Abstract
Conduction of changes in diameter plays an important role in the coordination of peripheral vascular resistance and, thereby, in the control of arterial blood pressure. It is thought that conduction of vasomotor signals relies on the electrotonic spread of changes in membrane potential from a site of stimulation through gap junctions connecting the cells of the vessel wall. To explore this idea, we stimulated a short segment of mouse cremasteric arterioles with an application, via micropipette, of ACh, an endothelium-dependent vasodilator, or pinacidil, an ATP-sensitive K+ channel opener. Vasodilations were evaluated at the stimulation site (local) and at 500, 1,000, and 2,000 μm upstream. The vasodilator response evoked by direct arteriolar hyperpolarization induced by pinacidil decayed rapidly with distance, as expected for the passive spread of an electrical signal. Deletion of the gap junction proteins connexin37 or connexin40 did not alter the conduction of pinacidil-induced vasodilation. In contrast to pinacidil, the vasodilator response activated by ACh spread along the entire vessel without decrement. Although the ACh-induced conducted vasodilation was similar in wild-type and connexin37 knockout mice, deletion of connexin40 converted the nondecremental conducted response activated by ACh into one similar to that of pinacidil, with a decline in magnitude along the vessel length. These results suggest that ACh activates a mechanism of regenerative conduction of vasodilator responses. Connexin40 is essential for the ACh-activated regenerative vasodilator mechanism. However, neither connexin40 nor connexin37 is indispensable for the electrotonic spread of hyperpolarizing signals.
Keywords: gap junction, connexin37, connexin40, conducted vasodilation, endothelium, mouse cremaster arterioles
systemic blood pressure is determined by cardiac output and the resistance to blood flow in small arteries and arterioles in the microcirculation. Precise regulation of blood flow distribution depends on the well-integrated regulation of vasomotor tone along the length of arterioles and on the coordination of different microvessel segments (7, 15, 17, 47). Consequently, longitudinal conduction of vasomotor signals has emerged as an important physiological mechanism involved in the control of blood flow distribution and coordination of vascular resistance within the microvascular network (13, 14).
Conducted vasomotor responses have been associated with the propagation of an electrical signal (7, 10, 15, 17, 47), usually attributed to electrotonic spread of changes in membrane potential via gap junctional connection between adjacent cells (17, 34). Both dilator and constrictor signals propagate electrotonically, but, in addition, endothelium-dependent vasodilations have been observed to propagate along the entire vessel without decay, suggesting the activation of a regenerative mechanism (6, 7, 10, 15, 33). The molecular basis for the regenerative propagation of a vasomotor signal along the arterioles remains to be determined.
Gap junctions are intercellular channels that connect the cytoplasm of adjacent cells, allowing the passage of small molecules (molecular mass <1,000 Da) and current (13, 35). Vascular gap junctions are assembled from one or more connexin (Cx) proteins: Cx37, Cx40, Cx43, and Cx45 (27, 42). Cx45 is expressed only in smooth muscle cells, whereas Cx37 is typically found only in endothelial cells. Although Cx40 and Cx43 are expressed in both cell types (16, 29, 42), Cx40 is located predominately in endothelial cells (16, 42), and, in the mouse, this Cx has been observed solely in the endothelium (7, 15, 38).
Gap junctions are critical in cell-to-cell communication in the cardiovascular system (13, 14). In particular, Cx40 seems to play a key role in the control of microvascular network function, because deletion of Cx40 has been associated with irregular vasomotion (8) and a reduced conduction of vasodilator responses induced by ACh, bradykinin (7, 46), or electrical stimulation (15) in mouse cremaster arterioles, which suggests that responses activated by endothelium-dependent vasodilators are conducted through gap junctions connecting endothelial cells. However, although Cxs have some overlap in function, it has been demonstrated that these proteins may also work in concert (13, 14, 36, 38). Therefore, we hypothesized that Cx37 and Cx40 have different functions in the conduction of vasodilator responses.
In this study, we used the ATP-sensitive K+ (KATP) channel opener pinacidil and the endothelium-dependent vasodilator ACh to examine characteristics of the conducted vasomotor responses triggered by direct hyperpolarization of the vessel wall or by a receptor-dependent mechanism in the mouse cremaster microcirculation in vivo. It has been shown that the vasodilation evoked by the activation of KATP channels is conducted through endothelial cells (40). Thus, we also analyzed the participation of Cx37 and Cx40 in the conduction mechanisms activated by pinacidil and ACh using Cx37 knockout (Cx37−/−) mice and Cx40 knockout (Cx40−/−) mice. Our findings indicate that the conduction induced by the two agonists is quite different. In contrast to pinacidil, the vasodilation evoked by ACh does not decay along the arteriolar length, and Cx40 seems to be critical only for the conduction of the nondecremental component of the ACh-activated vasodilator signal.
MATERIALS AND METHODS
Male C57 Bl/6 (wild type), Cx37−/− (38), and Cx40−/− (37) mice between 22 and 30 g were used. Experiments were conducted according to the Helsinki Declaration and the “Guiding Principles in the Care and Use of Laboratory Animals” endorsed by the American Physiological Society. All animal protocols were approved by the Animal Care and Use Committee of the University of Virginia. Tail DNA was used to genotype the animals following the experiment. The sequences of the primers used to detect Cx40 wild-type and knockout alleles were as follows: sense primer, 5′-TGGAGCCACAGTTGCAATGGT-3′; antisense primer, 5′-TCTCTGACTCCGAAAGGCAAG-3′; and neo primer, 5′-GCACGAGACTAGTGAGACGTG-3′. The sequences of the primers used to detect Cx37 wild-type and knockout alleles were as follows: sense primer, 5′-TGCTAGACCAGCTCCAGGAAC-3′; antisense primer, 5′-GTCCCTTCGTGCCTTTATCTC-3′; and neo primer, 5′-AGAGGCTATTCGGCTATGACTG-3′.
Mouse cremaster preparation.
Mice were anesthetized with pentobarbital sodium (40 mg/kg ip diluted in isotonic saline to 5 mg/ml) and placed on a Plexiglas board. Body temperature was maintained at 35–36°C with a heating pad throughout the experiment. The right cremaster muscle was exposed, opened by a longitudinal incision on its ventral surface, the testis and epididymis were excised after ligation of the supply vessels, and the cremaster was pinned out on a silicone rubber pedestal. The mouse was placed on the stage of an Olympus microscope (BX 50 WI, Gibraltar Platform), and the cremaster muscle was continuously superfused at 3 ml/min with bicarbonate-buffered saline solution [containing (in mM) 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, and 20.0 NaHCO3] kept at 35°C and equilibrated with 95% N2-5% CO2. The preparation was allowed to stabilize for 45–60 min before the experiment was begun. Throughout the experiment, supplemental doses of dilute pentobarbital sodium anesthetic in isotonic saline (10 mg/kg ip) were administrated as appropriate. At the end of the experiment, animals were euthanized by an anesthetic overdose.
Vessel diameters.
The cremaster muscle was transilluminated, and the microscope image was projected to a video camera (series 65, Dage-MTI) whose output was displayed on a monitor (model HR1000, Dage-MTI). Inner diameters of the arterioles were continuously measured using Diamtrak software (15, 47).
Arterioles of the second and third order were stimulated focally with a pressure-pulse ejection from a micropipette (inner diameter: 3–4 μm) of either the KATP channel opener pinacidil (100 μM) or ACh (10 μM). The duration of the stimulation period was set to induce a local vasodilation of ∼50% (pinacidil: 500–700 ms and ACh: 300 ms). In one group of experiments, the length of the pulse of ACh was extended to 700 ms to elicit maximal vasodilation at the stimulation site. Changes in diameter were measured first at the stimulation site (local) and then at locations 500, 1,000, and 2,000 μm upstream in four separate stimuli. To allow the recovery of the vasodilator response and the stabilization of the control diameter, the time interval between stimulations was set at 3 min for ACh and 5 min for pinacidil. Maximal diameter was estimated during superfusion of 1 mM adenosine. Variations in diameter were expressed as percentages of the maximal dilation possible (%maximum), which was calculated using the following equation: (Dst − Dcont)/(Dmax − Dcont) × 100, where Dst is the diameter after the stimulation, Dcont is the diameter before stimulation (control diameter), and Dmax is the maximal diameter.
Blood pressure measurements.
Tail-cuff blood pressure measurements were performed in a dark environment and determined in a “blinded” fashion using a computerized tail-cuff system (Visitech Systems, Cary, NC). Animals were conditioned to the measurement protocol for at least 7 days, and typically three replicate blood pressure measurement sets were averaged over the course of several days.
Immunofluorescence.
The vasculature of anesthetized mice was perfused through the left ventricle with warmed MOPS-buffered physiological salt solution (PSS) containing 1% FCS, 10 U/μl heparin, 10 μmol/l ACh, and 10 μmol/l sodium nitroprusside and fixed by perfusion of 2% paraformaldehyde in MOPS-buffered PSS. Cremaster muscles were removed and postfixed overnight. Tissues were dehydrated, embedded in paraffin, sectioned (5 μm), placed on charge-coated slides, and deparaffinized using standard procedures. Antigen retrieval was carried out by microwaving the slides in a citrate buffer. Sections were blocked with 0.5% BSA in PBS and incubated overnight at 4°C with rabbit primary antibody directed against Cx37 (ADI, TX) or Cx40 (ADI, TX) and then with Alexa 568-labeled goat anti-rabbit secondary antibody (Molecular Probes, OR) for 1 h at room temperature. The fluorescence signal was examined using an Olympus Fluoview confocal microscope.
Chemicals.
Adenosine, ACh, pinacidil, and chemicals of analytical grade were purchased from Sigma Chemical (St. Louis, MO). Pinacidil was prepared fresh daily in DMSO and further diluted in the superfusion solution to the final working concentration (DMSO <0.1%). The vehicle of pinacidil did not affect the vasomotor tone of the arterioles (data not shown).
Statistical analysis.
Results are presented as means ± SE. Comparisons between groups were made using one-way ANOVA plus a Newman-Keuls post hoc test. P < 0.05 was considered significant.
RESULTS
The maximum diameter of the mouse cremaster arterioles studied ranged from 19 to 58 μm. Although the vessels selected were of the same branching order in the three groups of animals, the maximum diameter of the arterioles from Cx40−/− mice was smaller than that of wild-type animals but not significantly different from that of Cx37−/− mice (Table 1). However, the mean resting diameter and degree of resting tone were similar in wild-type, Cx37−/−, and Cx40−/− mice (Table 1).
Table 1.
Characteristics of the mouse cremaster arterioles studied
| n | Maximum Diameter, μm | Resting Diameter, μm | Resting Tone, % | |
|---|---|---|---|---|
| Wild-type mice | 23 | 38.8±1.6 | 21.0±1.6 | 46.8±2.6 |
| Cx40 knockout mice | 19 | 30.4±1.4* | 16.8±0.8 | 44.7±1.6 |
| Cx37 knockout mice | 12 | 35.4±2.5 | 16.2±1.7 | 55.3±1.9 |
Values are means ± SE; n, no. of mice. Cx40, connexin40; Cx37, connexin37.
P < 0.05 vs. wild-type mice by one-way ANOVA plus the Newman-Keuls post hoc test.
Vasodilation induced by direct K+ channel activation.
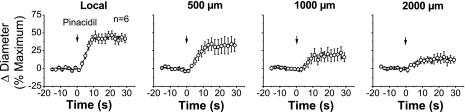
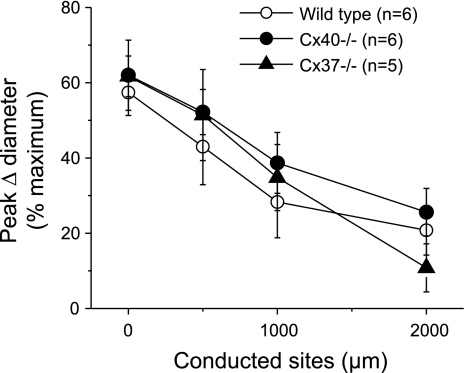
In wild-type animals, activation of KATP channels by a pulse of pinacidil evoked a slowly developing vasodilation that attained a maximum at ∼10 s after stimulation (Fig. 1) and returned to baseline gradually within 30–40 s (data not shown). The pinacidil-induced vasodilation was conducted along the vessel but decayed rapidly with a mechanical length constant (47) of 1.5 ± 0.4 mm (Fig. 1). Deletion of Cx40 or Cx37 did not affect the time course of the response to pinacidil (data not shown), and the mechanical length constants observed in arterioles from either Cx40−/− (2.0 ± 0.4 mm) or Cx37−/− (1.3 ± 0.6 mm) mice were similar to those of control animals (Fig. 2).
Fig. 1.
Time course of local and conducted vasodilation induced by pinacidil in wild-type mice. A short segment of the cremasteric arteriole was stimulated with a pressure-pulse ejection via micropipette of the ATP-sensitive K+ (KATP) channel opener pinacidil, and the resultant vasodilator responses were observed at the stimulation pipette site (local) and at locations 500, 1,000, and 2,000 μm upstream. Note that the changes in diameter decayed rapidly along the vessel length. Arrows indicate the time at which the stimulus was applied.
Fig. 2.
Analysis of the maximal response induced by pinacidil in wild-type (WT), connexin40 (Cx40) knockout (Cx40−/−), and connexin37 (Cx37) knockout (Cx37−/−) mice. A short segment of the arteriole was stimulated with a pulse of the KATP opener pinacidil ejected by pressure from a micropipette. The maximal vasodilator response induced by pinacidil was evaluated at the stimulation pipette site (local) and at locations 500, 1,000, and 2,000 μm upstream.
Vasodilation induced by ACh.
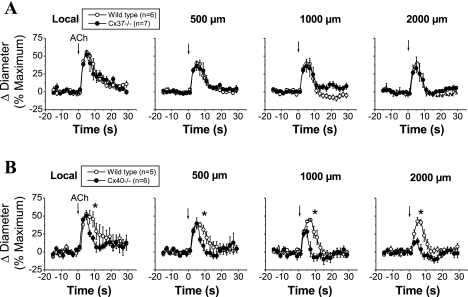
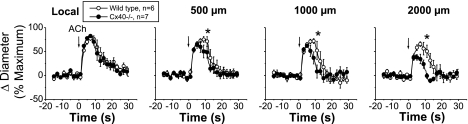
Stimulation with a pulse of ACh (300 ms) induced a very fast and transient vasodilator response that peaked at ∼6 s and rapidly returned to control diameter within 5–15 s (Fig. 3). The response to ACh was reduced from the local site to the 500-μm conducted site, but from this conducted site, the vasodilation was propagated along the entire arteriole without decay (Fig. 3), suggesting the activation of an endothelium-dependent regenerative vasodilator mechanism.
Fig. 3.
Time course of the local and conducted vasodilation induced by ACh in WT, Cx37−/−, and Cx40−/− mice. ACh was ejected by a pressure pulse (300 ms) via a micropipette to stimulate a short segment of the cremasteric arteriole, and the vasodilator response was analyzed at the stimulation site (local) and at locations at 500, 1,000, and 2,000 μm upstream. The vasodilator responses initiated by ACh in two different groups of experiments performed in WT animals were compared with the vasodilation observed in arterioles from Cx37−/− (A) and Cx40−/− (B) animals. Arrows indicate the time at which the stimulus was applied. *P < 0.05 vs. WT mice by one-way ANOVA plus the Newman-Keuls post hoc test.
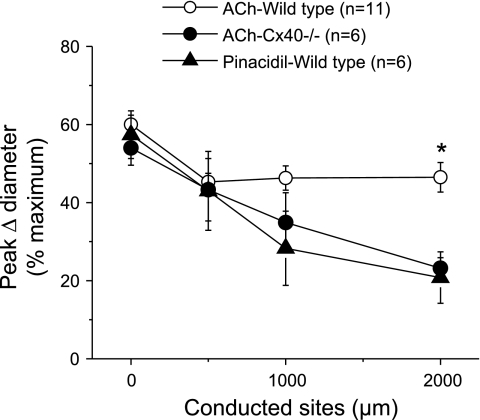
As observed with pinacidil stimulation, deletion of Cx37 did not affect the ACh-induced local or conducted vasodilator response (Fig. 3A). In contrast, endothelial cell communication via Cx40 seems to be critical for the regenerative propagation of the vasodilation. The conducted vasodilator response activated by ACh decreased rapidly with distance in Cx40−/− mice (Fig. 3B), and the mechanical length constant of the response to ACh in these animals (2.1 ± 0.3 mm) was similar to that observed with pinacidil stimulation in wild-type mice (Fig. 4). In addition, although the magnitude and time of the peak of the ACh-induced vasodilation were similar in Cx40−/− and control mice, the arteriolar diameter returned to baseline faster in Cx40−/− animals (Fig. 3B).
Fig. 4.
Comparison of the peak response induced by pinacidil with that initiated by ACh in WT and Cx40−/− mice. Maximal vasodilator responses of the data shown in Figs. 1 and 3 were compared. For this analysis, all the ACh-induced vasodilator responses shown in Fig. 3, A and B, were pooled together. *P < 0.05 vs. ACh-evoked vasodilation in WT mice by one-way ANOVA plus the Newman-Keuls post hoc test.
The pulse stimulation with ACh was set to induce an ∼50% vasodilation, and the deletion of Cx40 may have affected the threshold for triggering the regenerative propagation of the vasodilator response. To evaluate this possibility, we extended the pressure-pulse ejection of ACh from 300 to 700 ms to elicit a larger dilation. The increase in the intensity of the stimulation resulted in near-maximal vasodilation at the ACh application site (Fig. 5). Although the increase in diameter at the local site was similar in Cx40−/− and control mice, after deletion of Cx40, the response gradually became shorter and decayed along the vessel length as observed with the submaximal ACh stimulation (∼50% of maximum; Fig. 5).
Fig. 5.
Time course of the conduction of maximal vasodilation induced by ACh in WT and Cx40−/− mice. ACh was applied as described in Fig. 3, but the duration of the pressure pulse was increased to 700 ms to stimulate maximal vasodilation in the segment of the arteriole just underneath the micropipette. The vasodilator response was observed at the stimulation site (local) and at locations at 500, 1,000, and 2,000 μm upstream. Arrows indicate the time at which the stimulus was applied. *P < 0.05 vs. WT mice by one-way ANOVA plus the Newman-Keuls post hoc test.
Systolic blood pressure.
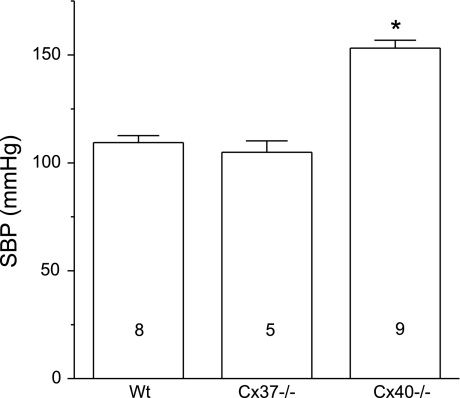
Wild-type and Cx37−/− mice were normotensive (Fig. 6), showing a blood pressure comparable with that described for C57 Bl/6 mice (28). In contrast, Cx40−/− animals exhibited marked hypertension (Fig. 6), which is consistent with pervious reports (7, 8, 25, 44) and supports the idea that Cx40-mediated coordination of vascular function contributes to the control of peripheral vascular resistance.
Fig. 6.
Systolic blood pressure (SBP) of WT, Cx37−/−, and Cx40−/− mice. Deletion of Cx37 did not alter the arterial blood pressure, but elimination of Cx40 resulted in marked hypertension. Numbers inside the bars indicate n values. *P < 0.05 vs. WT or Cx37−/− mice by one-way ANOVA plus the Newman-Keuls post hoc test.
Immunocytochemical analysis.
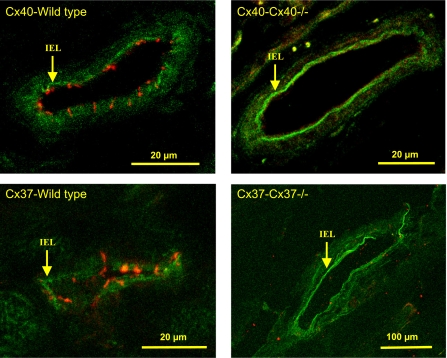
The cellular distribution of Cx37 and Cx40 in cremaster muscle arterioles was assessed by immunofluorescence. The signal for both gap junctional proteins Cx37 and Cx40 was apparently restricted to the endothelium, with no evident, positive label of arteriolar smooth muscle cells. As control, we also analyzed arterioles from Cx37−/− and Cx40−/− mice, which confirmed the deletion of Cx37 or Cx40 and the specificity of the immunocytochemical analysis (Fig. 7).
Fig. 7.
Cellular distribution of Cx37 and Cx40 in the arteriolar wall. Cx40 (top) and Cx37 (bottom) were detected in endothelial cells of the arterioles of WT mice (left). There was no immunoreactivity for Cx40 or Cx37 in arterioles of Cx40−/− and Cx37−/− animals, respectively (right). The fluorescent signal was not evident in smooth muscle cells of WT or knockout mice. Arrows indicate the internal elastic lamina (IEL).
DISCUSSION
The endothelium provides a preferential intercellular pathway for the conduction of vasodilator signals (3, 7, 11, 12, 15), and gap junctions are thought to be essential for the functional integration of endothelial cells. Cx37 and Cx40 are the main gap junction proteins present in the endothelium (7, 13–16, 38, 42), and we used two strains of knockout mice to study the participation of these proteins in the conduction of vasodilator responses induced by the KATP channel opener pinacidil and the endothelium-dependent vasodilator ACh. Our results indicate that neither Cx37 nor Cx40 plays a determining role in the conduction of the vasodilation evoked by opening of KATP channels. However, Cx40, but not Cx37, is essential for the efficient, nondecremental propagation of the vasodilator response activated by ACh.
Activation of KATP channels.
The vasodilation induced by openers of KATP channels, such as pinacidil, is associated with direct hyperpolarization of the vessel wall (2, 32, 40, 43), and the conduction of the resulting vasomotor responses is thought to rely on the passive, electrotonic spread of changes in membrane potential through gap junctions connecting cells along the length of the vessel wall (17, 34). Consistent with this idea, the local vasodilation evoked by pinacidil was conducted decrementally along the longitudinal axis of the stimulated arteriole (Fig. 1) with a mechanical length constant quite consistent with the electrical length constant determined by current injection in arterioles in vitro or in vivo (0.9–1.6 mm) (10, 18, 19).
Although KATP channels are known to be in both smooth muscle and endothelial cells (2, 4, 23), the hyperpolarization-mediated vasodilation elicited by the opening of KATP channels has been shown to spread through the endothelium of rat mesenteric arteries (40), and, in the mouse cremaster microcirculation, Cx37 and Cx40 appear to be localized exclusively in endothelial cells (Fig. 7). However, deletion of either Cx37 or Cx40 did not affect the local or conducted vasodilator response induced by KATP channel activation with pinacidil (Fig. 2). This finding may indicate that these Cxs are redundantly expressed and can compensate for each other, but the involvement of Cx43 in this process cannot be ruled out. Further experiments using knockout mice in combination with Cx mimetic peptides to study electrotonic potentials produced by current injection may help us to elucidate this problem.
Conducted vasodilation activated by ACh.
In contrast to the response induced by pinacidil, ACh stimulation activated a very fast vasodilation that dropped off only from the local site to the conducted site located 500 μm upstream, but it is noteworthy that from the 500-μm site, the response was propagated along the entire arteriole without apparent decay (Fig. 3), indicating that the response observed at the local site is a combination of two components: one that was restricted to the stimulation site and another that was conducted. The conducted vasodilation must then be assessed excluding the stimulation site, since the difference between the responses observed at the local and conducted sites may be misinterpreted as decay.
The conducted vasodilator response triggered by ACh has been associated with the electrotonic spread of the hyperpolarization initiated by the release of an endothelium-derived hyperpolarizing factor at the stimulation site (17, 20, 45). However, the response initiated by ACh was propagated over distances much longer than those predicted by the electrotonic model (Figs. 3 and 4), which suggests that ACh activated a regenerative vasodilator mechanism. Consistent with this idea, computer simulations have led to the proposal that activation of voltage-dependent conductances may be involved in the conduction of the responses initiated by ACh (5), and, interestingly, focal electrical stimulation of mouse cremaster arterioles activates a very rapid conducted vasodilation of similar characteristics of those induced by ACh (12, 15).
Direct measurements of membrane potential also support the idea that ACh activates a regenerative mechanism of propagation of vasodilator signals. In feed arteries of the hamster retractor muscle, analysis of the conduction of changes in membrane potential along the vessel has shown that the electrical length constant of ACh-induced hyperpolarization is longer than that for current injection (10). In addition, recently, Crane et al. reported that the hyperpolarizing current initiated by ACh in hamster cheek pouch arterioles in vivo can even increase during the first 1,000 μm of longitudinal conduction, which has led to the proposal that the regenerative mechanism may involve the opening of inward rectifier K+ (Kir) channels by hyperpolarization and by an elevation in extracellular K+ concentration (6, 24). However, conduction of the changes in diameter evoked by pinacidil argues against this hypothesis, since the hyperpolarization and the increase in extracellular K+ concentration produced by the activation of KATP channels would have been expected to have a similar effect on Kir channels and result in extended dilation. However, pinacidil-evoked conducted vasodilation decayed just as expected for the electrotonic spread of a hyperpolarizing current (Figs. 1 and 2). In addition, activation of Kir channels should also be expected during the hyperpolarization induced by current injection, as predicted by computational modeling of cell-to-cell communication (24). Taken together, these results suggest that the hyperpolarization of the vessel wall contributes to the electrotonic conduction of vasodilation but not to the propagation of the nondecremental component of the vasomotor signal activated by ACh. Probably, the ACh-induced hyperpolarization is enhanced along the vessel length by the activation of a regenerative vasodilator mechanism as has been recently described for the electrically induced conducted vasodilation (12).
The response to ACh might be conducted by either the endothelium or smooth muscle in arterioles (1, 3). However, the regenerative propagation of the vasodilation induced by ACh depended on the expression of Cx40 (Figs. 3 and 4), and, in cremasteric arterioles, Cx40 is only expressed in the endothelium (7, 15), which we confirmed in the present work (Fig. 7). Interestingly, deletion of Cx40 converted the nondecremental response activated by ACh into a rapidly decaying vasodilator signal (Figs. 3 and 4). In addition, the longitudinal reduction of the vasodilation elicited by ACh in Cx40−/− mice was similar to that observed with pinacidil stimulation (Fig. 4). Therefore, these results suggest that ablation of Cx40 selectively disrupted the regenerative propagation of a vasodilator signal via endothelial cells but without affecting the electrotonic component of the conducted response triggered by ACh, which may be mediated by others Cxs expressed either in the endothelium or smooth muscle. It is important to note, however, that Cx40 has been detected at myoendothelial junctions (22, 31), which raises the possibility that the gap junction communication between smooth muscle and endothelial cells may be involved in the regenerative mechanism. Although deletion of Cx40 may also affect the expression of Cx43 or Cx37 (21, 39), these Cxs probably are not involved in the regenerative vasodilator mechanism because the absence of Cx37 did not alter the conducted response induced by ACh (Fig. 3) and, in mouse cremaster muscle, Cx43 has been detected only in endothelial cells of large arterioles (30).
Deletion of Cx40 might have changed the axial resistance of the endothelial layer and, thus, the minimum threshold potential for triggering the activation of the regenerative vasodilator mechanism along the vessel length. To test this possibility, we stimulated arterioles with a longer pulse of ACh (700 ms) to evoke a larger response. Although the stronger ACh stimulation produced near-maximal vasodilation at the local site (Fig. 5), this response decayed along the vessel length in Cx40−/− animals exhibiting the same characteristics as those observed with ∼50% of maximal dilation induced by shorter pulses (300 ms) of ACh (compare Figs. 3 and 5), which supports the idea that Cx40 is an integral part of the regenerative vasodilator mechanism.
Cx37 and Cx40 play an important role in the development and function of the vascular system (26, 38, 39). However, only Cx40 seems to be involved in the coordination of vasomotor tone in the microcirculation and control of arterial blood pressure (Fig. 6). Although deletion of Cx40 has been associated with an increased plasma renin concentration, the hypertension observed in Cx40−/− mice appears to be mostly independent of angiotensin II (8, 44). These results support the notion that Cx40 is essential for the well-integrated regulation of peripheral vascular resistance.
In arterioles of the hamster cheek pouch, Budel et al. showed that the conducted vasodilation initiated by ACh is coupled to nitric oxide (NO) production (3), and, most recently, in mouse cremaster arterioles, this response was also observed to be followed by a slow, rapidly decaying vasodilator component that was associated with the spread of a Ca2+ wave (41). Although the Ca2+ wave-dependent vasodilation seemed to prolong the response to ACh observed at short distance from the stimulation site (300–400 μm), it was not involved in the vasodilator response propagated beyond 500 μm from the local site (41). It is important to note that the vasodilation induced by NO is not conducted along the length of arterioles (9, 20), and the hyperpolarization of endothelial cells is not coupled to an increase in intracellular Ca2+ in intact vessels (32, 40), as was proposed by Budel et al. (3) to explain the activation of endothelial NO synthase (eNOS) at the conducted sites. In addition, as mentioned above, focal stimulation with depolarizing pulses of current activates a nondecremental conducted vasodilator response of similar characteristics to those initiated by ACh (15). Interestingly, the conducted response induced by electrical stimulation of mouse cremaster arterioles is also coupled to NO production and is associated to the activation of voltage-dependent Na+ and Ca2+ channels in endothelial cells (12). Therefore, these data are consistent with the activation of a voltage-dependent regenerative vasodilator signal by ACh that is, in turn, coupled to eNOS activation and endothelial cell hyperpolarization.
In summary, direct hyperpolarization of the arteriolar wall by the opening of KATP channels induces a vasodilation that decays along the vessel length in a manner consistent with the electrotonic spread of an electrical signal. Conduction of this vasodilator response does not depend on the presence of Cx37 or Cx40. In contrast, the vasodilation induced by ACh is propagated along the entire vessel without an apparent reduction in magnitude or duration of the response, strongly suggesting that ACh receptors are coupled to the activation of a regenerative vasodilator mechanism. The regenerative component of the response initiated by ACh was selectively blocked by deletion of Cx40 but not Cx37, which indicates that Cx40 gap junctions play an essential role in the function of the regenerative vasodilator mechanism activated by ACh.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-53318 (to B. R. Duling), a grant from Vicerrectoría Adjunta de Investigación y Doctorado de la Pontificia Universidad Católica de Chile (to X. F. Figueroa), Fondo Nacional de Desarrollo Científico y Tecnológico Grant 11060289 (to X. F. Figueroa).
Acknowledgments
Cx40 and Cx37 knockout mice were generously provided by Alexander M. Simon and David L. Paul. We thank Kathleen H. Day for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol 278: H604–H612, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Brayden JE Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 29: 312–316, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res 93: 61–68, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Al Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285: C959–C967, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Crane GJ, Hines ML, Neild TO. Simulating the spread of membrane potential changes in arteriolar networks. Microcirculation 8: 33–43, 2001. [PubMed] [Google Scholar]
- 6.Crane GJ, Neild TO, Segal SS. Contribution of active membrane processes to conducted hyperpolarization in arterioles of hamster cheek pouch. Microcirculation 11: 425–433, 2004. [DOI] [PubMed] [Google Scholar]
- 7.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000. [DOI] [PubMed] [Google Scholar]
- 8.de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13: 169–177, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Doyle MP, Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol 272: H1364–H1371, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Emerson GG, Neild TO, Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol 283: H102–H109, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol 293: H1371–H1383, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda ) 19: 277–284, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa XF, Isakson BE, Duling BR. Vascular gap junctions in hypertension. Hypertension 48: 804–811, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res 92: 793–800, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res 83: 636–643, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand 167: 11–21, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Hirst GD, Edwards FR, Gould DJ, Sandow SL, Hill CE. Electrical properties of iridial arterioles of the rat. Am J Physiol Heart Circ Physiol 273: H2465–H2472, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol 280: 87–104, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoepfl B, Rodenwaldt B, Pohl U, de Wit C. EDHF, but not NO or prostaglandins, is critical to evoke a conducted dilation upon ACh in hamster arterioles. Am J Physiol Heart Circ Physiol 283: H996–H1004, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol 290: H1199–H1205, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97: 44–51, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Jackson WF Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol 291: H1319–H1328, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int 72: 814–822, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kruger O, Beny JL, Chabaud F, Traub O, Theis M, Brix K, Kirchhoff S, Willecke K. Altered dye diffusion and upregulation of connexin37 in mouse aortic endothelium deficient in connexin40. J Vasc Res 39: 160–172, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development 127: 4179–4193, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA 98: 9989–9994, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth-muscle and endothelium in-vivo. Am J Physiol Heart Circ Physiol 268: H729–H739, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol 97: 1152–1158, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97: 399–407, 2005. [DOI] [PubMed] [Google Scholar]
- 32.McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium 38: 23–33, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Neild TO, Crane GJ. Cellular coupling and conducted vasomotor responses. Clin Exp Pharmacol Physiol 29: 626–629, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Pacicca C, Schaad O, Beny JL. Electrotonic propagation of kinin-induced, endothelium-dependent hyperpolarizations in pig coronary smooth muscles. J Vasc Res 33: 380–385, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Simon AM, Goodenough DA. Diverse functions of vertebrate gap junctions. Trends Cell Biol 8: 477–483, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol 8: 295–298, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251: 206–220, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116: 2223–2236, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Takano H, Dora KA, Spitaler MM, Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol 556: 887–903, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. [DOI] [PubMed] [Google Scholar]
- 42.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol 112: 479–486, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Videbaek LM, Aalkjaer C, Hughes AD, Mulvany MJ. Effect of pinacidil on ion permeability in resting and contracted resistance vessels. Am J Physiol Heart Circ Physiol 259: H14–H22, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Welsh DG, Segal SS. Role of EDHF in conduction of vasodilation along hamster cheek pouch arterioles in vivo. Am J Physiol Heart Circ Physiol 278: H1832–H1839, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res 101: 1292–1299, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Xia J, Duling BR. Electromechanical coupling and the conducted vasomotor response. Am J Physiol Heart Circ Physiol 269: H2022–H2030, 1995. [DOI] [PubMed] [Google Scholar]