Abstract
This study tested whether leptin restores sympathetic-vagal balance, heart rate (HR) variability, and cardiac baroreflex sensitivity (BRS) in streptozotocin (STZ)-induced diabetes. Sprague-Dawley rats were instrumented with arterial and venous catheters, and a cannula was placed in the lateral ventricle for intracerebroventricular (ICV) leptin infusion. Blood pressure (BP) and HR were monitored by telemetry. BRS and HR variability were estimated by linear regression between HR and BP responses to phenylephrine or sodium nitroprusside and autoregressive spectral analysis. Measurements were made during control period, 7 days after induction of diabetes, and 7 days after ICV leptin infusion. STZ diabetes was associated with hyperglycemia (422 ± 17 mg/dl) and bradycardia (−79 ± 4 beats/min). Leptin decreased glucose levels (165 ± 16 mg/dl) and raised HR to control values (303 ± 10 to 389 ± 10 beats/min). Intrinsic HR (IHR) and chronotropic responses to a full-blocking dose of propranolol and atropine were reduced during diabetes (260 ± 7 vs. 316 ± 6, −19 ± 2 vs. −43 ± 6, and 39 ± 3 vs. 68 ± 8 beats/min), and leptin treatment restored these variables to normal (300 ± 7, −68 ± 10, and 71 ± 8 beats/min). Leptin normalized BRS (bradycardia, −2.6 ± 0.3, −1.7 ± 0.2, and −3.0 ± 0.5; and tachycardia, −3.2 ± 0.4, −1.9 ± 0.3, and −3.4 ± 0.3 beats·min−1·mmHg−1 for control, diabetes, and leptin) and HR variability (23 ± 4 to 11 ± 1.5 ms2). Chronic glucose infusion to maintain hyperglycemia during leptin infusion did not alter the effect of leptin on IHR but abolished the improved BRS. These results show rapid impairment of autonomic nervous system control of HR after the induction of diabetes and that central nervous system actions of leptin can abolish the hyperglycemia as well as the altered IHR and BRS in STZ-induced diabetes.
Keywords: diabetes mellitus, autonomic nervous system, heart rate variability, streptozotocin
diabetes is associated with metabolic and cardiovascular complications that lead to increased risk for cardiovascular and kidney disease (4). One of the many complications observed in diabetes that increases cardiovascular risk is impaired autonomic function (12, 24, 25). The autonomic dysfunction affects the modulation of the sinus-atrial node (18, 22) and causes changes in heart rate (HR) variability (14, 21, 26). Diabetes is also associated with impairment of baroreflex sensitivity (9, 24), which reduces the buffering of beat-to-beat alterations in arterial pressure (18, 32) and may contribute to the development of target organ injury (12). In experimental models of insulin deficiency, functional alterations in the control of autonomic nervous system activity, HR, and baroreceptor sensitivity have been demonstrated to occur as early as 5 to 7 days postinduction of diabetes (24), whereas structural changes in tissues and organs develop after longer periods of diabetes (14).
Previous studies from our laboratory and others have demonstrated that leptin, a hormone produced by fat cells that regulates food intake and thermogenesis (18, 35), markedly improves glucose utilization (29, 38, 41). Although leptin has been shown to enhance insulin sensitivity in humans (30) and rodents (39), a major part of the effects of leptin on glucose homeostasis is independent of insulin. Chronic leptin infusion, for example, completely restores euglycemia in insulin-deficient diabetic rats (2, 6, 15), and this effect appears to be mediated mainly by the direct actions of leptin in the central nervous system (CNS) (6, 15, 29).
In addition to its CNS actions on metabolism and appetite, leptin also plays a major role in the regulation of sympathetic nervous system (SNS) activity and cardiovascular function (1, 3, 10, 15, 38). In a recent study, we showed that chronic intracerebroventricular (ICV) or intravenous (IV) leptin infusion not only normalized blood glucose levels and reduced the hyperphagia in streptozotocin (STZ)-induced diabetes in rats, but it also reversed the bradycardia caused by the induction of diabetes (6).
The present study tested whether leptin ameliorates the cardiovascular functional changes associated with short-term diabetes including autonomic dysfunction and impaired baroreflex sensitivity in STZ-induced diabetes, a widely used model of diabetes. Since chronic adrenergic blockade prevented only part of the effects of leptin to increase HR back to prediabetic values, we also determined whether the remaining effect of leptin to raise HR was mediated by reduced cardiac parasympathetic nervous system (PNS) tone or by an increase in intrinsic HR (IHR), which has been shown to be reduced in this diabetic model (20, 24).
METHODS
Animals
Experiments were performed in male Sprague-Dawley rats (n = 20; Harlan, Indianapolis, IN) weighing between 340 and 360 g. The experimental protocol and procedures were submitted and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Surgical Protocols
Implantation of the telemetry transmitter, femoral arterial, and venous catheters and ICV cannula.
Rats were anesthetized with pentobarbital sodium (50 mg/kg), and atropine sulfate (0.1 mg/kg) was used to prevent excess airway secretion. With the use of aseptic techniques, a laparotomy was performed, and the catheter of the pressure telemetry transmitter (Model TA11PAC40; Data Sciences International) was inserted into the abdominal aorta, distal to the kidneys, for continuous 24-h/day blood pressure (BP) and HR measurements. The catheter was fixed in the aorta with a small drop of cyanoacrylate adhesive, and the transmitter was secured to the abdominal wall by sutures. Arterial and venous catheters were also inserted into the left femoral artery and vein for blood sampling and IV infusion. The catheters were exteriorized in the scapular region through a subcutaneously implanted stainless steel button.
After implantation of the telemetry transmitter and arterial and venous catheters, a stainless steel cannula (21 gauge; 10-mm long) was placed into the right lateral cerebral ventricle using coordinates previously described (6). The guide cannula was anchored into place with two stainless steel machine screws, a metal cap, and dental acrylic, and a stylet was inserted to seal the cannula until use. During stereotaxic manipulation, anesthesia was maintained with 0.5% isoflurane. Eight days after recovery from surgery, accuracy of the cannula placement was tested by dipsogenic response (immediate drinking of at least 5 ml of water in 10 min) to an ICV injection of 100 ng of angiotensin II.
Hemodynamic measurements.
After recovery from anesthesia, rats were housed in metabolic cages for determination of daily food and water and urine output. The arterial and venous catheters were connected to a dual-channel infusion swivel (Instech). The rats received food intake and water ad libitum during the study. The venous catheter was connected to a syringe pump for continuous IV infusion of saline (0.9%; 21 ml/day) immediately after the placement of the rats into the metabolic cages. Total sodium intake was maintained constant at ∼3.1 meq/day via the continuous saline infusion combined with sodium-deficient rat chow (0.006 nmol/g food; Harlan Teklad). The rats were allowed to recover for 8 to 10 days before control measurements were recorded. BP and HR data were analyzed using Dataquest ART software (Data Sciences International). A small amount of blood (25 μl) collected from the arterial catheter was used to determine blood glucose levels using glucose strips (Reli On Ultima).
Experimental Protocols
After 5 days of stable control measurements, diabetes was induced by a single IV injection of STZ (50 mg/kg; Sigma). Seven days after STZ injection, rats (n = 15) received a continuous ICV infusion of leptin (0.021 μg·kg−1·min−1) for 7 days via osmotic minipump (0.5 μl/h; Alzet). The minipump was implanted subcutaneously between the scapulae under isoflurane anesthesia and connected to the ICV cannula via tygon tubing. Our laboratory has previously shown that this rate of ICV leptin infusion has no significant effect on plasma leptin levels, indicating that leptin infused into the cerebral ventricles did not spill over into the circulation (6).
In an additional group of rats (n = 5), the IV saline infusion was replaced by a solution containing 35 ml of 50% glucose solution and 10 ml of 0.9% saline (infusion rate of 42 ml/day) to maintain blood glucose levels above 350 mg/dl. The replacement of the 0.9% saline to glucose solution occurred at the beginning of the leptin treatment period and continued until the animals were euthanized.
Cardiac Sympathetic-Vagal Balance
Cardiac SNS and PNS tone under resting conditions were estimated by the chronotropic effects of full-blocking doses of propranolol (4 mg/kg), a β-adrenergic receptor antagonist, and atropine (2 mg/kg), a muscarinic receptor blocker at a volume per injection of 0.2 ml or less. Propranolol and atropine injections were performed at the end of the control period, 7 days after STZ injection, and on day 7 of leptin infusion. After resting BP and HR were recorded, propranolol was injected first, and 15 min later, atropine was injected. The IHR was evaluated after simultaneous blockade with propranolol and atropine.
Baroreflex Analysis
After 10–15 min of baseline BP recording, the animals received a single IV bolus (0.2 ml) of phenylephrine (16 μg/kg) or sodium nitroprusside (32 μg/kg). An appropriate interval between the drugs (10–20 min) was allowed for BP and HR to return to baseline levels. The maximal response of mean arterial pressure (MAP) to phenylephrine or sodium nitroprusside and the corresponding maximal reflex change in HR were plotted for each drug. The slope of the linear regression calculated based on the reflex change in HR to a given change in MAP was used as the index of baroreflex sensitivity.
HR Variability Determined by Spectral Analysis
Pulsatile BP recordings were analyzed using customized software designed to detect inflection points of periodic waves. Beat-by-beat time series of systolic BP were generated, and series of pulse interval (PI) were obtained by measuring the intervals between consecutive systolic BP values. PI values were considered as HR and used to calculate HR variability. Series of BP and PI were visually inspected to exclude nonstationary segments. Stationary segments of time series had their means and variances calculated and were submitted to autoregressive spectral analysis. Autoregressive spectral analysis was performed with parameters estimated by Levinson-Durbin recursion, with order chosen according to Akaike's criterion (25). The power of each relevant oscillatory component in absolute units was quantified in low frequency (LF; 0.25–0.75 Hz) and high frequency (HF; 0.75–3.0 Hz). The LF component reflects both sympathetic and parasympathetic activities to the heart, whereas the HF component is accepted as a marker of parasympathetic modulation to the heart (23).
Statistical Analysis
Data are reported as means ± SE. Baseline values of MAP, HR, and glucose were compared by one-way ANOVA for repeated measurements followed by Tukey's post hoc test. Variance, LF, HF power of systolic pressure, and PI were compared using variance on ranks followed by Mann-Whitney's post hoc test. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Effects of Diabetes and Chronic ICV Leptin Infusion on Food Intake, Glucose Levels, BP, and HR
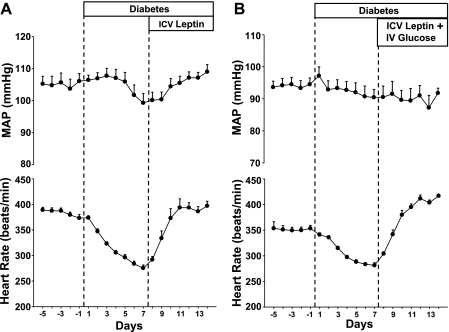
Induction of insulin-deficient diabetes by STZ injection caused hyperphagia (19 ± 1 vs. 42 ± 2 g/day average for the last day of control and 7 days after the induction of diabetes, respectively) and marked hyperglycemia (92 ± 7 vs. 422 ± 17 mg/dl; Fig. 1), which were associated with increased water intake (from 7 ± 1 to 232 ± 30 ml/day) and urine production (from 22 ± 6 to 268 ± 30 ml/day). STZ-induced diabetes was also associated with pronounced bradycardia (from 372 ± 7 to 275 ± 6 beats/min; Fig. 2A). MAP gradually decreased reaching significance (P < 0.05) on day 7 after the induction of diabetes (Fig. 2A).
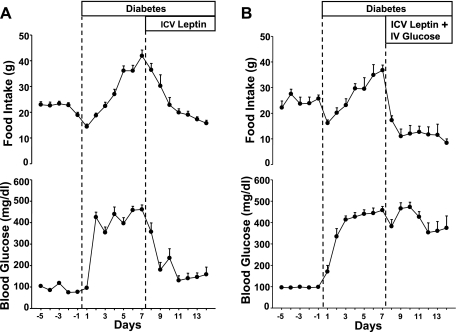
Fig. 1.
Responses to chronic intracerebroventricular (ICV) leptin infusion (0.021 μg·kg−1·min−1; A) or chronic ICV leptin infusion and intravenous (IV) glucose infusion (B) on food intake and glucose levels in streptozotocin (STZ)-diabetic rats. Data are expressed as means ± SE (n = 12).
Fig. 2.
Responses to chronic ICV leptin infusion (0.021 μg·kg−1·min−1; A) or chronic ICV leptin infusion and IV glucose infusion (B) on mean arterial pressure (MAP) and heart rate, measured 24 h/day, in STZ-diabetic rats. Data are expressed as means ± SE (n = 12).
Chronic ICV leptin infusion for 7 days reduced food intake to control values (18 ± 1 g/day, average of the last 4 days of leptin infusion; P < 0.05 compared with the day 7 postinduction of diabetes) and completely reversed the hyperglycemia (131 ± 20 mg/dl; P < 0.001; Fig. 1A). The reduced food intake and normalization of glycemic levels during leptin treatment were also associated with the marked reduction in water intake (19 ± 3 ml/day; P < 0.001) and urine production (57 ± 4 ml/day; P < 0.001). Chronic leptin ICV infusion also fully reversed bradycardia caused by the induction of diabetes, and HR quickly rose back to prediabetic values (396 ± 8 beats/min; P < 0.05; Fig. 2A). Leptin infusion also returned MAP to control values (Fig. 2A).
In the group where blood glucose were kept at hyperglycemic levels by continuous IV glucose infusion (Fig. 1B), chronic ICV leptin infusion reduced food intake from 37 ± 2 to 8 ± 1.5 g/day (P < 0.001; Fig. 1B) and completely reversed the bradycardia caused by the induction of diabetes (Fig. 2B). This indicates that the effects of leptin to raise HR in diabetic rats were independent of the antidiabetic actions of leptin. No major changes in MAP were observed in this group (Fig. 2B).
Effects of Diabetes and Chronic ICV Leptin Infusion on Cardiac Sympathetic and Parasympathetic Tone and IHR
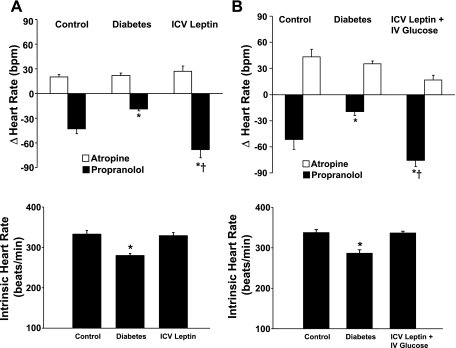
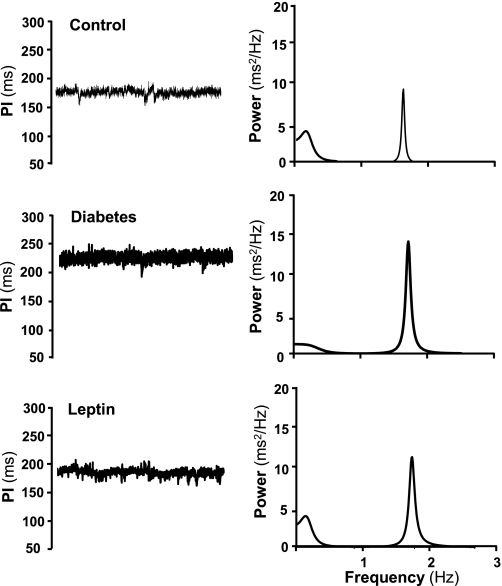
Diabetes caused a rapid impairment of cardiac sympathetic tone evaluated 7 days after STZ injection by the HR responses to β-adrenergic blockade (Fig. 3A) and by spectral analysis, which demonstrated a significant attenuation of the LF component of the spectra (Table 1 and Fig. 4). No change was observed in the HR response to atropine injection, suggesting that cardiac parasympathetic tone was not altered 7 days after the induction of diabetes (Fig. 3A). The spectral analysis method, however, indicated increased parasympathetic tone to the heart (evidenced by increased HF component of the spectra) after the onset of diabetes (Table 1 and Fig. 4). Chronic ICV leptin treatment raised cardiac sympathetic tone to levels above control while not significantly altering parasympathetic tone to the heart (Fig. 3A). Leptin treatment also increased the LF/HF ratio to prediabetic values, which were markedly reduced 7 days after the induction of diabetes (Table 1).
Fig. 3.
Cardiac sympathetic and parasympathetic tone and intrinsic heart rate evaluated on day 4 of control, day 7 after induction of diabetes, and on the last day of chronic ICV leptin infusion (0.021 μg·kg−1·min−1; A) or on the last day of chronic ICV leptin infusion and IV glucose infusion (B). Data are expressed as means ± SE (n = 8). *P < 0.05 vs. control; †P < 0.05 vs. diabetes. bpm, Beats per minute.
Table 1.
Spectral analysis data of systolic arterial pressure and pulse interval during control, diabetes, and after chronic ICV leptin infusion
| Variables | Control | Diabetes | Leptin |
|---|---|---|---|
| Systolic arterial pressure | |||
| Mean, mmHg | 127±2.0 | 121±2.0 | 134±3.0 |
| Variance, mmHg2 | 11±1.0 | 8.0±1.0 | 13±1.0 |
| LF, mmHg2 | 6.5±0.4 | 3.9±0.7 | 7.7±0.7 |
| HF, mmHg2 | 2.6±0.2 | 1.5±0.4 | 3.0±0.2 |
| Pulse interval | |||
| Mean, ms | 167±4.0 | 207±11*† | 140±3.5 |
| Variance, ms2 | 12±1.5 | 23±4.0*† | 11±1.5 |
| LF, ms2 | 3.0±1.5 | 1.7±0.4*† | 3.5±0.5 |
| HF, ms2 | 5.0±1.5 | 16.5±3.5*† | 5.0±0.5 |
| LF/HF | 0.7±0.1 | 0.11±0.02*† | 0.9±0.1 |
Data are means ± SE; n = 8/group. Values were determined on day 4 of control, day 7 after induction of diabetes, and at the last day of chronic intracerebroventricular (ICV) leptin infusion (0.021 μg·kg−1·min−1). LF and HF, low and high frequency power components of the spectral analysis, respectively.
P < 0.05 vs. control;
P < 0.05 vs. leptin.
Fig. 4.
Representative time series of systolic arterial pressure (in mmHg) and pulse interval (PI; in ms) determined at day 4 of control, day 7 after induction of diabetes, and at the last day of chronic ICV leptin infusion (0.021 μg·kg−1·min−1).
Diabetes was also associated with an average 50 beats/min decrease in the IHR (P < 0.05) measured after sympathetic and parasympathetic inputs to the heart were removed by propranolol and atropine injections (Fig. 3A). This reduction in IHR was completely reversed by leptin ICV infusion (Fig. 3A).
The CNS effects of leptin to restore cardiac sympathetic tone and IHR to control values were mainly independent of the ability of leptin to normalize glycemic levels, since IV glucose infusion, to keep circulating glucose values close to diabetic levels, did not significantly alter the effects of leptin to raise sympathetic tone and IHR to values similar to those observed in rats receiving IV saline infusion (Fig. 3, A and B).
Effects of Diabetes and Chronic ICV Leptin Infusion on HR Variability and Baroreceptor Sensitivity
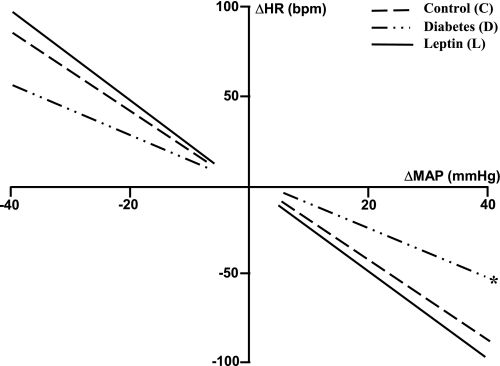
The spectral analysis also showed that diabetes was associated with increased PI, an indication of the bradycardia, and increased HR variability (Table 1). Diabetes also reduced baroreflex sensitivity, affecting the tachycardic and bradycardic reflex responses to a bolus injection of sodium nitroprusside (from 3.2 ± 0.4 to 1.9 ± 0.3 beats/min/mmHg; P < 0.05) and phenylephrine (from 2.6 ± 0.3 to 1.7 ± 0.2 beats·min−1·mmHg−1; P < 0.05), respectively (Table 2 and Fig. 5), resulting in the decreased slope of the linear regression curve plotting changes in HR to a given change in MAP (Fig. 5). This impairment in baroreflex sensitivity and the increased HR variability observed 7 days after the induction of diabetes were completely reversed to prediabetic values by chronic ICV leptin infusion (Fig. 5 and Table 1). Contrary to the effects of leptin on cardiac sympathetic tone and IHR, the improvement of baroreceptor sensitivity during ICV leptin treatment was abolished by an IV glucose infusion to maintain the hyperglycemia (Table 2).
Table 2.
Baroreflex sensitivity analyzed by linear regression curve during control, diabetes, and after chronic ICV leptin infusion and ICV leptin + IV glucose infusion
| Experimental Groups | Tachycardia | Bradycardia |
|---|---|---|
| ICV leptin + IV saline | ||
| Control | −3.2±0.4 | −2.6±0.3 |
| Diabetes | −1.9±0.3* | −1.7±0.2* |
| ICV leptin infusion | −3.4±0.3 | −3.0±0.3 |
| ICV leptin + IV glucose | ||
| Control | −3.3±0.5 | −2.8±0.3 |
| Diabetes | −1.8±0.1* | −1.8±0.3* |
| ICV leptin + IV glucose infusion | −2.0±0.1* | −2.3±0.5* |
Data are means ± SE; n = 8/ICV leptin + intravenous (IV) saline group and 5/ICV leptin + IV glucose group.
P < 0.05.
Fig. 5.
Reflex changes in heart rate (HR) to induced changes in MAP after phenylephrine (16 μg/kg) and sodium nitroprusside (32 μg/kg) evaluated on day 4 of control, day 7 after induction of diabetes, and on the last day of chronic ICV leptin infusion (0.021 μg·kg−1·min−1). Lines represent the linear regression between changes in HR and MAP. Data are expressed as means ± SE (n = 8).
Diabetes or chronic leptin treatment did not alter arterial pressure variability or the LF and HF power components of arterial pressure regulation evaluated by spectral analysis (Table 1).
DISCUSSION
In the present study, we show that leptin has a marked effect via its direct actions on the CNS that not only restores euglycemia and reverses the hyperphagia and bradycardia associated with insulin-deficient diabetes but also completely restores cardiac sympathetic tone and reduces the increased HR variability to levels comparable with nondiabetic rats. Induction of diabetes also caused a substantial decrease in the IHR that was completely reversed by ICV leptin treatment. CNS actions of leptin to normalize baroreflex sensitivity in insulin-deficient diabetes were mediated mainly by the associated restoration of euglycemia.
The bradycardia observed in STZ-induced diabetes has been suggested to reflect changes in the electrophysiological properties of the sinus node, increased cardiac vagal activity, or decreased cardiac sympathetic tone (19, 24). We recently showed that chronic adrenergic receptor antagonism blunted ∼50% of the rise in HR during leptin infusion in STZ-diabetic rats (6), whereas in the present study we observed a marked reduction in cardiac sympathetic tone after the induction of diabetes that was also completely reversed by leptin treatment. Taken together, these results indicate that at least one-half of the bradycardia observed in STZ-diabetic rats is mediated by reduced cardiac sympathetic tone. In the present study, we did not find any significant change in cardiac parasympathetic tone, evaluated by the increase in HR after a full-blocking dose of atropine, after the induction of diabetes or during leptin infusion. However, IHR, which is the HR after sympathetic and parasympathetic inputs to the heart have been blocked by propranolol and atropine, was markedly reduced 7 days after the induction of diabetes and ICV leptin infusion raised the IHR back to normal prediabetic values. These observations suggest that marked bradycardia observed in this experimental model of diabetes is mediated by a reduction in cardiac SNS activity and by a decrease in the intrinsic pacemaker activity of the heart. Our results also indicate that chronic CNS actions of leptin can completely return HR back to prediabetic values by restoring normal cardiac sympathetic tone and by normalizing IHR. Our results do not exclude the possibility that prolonged diabetes may induce other changes that contribute to the control of HR, such as altering vagal tone to the heart. We, however, did not observed any significant change in cardiac parasympathetic tone during the first two weeks postinduction of diabetes. Whether chronic ICV leptin can also ameliorate these cardiovascular alterations after prolonged diabetes, which could lead to neuropathy, is unknown and remains an important area for further investigation.
The mechanisms by which leptin increases SNS activity and IHR in diabetic rats are still unclear. However, activation of the hypothalamic proopiomelanocortin (POMC) system and its receptor types 3 and 4 (MC3/4R) appear to play an important role. Studies with blockade of the POMC-MC3/4R pathway using pharmacological agents or transgenic mice that do not have functional MC4R receptors have shown that an intact POMC-MC3/4R pathway is required for leptin-induced sympathetic activation and to increase arterial pressure and HR (7, 40). Another possibility that was tested in our study is that leptin may also normalize IHR by restoring normoglycemia in diabetic rats since most cardiac electrophysiological disturbances in STZ-diabetic rats can be reversed by the normalization of glucose levels (8). Exposure of myocytes to high glucose levels, but not other nonmetabolizable sugars, also prolongs the relaxation phase of the action potential (34). Our results, however, indicate that the CNS effects of leptin to normalize cardiac sympathetic tone and IHR to control values were mainly independent of the ability of leptin to normalize glycemic levels since maintenance of the hyperglycemia during leptin treatment did not significantly alter the effects of leptin to raise sympathetic tone and IHR back to values observed before the development of diabetes. These data suggest that mechanism(s) other than the normalization of circulating glucose levels mediate these effects of leptin. However, further studies are necessary to determine the precise mechanisms by which leptin may alter pacemaker activity and autonomic outflow to the heart in diabetic rats.
Studies in diabetic patients and experimental animal models of diabetes have demonstrated an impairment of baroreflex-mediated HR responses to increases or decreases in BP (5, 11, 21, 22, 31). Therefore, another important goal of the present study was to determine the efficacy of chronic leptin infusion in ameliorating the impaired baroreceptor responses in diabetic rats. We found that STZ-induced diabetes is associated with impaired tachycardic and bradycardic baroreflex responses as early as 7 days after the induction of diabetes. We also found that chronic ICV leptin infusion augmented baroreflex sensitivity back to normal in STZ-treated rats. Chang and Lund (5) showed that insulin replacement in STZ-diabetic rats also enhanced baroreflex sensitivity, suggesting that the normalization of glucose levels may be an important component by which leptin restored baroreflex function in the present study. It is also possible that the augmented sympathetic tone during leptin treatment contributes, at least in part, to the improvement of baroreceptor function in our diabetic rats. Although we showed that the return of cardiac sympathetic tone and IHR to control values is independent of the action of leptin on glucose levels, amelioration of the hyperglycemia appears to play a major role in mediating the effects of leptin to improve baroreceptor sensitivity in diabetic rats.
The spectral analysis data also showed a reduction in the LF component of the power spectra, indicating a reduction in cardiac sympathetic tone 7 days after the induction of diabetes. Similar results were obtained using propranolol to assess sympathetic control of HR. Analysis of the HF component of the spectra indicated an increase in parasympathetic control of HR after the induction of STZ diabetes, but our results with atropine injections provided no evidence for altered parasympathetic control of HR in STZ-treated rats. One possible explanation is that the pharmacological method evaluates the maximum response of HR after the withdrawal of parasympathetic tone by a full-blocking dose of atropine, whereas the spectral analysis evaluates more subtle changes in cardiac parasympathetic tone in the presence of normal sympathetic and parasympathetic inputs to the heart.
Another aspect of diabetes is that cardiovascular and metabolic functions gradually worsen with time and according to the severity of the hyperglycemia, which could explain why some studies have reported reduced sympathetic or parasympathetic tone or both. Dall′Ago et al. (9) showed an impairment of parasympathetic tone to the heart in diabetic rats, whereas McDowell et al. (26) observed normal vagal response to vagal electrical stimulation, and clinical studies have found impaired vagal and sympathetic control of the heart rate (11). One major advantage of our study compared with most previous studies is that we used chronically implanted telemetry probes to continuously monitor arterial pressure and HR throughout the length of our experiment so that each animal served as its own control for the effects of diabetes or chronic ICV leptin infusion. Our findings indicate that short-term diabetes, lasting for 2 wk or less, is associated mainly with sympathetic rather than parasympathetic dysfunction.
The finding of the present study that the induction of diabetes was associated with increased HR variability is similar to the observations by Schaan et al. (36). However, some studies have found reduced HR variability in the STZ-diabetic model (16, 23, 28). These differences may be related to differences in the duration of diabetes, the degree of hyperglycemia, different species, and other factors that may influence HR variability such as the development of neuropathy with prolonged severe diabetes. In the present study, each animal served as its own control, which is an important factor that strengthens our findings.
In the present study, we mainly focused on the effects of leptin in short-term diabetes to determine whether chronic ICV leptin treatment could reverse the functional cardiovascular alterations known to develop during the onset of diabetes. Whether leptin would also exert similar effects after long-term exposure to diabetes remains to be determined. In a recent study, Park et al. (32) showed that leptin replacement therapy for one year in patients that had developed type 1 diabetes seven years before the study markedly reduced their insulin requirements and was also associated with significant improvement of most of the metabolic parameters examined (32). However, the effects of leptin of cardiovascular parameters were not investigated, and little information is available regarding the cardiovascular actions of chronic leptin therapy in diabetic subjects.
Although the mechanisms by which the CNS actions of leptin markedly reduced plasma glucose levels and food intake in diabetic rats are unknown, activation of the POMC-MC3/4R pathway and inhibition of the hypothalamic neuropeptide Y (NPY) system may play a major role. Pharmacological inhibition of the MC3/4R, via an ICV infusion of the antagonist, completely prevented the effects of leptin to reduce appetite and to increase insulin sensitivity in rats (7). Furthermore, induction of diabetes with STZ is associated with reductions in POMC levels in the hypothalamus (17). Conversely, NPY levels are markedly increased in STZ-diabetic mice (39a), and NPY-deficient mice have hyperglycemia comparable with that observed in wild-type mice after the induction of diabetes but do not show any increase in food intake (39a). Since leptin activates the POMC pathway while inhibiting the NPY system, the increased POMC/NPY ratio may be a key factor contributing to the antidiabetic and appetite actions of leptin observed in the present study.
In summary, we showed that leptin has powerful CNS actions that completely restore glucose and appetite regulation in STZ-diabetic rats. In addition to its metabolic actions, leptin also appears to have major cardiovascular effects mediated by the CNS that reversed the bradycardia, the HR variability, and the decreased IHR to prediabetic values. The improvement of baroreflex sensitivity in STZ-diabetic rats during ICV leptin infusion appears to be largely mediated by the CNS effects of leptin to normalize blood glucose levels. The CNS mechanisms triggered by chronic leptin infusion that mediate these metabolic and cardiovascular effects are unknown. Moreover, the link between the CNS and the peripheral organs leading to the normalization of glucose levels is also unknown and remains to be elucidated. Future studies are needed to better understand how the brain regulates peripheral glucose homeostasis in diabetic models with insulin deficiency. The results from the present study also suggest that the CNS may be an important target for novel therapies to improve the metabolic and cardiovascular outcome of current antidiabetic treatments.
GRANTS
Our research was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-51971 and by a Scientist Development Grant from the American Heart Association (to A. A. da Silva).
Acknowledgments
We thank Stephanie Evans, Calvin Torrey, and Adrian Dreher for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe H, Minokoski Y, Shimazu T. Effects of beta 3-adrenergic agonist BRL35135A, on glucose uptake in rat skeletal muscle in vitro and in vivo. J Endocrinol 139: 479–486, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Akirav EM, Chan O, Inouye K, Riddell MC, Mathews SG, Vranic M. Partial leptin restoration increases hypothalamic-pituitary adrenal activity while diminishing weight loss and hyperphagia in streptozotocin diabetic rats. Metabolism 53: 1558–1564, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Centers for diseases control and prevention (CDC). Prevalence of chronic kidney disease and associated risk factors—United States, 1997–2004. MMWR Morb Mortal Wkly Rep 161: 161–165, 2007. [PubMed] [Google Scholar]
- 5.Chang KSK, Lund DD. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J Mol Cell Cardiol 18: 617–624, 1986. [DOI] [PubMed] [Google Scholar]
- 6.da Silva AA, Tallam LS, Liu J, Hall JE. Chronic antidiabetic and cardiovascular actions of leptin: role of CNS and increased adrenergic activity. Am J Physiol Regul Integr Comp Physiol 291: R1271–R1282, 2006. [DOI] [PubMed] [Google Scholar]
- 7.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4 receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 43: 1312–1317, 2004. [DOI] [PubMed] [Google Scholar]
- 8.D′Amico M, Marfella R, Nappo F, Di Filippo C, De Angelis L, Berrino L, Rossi F, Giugliano D. High glucose induces ventricular instability and increases vasomotor tone in rats. Diabetologia 44: 464–470, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Dall′Ago P, Silva VOK, De Angelis KDL, Irigoyen MC, Fazan R Jr, Salgado HC. Reflex control of arterial pressure and heart rate in short-term streptozotocin diabetic rats. Braz J Med Biol Res 35: 843–849, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes 46: 2040–2043, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Eckberg DL, Harkins SW, Fritsch JM, Musgrave GE, Gardner DF. Baroreflex control of plasma norepinephrine and heart period in healthy subjects and diabetic patients. J Clin Invest 78: 366–374, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eto M, Toba K, Akishita M, Kozakik K, Watanabe T, Kim S, Hashimoto M, Sudoh Yoshizumi MN, Ouchi Y. Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat of blood pressure variability. Hypertens Res 26: 991–998, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Fazan R, Ballejo G, Salgado MCO, Moraes MFD, Salgado HC. Heart rate variability and baroreceptor function in chronic diabetic rats. Hypertension 30: 632–635, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fazan VPS, Salgado HC, Barreira AA. Aortic depressor nerve myelinated fibers in acute and chronic experimental diabetes. Am J Hypertens 19: 253–260, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin enhancing glucose uptake in peripheral tissues after hypothalamic injection of leptin in rats. Diabetes 48: 1706–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto M, Harada T, Ishikawa T, Obata M, Shibutani Y. Investigation on diabetic neuropathy assessed by power spectral analysis of heart rate variability in WBN/Kob rats. J Electrocardiol 34: 243–250, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Havel PJ, Sindelar DK, Baskin DG, Dallman MF, Weigle DS, Schwart MW. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes 49: 244–252, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiovascular actions of leptin. Hypertension 30: 619–623, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J Auton Nerv Syst 69: 21–30, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hicks KK, Seifen E, Stimers JR, Kennedy RH. Diabetes with and without ketoacidosis on right atrial pacemaker rate and autonomic responsiveness. Am J Physiol Heart Circ Physiol 273: H1888–H1893, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Hilsted J Pathophysiology in diabetic autonomic neuropathy: cardiovascular, hormonal and metabolic studies. Diabetes 31: 730–737, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Homma S, Yamazaki Y, Karakida T. Blood pressure and heart rate relationship during cervical sympathetic and vagus nerve stimulation in streptozotocin diabetic rats. Brain Res 629: 342–344, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Howarth FC, Jacobson M, Naseer O, Adeghate E. Short-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol 90: 237–245, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Maeda CY, Fernandes TG, Timm HB, Irigoyen MC. Autonomic dysfunction in short-term experimental diabetes. Hypertension 26: 1100–1104, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Massimini M, Porta A, Mariotti M, Malliani A, Montano N. Heart rate variability is encoded in the spontaneous discharge of thalamic somatosensorial neurons in cat. J Physiol 526: 387–396, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell TS, Chapleau MW, Hajduczok G, Abboud FM. Baroreflex dysfunction in diabetes mellitus. I. Selective impairment of parasympathetic control of heart rate. Am J Physiol Heart Circ Physiol 266: H235–H243, 1994. [DOI] [PubMed] [Google Scholar]
- 27.McDowell TS, Hajduczok G, Abboud FM, Chapleau MW. Baroreflex dysfunction in diabetes mellitus. II. Site of baroreflex impairment in diabetic rabbits. Am J Physiol Heart Circ Physiol 266: H244–H249, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Mesangeau D, Laude D, Elghozi JL. Early detection of cardiovascular autonomic neuropathy in pig using blood pressure and heart rate variability. Cardiovasc Res 45: 889–899, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Minokoshi Y, Haque MS, Shimazu T. Microinjection into the hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48: 287–291, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Oral EA, Simha V, Ruiz E, Andrewelt A, Premkumar A, Snell P, Wagner AJ, Depaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346: 570–578, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Page MM, Watkins PJ. Cardio-respiratory arrest and diabetic autonomic neuropathy. Lancet 1: 15–16, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Park JY, Chong AY, Cochran EK, Kleiner DE, Haller MJ, Schatz DA, Gorden P. Type 1 diabetes associated with acquire generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab 93: 26–31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren J, Gintant GA, Miller RE, Davidoff AJ. High extracellular glucose impairs cardiac E-C coupling in a glycosilation-dependent manner. Am J Physiol Heart Circ Physiol 273: H2876–H2883, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MW, Woods SC, Porte D Jr, Seely RJ, Braskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Shaan BD, Maeda CY, Medeiros S, Moraes RS, Ferlin E, Fernandes TG, Ribeiro JP, Schmid H, Irigoyen MC. Time course of changes in heart rate and blood pressure variability in streptozotocin-induced diabetic rats treated with insulin. Braz J Med Biol Res 30: 1081–1086, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension 32: 409–414, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401: 73–76, 1999. [DOI] [PubMed] [Google Scholar]
- 39a.Sindelar DK, Mystkowski P, Marsh DJ, Palmiter RD, Schwart MW. Attenuation of diabetic hyperphagia in neuropeptide Y−/− deficient mice. Diabetes 3: 778–783, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Wang JL, Chinookoswong N, Scully S, Qi M, Shi ZQ. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology 140: 2117–2124, 1999. [DOI] [PubMed] [Google Scholar]