR) was dramatically enhanced in healthy rat hearts overexpressing SERCA1. This is in contrast to what has been reported for transgenic mice overexpressing SERCA1. Huke et al. (17) reported that the β-adrenergic response was dramatically reduced in isolated transgenic hearts relative to the response of nontransgenic hearts. They suggested that the improvements observed with SERCA1 overexpression at baseline may have been maximal. Their finding is consistent with the level of SERCA2a and phospholamban expressed in those hearts. Unlike SERCA1, SERCA2a is regulated by the PKA-dependent phosphorylation of phospholamban in response to catecholamines. This stimulation of the Ca2+ uptake rate by catecholamines appears to be the main means by which β-adrenergic agonists accelerate relaxation in the heart (28). With the total SERCA content increased by 150% in SERCA1 transgenic animals, the endogenous SERCA2 content was reduced by 50% (17, 25, 30). It is reasonable to predict a reduction in the adrenergic response of the catecholamine-sensitive phospholamban/SERCA2 interaction. In our in vivo model, whereas the total SERCA content was increased by 34%, SERCA2a content was not reduced, and the heart remained responsive to the adrenergic challenge.
Importantly, the LVH_SR1_ISO group also remained responsive to the adrenergic challenge. It has been widely reported that β-adrenergic receptor density and responsiveness in the failing heart reduces as the heart progresses toward late-stage failure (4, 57). At the early stage of the disease (10 wk postbanding), the adrenergic response is still intact. We found that the response was not compromised with SERCA1 expression. In fact, the RPP was normalized in the LVH_SR1_ISO group relative to the SHM_PBS_ISO group. Consistent with the baseline results, the improvement in the RPP in LVH_SR1_ISO hearts was linked solely to an increase in HR and not LVDP or the relaxation rate.
Exogenous fatty acid contributes less to mitochondrial ATP production with SERCA1 overexpression in healthy and hypertrophic hearts at higher workloads.
Whereas a number of studies have reported functional changes in the SERCA-overexpressing heart, only a few studies have examined metabolic changes (2, 8). In one such study, Belke et al. (2) followed fatty acid and glucose oxidation in an isolated heart preparation from transgenic mice overexpressing SERCA2. They found that the balance of substrate oxidation shifted from fats (palmitate) to carbohydrates (glucose) with SERCA overexpression at baseline workloads without an increase in glycolytic rates. They linked the shift to a measured increase in pyruvate dehydrogenase activity and mitochondrial calcium. They concluded that the higher mitochondrial calcium levels stimulated the calcium-sensitive pyruvate dehydrogenase complex to increase pyruvate oxidation.
Although we observed no shift in substrate selection at baseline workloads with SERCA1 expression, oxidation did shift from fats to carbohydrates in both groups at higher workloads. In agreement with our previous report (42), this shift was not observed in PBS groups going from low to high workloads. That is, the balance between fatty acid and carbohydrate oxidation did not change with increases in workload in nonfailing, isolated, retrograde-perfused hearts (42). Therefore, the shift with SERCA expression in the SHM group represents a more efficient selection of substrates (in terms of oxygen cost) that accompanies the improved contractile performance. In the LVH group, this shift may have therapeutic value. Several studies have reported that drugs (ranolazine and trimetazine) designed to force this shift in heart disease are accompanied by improved cardiac function and an increase in the myocardial energy potential (PCr/ATP) (10, 11, 51, 58).
The energetic state (PCr/ATP) of healthy and hypertrophic hearts is sustained with enhanced function after the overexpression of SERCA1.
31P NMR is the only noninvasive technique capable of measuring PCr, ATP, and inorganic phosphate in the intact beating heart. PCr/ATP is one indicator of the energetic state of the myocardium, and a reduction in PCr/ATP is interpreted as a loss of energy reserve (for a review, see Ref. 53). Our group and others have shown a drop in PCr content and PCr/ATP in the failing myocardium, consistent with a drop in total creatine content and the creatine kinase reaction velocity (41, 56). Importantly, it has been reported that the reduced energy state of the failing heart is improved by overexpressing the SERCA2a isoform in aortic banded rat hearts (8). Whether this improvement could be sustained for extended periods of stress (i.e., β-adrenergic stimulation, 40 min) was not determined. In addition, the hearts in the previous study were retrograde perfused with glucose alone, and PCr/ATP actually declined in the normal SHM group with SERCA overexpression. Thus, the energetic costs involved in improving contractile function with SERCA are still incompletely understood.
Our 31P NMR measures of PCr and ATP were performed under a more physiological provision of substrates (glucose and palmitate) at both basal and high workloads. In contrast to the previous energetic study cited above (8), PCr/ATP (1.7) was not compromised in the SHM_SR1 group compared with the SHM_PBS group (1.8). This energetic state was sustained in both SHM groups during the adrenergic challenge, even though the SHM_SR1_ISO group demonstrated significantly greater function than the SHM_PBS_ISO group (Table 3).
In the banded group at baseline, PCr/ATP was low (LVH_PBS group: 1.4), as expected for this stage of the disease (41, 53, 56). In contrast to results of the previously reported study for SERCA2a (8), overexpression of the SERCA1 isoform in banded animals did not improve the ratio (LVH_SR1 group: 1.2, nonsignificant difference) even though function was enhanced. Importantly, PCr/ATP did not reduce further throughout the 40-min adrenergic challenge despite enhanced function.
The maintenance of the energetic ratio does not necessarily indicate that metabolic processes are intact and meeting demands. Measuring PCr/ATP values has the disadvantage that it underestimates the change in PCr when ATP levels are also reduced. Therefore, the quantification of PCr and ATP is valuable. Although direct quantification of high-energy metabolite concentrations was beyond the scope of this study, we did assess the percent change in PCr and ATP levels going from baseline to high workloads (i.e., baseline PCr and ATP levels were used as an internal reference). After 40 min of adrenergic challenge, both PCr and ATP were 10% lower in the SHM_SAL_ISO group relative to pre-ISO values, 20% reduced in the SHM_SR1_ISO group, 22% in the LVH_SAL_ISO group, and 19% in the LVH_SR1_ISO group. This drop in ATP raises concerns about the energy available to drive ATP-ase reactions.
Others have raised concerns that the overexpression of SERCA in the heart could limit the free energy (ΔG) of ATP/ADP needed to drive enzymatic reactions (8). In the normal heart, ΔG is −58 kJ/mol (55). The ATP-ase SERCA will stop functioning properly below a threshold value for ΔG of about −52 kJ/mol (8, 55). If ATP levels decrease and ADP levels rise, ΔG needed to drive SERCA could be compromised, and, in turn, relaxation rates slowed and contractility reduced. Our findings indicate that this is not an issue. Indeed, we did observe a drop in ATP levels with the adrenergic challenge in the isolated hearts, but the response to SERCA overexpression was not compromised. In fact, function (RPP) was enhanced.
TCA cycle flux and α-KG-malate transport activity.
We examined whether a change in calcium levels due to hypertrophy or SERCA overexpression could influence the calcium-sensitive dehydrogenase of the TCA cycle and impact metabolite exchange between the mitochondria and cytosol. More specifically, we have shown, in a previous study, that an increase in average cellular calcium levels increased the affinity of calcium-sensitive α-KG dehydrogenase for the substrate α-KG, thereby outcompeting the α-KG-malate transporter for α-KG (13, 26, 40). The α-KG-malate transporter is part of the malate-aspartate shuttle and is key to bringing reducing equivalents generated by glycolysis into the mitochondria for ATP production (13, 26). As measured by 13C NMR, α-KG-malate transport was reduced in the previous study at the higher calcium levels for a given workload. A loss in malate-aspartate shuttle activity for a given workload represents a loss in efficient utilization of metabolic substrates.
It has been widely reported that pressure-overload hypertrophy is characterized by cytosolic calcium overload as the disease progresses to late-stage failure (3, 7, 12). Thus, one might speculate that the α-KG-malate transport rate may be reduced in the LVH_PBS group relative to the SHM_PBS group as a consequence of calcium overload. However, in support of our previous report (27), α-KG-malate transport rates (F1; Table 3) were nearly equal between LVH_PBS and SHM_PBS groups (nonsignificant difference, P > 0.05). In our previous study (27), we reported that the rate was increased twofold at 2 wk postbanding but returned to normal rates by 10 wk. It is unknown if F1 continues to drop as the disease progresses from compensated hypertrophy to the later stages of cardiac failure. This may, in fact, account for the slightly lower trend (nonsignificant) observed for the LVH group in this study. In addition, we show here, for the first time, that F1 did not increase with adrenergic challenge or VTCA (i.e., with ISO) in both SHM and LVH groups.
We did questioned whether the overexpression of SERCA could influence cytosolic and mitochondrial calcium levels enough to affect the activity of the α-KG-malate transporter. Indeed, Belke and colleagues (2) reported that mitochondrial calcium was greater in paced cardiomyocytes isolated from transgenic mice overexpressing SERCA2. Thus, there is the potential that F1 could be reduced and limit mitochondrial oxidation of NADH produced via glycolysis. As shown in Table 3, we found α-KG-malate transport rates (F1) were not significantly affected by SERCA1 overexpression. In particular, F1 was similar for SHM_PBS and SHM_SR1 groups. Likewise, LVH_PBS and LVH_SR1 groups were unchanged. This finding indicates that metabolic efficiency, in terms of glycolytic NADH transport into the mitochondria, was not compromised with the overexpression of SERCA1 in SHM and LVH hearts.
Limitations.
The ex vivo hearts were not paced in the spectrometer. HR and cardiac function were recorded parallel to NMR measures of metabolism inside the 9.4-T NMR magnet throughout the duration of the protocol. Pacing the isolated hearts inside the magnet would require positioning metal pacing leads directly on the heart and within the NMR-radio frequency (RF) coil. This approach is highly susceptible to RF noise and is not routinely used. Importantly, we found that nonpaced HRs increased with the overexpression of SERCA1. Had HRs been paced and normalized to the slower control rates (similar to experimental conditions set for isolated cardiomyocytes experiments), LVDPs may have increased (force-frequency) and influenced the outcome and conclusions of the study. While paced experiments would provide additional insight into this study, the nonpaced experiments underscore the role of SERCA overexpression on intrinsic HRs, which is poorly understood.
A second limitation involved the decision to use PBS rather than LacZ, green fluorescent protein (GFP), or empty virus as the control group in these experiments. This decision was based on several previous studies that indicated that reporter genes expressed in cardiomyocytes influence function. Weisser-Thomas et al. (62) reported a significant reduction in contraction amplitude in cardiomyocytes after Ad.cmv.LacZ infection relative to noninfected cells. Similarly, Izumo and Shioi (20) found that GFP could produce contractile dysfunction in a transgenic mouse heart in which GFP was expressed, and Lafont et al. (24) observed vasomotor dysfunction with Ad.LacZ in rabbit arteries. Consequently, contractility data for SERCA gene transfer could be misinterpreted if compared with LacZ or GFP controls. These effects have yet to be resolved for intact functioning healthy/failing hearts, and they were not an aim of this study.
Summary.
Although previous cardiomyocyte data and nonhypertrophic transgenic mouse models have revealed important and positive functional and metabolic responses to SERCA1 isoform overexpression, this study extends those earlier findings to include the intact functioning hypertrophic heart expressing SERCA1 under both in vivo and ex vivo conditions. In support of previous work, we found that the overexpression of SERCA1 in isolated heart preparations resulted in enhanced function and sustained energy potentials. At higher workloads, SERCA1 expression influenced energy metabolism by increasing glucose oxidation, thereby potentially making overall energy production more efficient. However, under the fully loaded in vivo condition, function was not affected in healthy hearts overexpressing SERCA1 at basal workloads, and hypertrophic hearts revealed depressed function with SERCA1 expression. Further investigation is required to determine if this is a consequence of whole body compensatory mechanisms or a limitation in the competitive handling of calcium between the SR versus myofilaments.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-079415 (to J. M. O'Donnell), American Heart Association National Scientist Development Grant 0230099N (to J. M O'Donnell), NHLBI Program Project Grant HL-62426 (to D. L. Geenen), and the University of Illinois at Chicago Animal Core (D. L. Geenen, Core Director) for the Center for Cardiovascular Research.
Acknowledgments
The SERCA1 adenovirus was a kind gift from Dr. G. Inesi. We gratefully acknowledge Dr. Douglas Lewandowski for advice and support during the course of this study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arai M, Suzuki Tadashi Nagai R. Sarcoplasmic reticulum genes are up regulated in mild cardiac hypertrophy but down regulated in severe cardiac hypertrophy induced by pressure overload. J Mol Cell Cardiol 28: 1583–1590, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol 292: H1755–H1763, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85: 1046–1055, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Minobe WA, Reynolds MV, Port JD, Rasmussen R, Ray PE, et al. Reduced b1 receptor messenger RNA abundance in the failing human heart. J Clin Invest 92: 2737–2745, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavagna M, O'Donnell JM, Sumbilla C, Inesi G, Klein MG. Exogenous Ca2+-ATPase isoform effects on Ca2+ transients of embryonic chicken and neonatal rat cardiac myocytes. J Physiol 528: 53–63, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ. Moderate severity heart failure does not involve a downreguation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol 287: H1538–H1543, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Del Monte F, Harding S, Schmidt U, Matsui T, Kang Z, William GD, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 100: 2308–2311, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation 104: 1424–1429, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Monte F, Butler K, Boecker W, Gwathmey JK, Hajjar RJ. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics 9: 49–56, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Fragasso G, Perseghin G, DeCobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, DelMaschio A, Margonato A. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 27: 942–948, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fragasso G Inhibition of free fatty acids metabolism as a therapeutic target in patients with heart failure. Int J Clin Pract 61: 603–610, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 61: 701–76, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Hansford RG Dehydrogenase activation by Ca2+ in cells and tissues. J Bioenerg Biomembr 23: 823–854, 1991. [DOI] [PubMed] [Google Scholar]
- 14.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, Bluhm WF, Meyer M, Sayen MR, Swanson E, Dillman WH. Overexpression of the rat SR Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest 100: 380–389, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinken AC, Solaro RJ. A dominant role of cardiac molecular motors in the intrinsic regulation of ventricular ejection and relaxation. Physiology (Bethesda) 22: 73–80, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hiranandani N, Raman S, Kalyanasundaram A, Periasamy M, Janssen PML. Frequency-dependent contractile strength in mice over- and underexpressing the sarco(endo)plasmic reticulum calcium-ATPase. Am J Physiol Regul Integr Comp Physiol 293: R30–R36, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Huke S, Prasad V, Nieman ML, Nattamai KJ, Grupp IL, Lorenz JN, Periasamy M. Altered dose response to β-agonists in SERCA1-expressing hearts ex vivo and in vivo. Am J Physiol Heart Circ Physiol 283: H958–H966, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Inesi G, Lewis D, Sumbilla C, Nandi A, Strock C, Huff KW, Rogers TB, Johns DC, Kessler PD, Ordahl CP. Cell-specific promoter in adenovirus vector for transgenic expression of SERCA 1 ATPase in cardiac myocytes. Am J Physiol Cell Physiol 274: C655–C663, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Inesi G, Cavagna M, O'Donnell JM, Sumbilla C, Zhong L, Ma H, Klein MG. Adenovirus-mediated gene transfer of SERCA isoforms. In: Molecular Approaches to the Therapy of Heart Failure, edited by Hasenfuss G and Marban E. Darmstadt, Germany: Springer-Steinkopff, 2000.
- 20.Izumo S, Shioi T. Cardiac transgenic and gene-targeted mice as models of cardiac hypertrophy and failure: a problem of (new) riches. J Card Fail 4: 263–270, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Janssen PML, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol 43: 523–531, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen PM, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol 282: H499–H507, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kawase Y, Ly GQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 51: 1112–1119, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Lafont A, Loirand G, Pacaud P, Vilde F, Lemarchand P, Escande D. Vasomotor dysfunction early after exposure of rabbit arteries to an adenoviral vector. Hum Gene Ther 10: 1033–1040, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Lalli MJ, Yong J, Prasad V, Hashimoto K, Plank D, Babu GJ, Kirkpatrick D, Walsh RA, Sussman M, Yatani A, Marban E, Periasamy M. Sarcoplasmic reticulum Ca2+ ATPase (SERCA1) 1a-structurally substitutes for SERCA2a in the cardiac sarcoplasmic reticulum and increases cardiac Ca2+ handling capacity. Circ Res 89: 160–167, 2001. [DOI] [PubMed] [Google Scholar]
- 26.LaNoue KF, Nicklas WJ, Williamson JR. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem 245: 102–111, 1970. [PubMed] [Google Scholar]
- 27.Lewandowski ED, O'Donnell JM, Scholz TD, Sorokina N, Buttrick PM. Recruitment of NADH Shuttling in pressure overloaded and hypertrophic rat hearts. Am J Physiol Cell Physiol 292: C1880–C1886, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, DeSantiago J, Chu G, Kranias EG, Bers SM. Phosphorylation of phospholamban and troponin I in β-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol 278: H769–H779, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Logeart D, Vinet L, Ragot T, Heimburger M, Louedec L, Michel JB, Escoubet B, Mercadier JJ. Percutaneous intracoronary delivery of SERCA gene increases myocardial function: a tissue Doppler imaging echocardiographic study. Am J Physiol Heart Circ Physiol 291: H1773–H1779, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, Grupp G, Lytton J, Walsh RA, Periasamy M. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res 83: 889–897, 1998. [DOI] [PubMed] [Google Scholar]
- 31.MacGowan GA The myofilament force-calcium relationship as a target for positive inotropic therapy in congestive heart failure. Cardiovasc Drugs Ther 19: 203–210, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Malloy CR, Sherry AD, Jeffrey FMH. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem 263: 6965–6971, 1989. [PubMed] [Google Scholar]
- 33.Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium 25: 277–290, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventrcular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA 97: 793–798, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donnell JM, Alpert NM, White L, Lewandowski ED. Coupling of mitochondrial fatty acid uptake to oxidative flux in the intact heart. Biophys J 82: 11–18, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell JM, Lewandowski ED. Efficient, cardiac-specific adenoviral gene transfer by isolated retrograde perfusion in vivo. Gene Ther 12: 958–965, 2005. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab 290: E448–E455, 2006. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell JM, Lewandowski ED. Controlling specificity and efficiency of adenoviral gene transfer in heart by catheter-based coronary perfusion. In: Gene Therapy: Prospective Assessment in Its Societal Context, edited by Niewohner J and Tannert C. Amsterdam, Netherlands: Elsevier, 2006, p. 33–46.
- 39.O'Donnell JM, Sumbilla C, Hailun M, Farrance I, Cavagna M, Klein M, Inesi G. Tight control of exogenous SERCA expression is required to obtain acceleration of calcium transients with minimal cytotoxic effect in cardiac myocytes. Circ Res 88: 415–421, 2001. [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell JM, Doumen C, LaNoue KF, White LT, Yu X, Alpert NM, Lewandowski ED. Dehydrogenase regulation of metabolite oxidation and efflux from mitochondria in intact hearts. Am J Physiol Heart Circ Physiol 274: H467–H476, 1998. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell JM, Narayan P, Bailey MQ, Abduljalil AM, McCune S, Altschuld R, Robitaille PM. 31P-NMR analysis of congestive heart failure in the SHHR/Mcc-facp rat heart. J Mol Cell Cardiol 30: 235–241, 1998. [DOI] [PubMed] [Google Scholar]
- 42.O'Donnell JM, Fields A, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Physiol 44: 315–322, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez NG, Hashimoto K, McCune S, Altschuld RA, Marban E. Origin of contractile dysfunction in heart failure: calcium cycling versus myofilaments. Circulation 99: 1077–1083, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35: 430–442, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J Mol Cell Cardiol 33: 1053–1063, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro HB, Okoshi K, Cicogna AC, Bregagnollo EA, Rodrigues MA, Padovani CR, Aragon FF, Jamas E, Okoshi MP. Follow-up study of morphology and cardiac function in rats undergoing induction of supravalvular aortic stenosis. Arq Bras Cardiol 81: 569–575, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Rubio M, Bodi I, Fuller-Bicer GA, Hahn HS, Periasamy M, Schwartz A. Sarcoplasmic reticulum adenosine triphosphatase overexpression in the L-type Ca2+ channel mouse results in cardiomyopathy and Ca2+ induced arrhythmogensis. J Cardiovasc Pharmacol Ther 10: 235–249, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is down regulated in the failing heart. Circulation 94: 2837–2842, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Sanyal S, Jennings T, Dowse H, Ramaswami M. Conditional mutations in SERCA, the sarco-endoplasmic reticulum Ca2+-ATPase, alter heart rate and rhythmicity in Drosophila. J Comp Physiol 176: 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 155: 2033–2041, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev 7: 115–130, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Sumbilla C, Cavagna M, Zhong L, Ma H, Lewis D, Farrance I, Inesi G. Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes. Am J Physiol Heart Circ Physiol 277: H2381–H2391, 1999. [DOI] [PubMed] [Google Scholar]
- 53.TenHove M, Neubauer S. MR spectroscopy in heart failure–clinical and experimental findings. Heart Fail Rev 12: 48–57, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Teucher N, Prestle J, Seidler T, Currie S, Elliott EB, Reynolds DR, Schott P, Wagner S, Kogler H, Inesi G, Bers DM, Hasenfuss G, Smith GL. Excessive sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression causes increased sarcoplasmic reticulum Ca2+ uptake but decreases myocyte shortening. Circulation 110: 3553–3559, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Tian R, Halow JM, Meyer M, Dillmann WH, Figueredo VM, Ingwall JS, Camacho SA. Thermodynamic limitation for Ca2+ handling contributes to decreased contractile reserve in rat hearts. Am J Physiol Heart Circ Physiol 275: H2065–H2071, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Tian R, Ingwall JS. The molecular energetics of the failing heart from animal models–small animal models. Heart Fail Rev 4: 245–253, 1999. [Google Scholar]
- 57.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β-adrenergic receptors in the failing human heart. Circulation 87: 454–463, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Van Bilsen M, Smeets PJH, Gilde AJ, van der Vusse GJ. Metabolic remodeling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res 61: 218–226, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Vangheluwe P, Tjwa M, Van Den Bergh A, Louch WE, Beullens M, Dode L, Carmeliet P, Dranias E, Herijgers P, Sipido KR, Raeymaekers L, Wuytack F. A SERCA2 pump with an increased Ca2+ affinity can lead to severe cardiac hypertrophy, stress intolerance and reduced life span. J Mol Cell Cardiol 41: 308–317, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Vatner DE, Ingwall JS. Effects of moderate pressure-overload cardiac hypertrophy on the distribution of creatine kinase isozymes. Proc Soc Exp Biol Med 175: 5–9, 1984. [DOI] [PubMed] [Google Scholar]
- 61.Viner RI, Ferrington DA, Willioms TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J 340: 667–669, 1999. [PMC free article] [PubMed] [Google Scholar]
- 62.Weisser-Thomas J, Dieterich E, Janssen PML, Schmidt-Schweda S, Maier LS, Sumbilla C, Pieske B. Method-related effects of adenovirus-mediated LacZ and SERCA1 gene transfer on contractile behavior of cultured failing human cardiomyocytes. J Pharm Tox Meth 51: 91–103, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Wolosker H, Rocha JBT, Engelender S, Panizzutti R, Miranda JD, de Meis L. Sarco/endoplasmic reticulum Ca2+-ATPase isoforms: diverse responses to acidosis. Biochem J 321: 545–550, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright MJ, Rosenthal E, Stewart L, Wightman LML, Miller AD, Latchman DS, Marber MS. β-Galactosidase staining following intracoronary infusion of cationic liposomes in the in vivo rabbit heart is produced by microinfarction rather than effective gene transfer: a cautionary tale. Gene Ther 5: 301–308, 1998. [DOI] [PubMed] [Google Scholar]
- 65.Yu X, White LT, Doumen C, Damico LA, LaNoue KF, Alpert NM, Lewandowski ED. Kinetic analysis of dynamic 13C NMR spectra: metabolic flux, regulation, and compartmentation in hearts. Biophys J 69: 2090–2102, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Alpert NM, Lewandowski ED. Modeling enrichment kinetics from dynamic 13C NMR spectra: theoretical analysis and practical considerations. Am J Physiol Cell Physiol 272: C2037–C2048, 1997. [DOI] [PubMed] [Google Scholar]