Abstract
The sympathetic nervous system and renin-angiotensin system are both thought to contribute to the development and maintenance of hypertension in experimental models such as the spontaneously hypertensive rat (SHR). We demonstrated that periarterial nerve stimulation (NS) increased the perfusion pressure (PP) and neuropeptide Y (NPY) overflow from perfused mesenteric arterial beds of SHRs at 4–6, 10–12, and 18–20 wk of age, which correspond to prehypertensive, developing hypertensive, and maintained hypertensive stages, respectively, in the SHR. NS also increased PP and NPY overflow from mesenteric beds of Wistar-Kyoto (WKY) normotensive rats. NS-induced increases in PP and NPY were greater in vessels obtained from SHRs of all three ages compared with WKY rats. ANG II produced a greater increase in PP in preparations taken from SHRs than WKY rats. ANG II also resulted in a greater increase in basal NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old SHRs than age-matched WKY rats. ANG II enhanced the NS-induced overflow of NPY from SHR preparations more than WKY controls at all ages studied. The enhancement of NS-induced NPY overflow by ANG II was blocked by the AT1 receptor antagonist EMD-66684 and the angiotensin type 2 receptor antagonist PD-123319. In contrast, ANG II greatly enhanced norepinephrine overflow in the presence of PD-123319. Both captopril and EMD-66684 decreased neurotransmitter overflow from SHR mesenteric beds; therefore, we conclude that an endogenous renin-angiotensin system is active in this preparation. It is concluded that the ANG II-induced enhancement of sympathetic nerve stimulation may contribute to the development and maintenance of hypertension in the SHR.
Keywords: hypertension, norepinephrine, sympathetic neurotransmission, mesenteric artery
sympathetic nerves synthesize and release the neurotransmitters norepinephrine (NE), neuropeptide Y (NPY), and ATP (51, 57). When administered to tissue preparations innervated by sympathetic nerves, each mimics a specific phase of sympathetic nerve stimulation (42, 51). All three mediators exert prejunctional and postjunctional effects at the vascular neuroeffector junction. Postjunctionally, NE, NPY, and ATP produce contraction of vascular smooth muscle through the activation of α1-, Y1, and P2X receptors, respectively. Prejunctionally, these mediators activate α2-, Y2, and P2Y receptors, respectively, resulting in feedback inhibition of their own release as well as the release of each other (62, 66).
In addition to the activity of the sympathetic nervous system (SNS) and its three cotransmitters, the renin-angiotensin system (RAS) also plays a very important role in the regulation of vascular tone. Originally the RAS was viewed solely as a hormonal circulating system involved in the regulation of blood pressure along with salt and water homeostasis. However, more recent evidence has identified distinct components of the RAS that are active locally in the vasculature, heart, and other tissues (9, 54). ANG II has long been considered the main biologically active product of the RAS. ANG II produces multiple actions including direct vasoconstriction and release of aldosterone from the adrenal cortex via the activation of AT1 receptors. In addition, ANG II enhances sympathetic neurotransmission (8, 10, 17, 36, 40, 43, 44, 46, 58, 72, 75), which contributes to the vasopressor effects of the peptide.
Essential hypertension is a chronic, progressive disease that involves numerous neuronal, endocrine, and pathophysiological alterations. Findings to date implicate both the SNS and RAS in contributing to the development and maintenance of hypertension in both humans and experimental models such as the spontaneously hypertensive rat (SHR) (17, 22, 30, 70). There is evidence for dysfunction at both the pre- and postjunctional levels of sympathetic neurotransmission at the vascular neuroeffector junction that involve both NPY and ANG II. For example, we (67, 70) have previously observed that the inhibitory effect of NPY on NE release is attenuated in blood vessels obtained from the SHR. In addition, the facilitatory effect of ANG II on NE release is enhanced from blood vessels of SHRs compared with those obtained from normotensive controls (3, 47, 68). Moreover, the vasoconstrictor actions of both NPY and ANG II were higher in blood vessels obtained from SHRs. In fact, the vasoconstrictor action of NPY increased along with the development of elevated blood pressure in SHRs (49, 76). The perfused mesenteric arterial bed contains a dense sympathetic innervation and represents an excellent model of the vascular neuroeffector junction. SHRs have been extensively studied, and it is well established that these animals develop hypertension as they age. Although there may indeed be differences between SHRs and Wistar-Kyoto (WKY) rats even at a very early age, from birth to 8 wk, the blood pressure of the SHR is comparable with the normotensive genetic control (47, 59, 68). The blood pressure of the SHR then begins to rise sharply until the rats are 10–12 wk old. The increase in blood pressure of the SHR then slows and, at ∼18–20 wk, stabilizes (71).
The objectives of the present investigation were severalfold. First, we examined the effect of nerve stimulation on the increase in perfusion pressure (PP) and NPY overflow from the mesenteric arterial beds of SHRs at a time before the development of hypertension (4–6 wk old), during the development of hypertension (10–12 wk old), and after the establishment of hypertension (18–20 wk old) and to compare it with the effect on age-matched normotensive controls. Second, we investigated the role of ANG II in the regulation of NPY overflow from SHR preparations during the above stages and compare its effect with that on preparations obtained from age-matched normotensive controls. Finally, we investigated which AT receptor subtype is involved and whether or not an endogenous RAS is active in our model. In this report, we show that the modulation of NPY overflow by ANG II may contribute to the development and maintenance of hypertension in SHRs.
MATERIALS AND METHODS
Materials
ANG II, captopril, EMD-66684 (AT1 receptor antagonist), PD-123319 (AT2 receptor antagonist), protease inhibitor cocktail (for mammalian cell and tissue extracts), and Krebs buffer salts were all purchased from Sigma (St. Louis, MO). The NPY enzyme immunoassay kit was purchased from Peninsula Laboratories (San Carlos, CA).
Animals
We used age-matched WKY rats and SHRs obtained from Harlan in all our experiments. They were 4–6, 10–12, and 18–20 wk old so as to match with the prehypertensive, young hypertensive, and old hypertensive stages of the SHR, respectively. Animals were housed at 2–4 animals/cage in constant temperature and with a 12:12-h light-dark cycle room. Rats were fed standard rat chow and water at will, and they were under the supervision of the Department of Comparative Medicine. All the experimental procedures carried out were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the Saint Louis University Health Sciences Center.
Isolated Perfused Mesenteric Preparation of the Rat
Surgery.
Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium at a dose of 50 mg/kg. The abdomen was opened, and the intestine was excised by ligations of the descending colon proximal to the rectum, duodenum distal to the stomach, and superior mesenteric artery distal to the abdominal aorta. The isolated intestine with the mesentery attached was then spread in a petri dish containing Krebs physiological buffer [composed of (in mM) 120 NaCl, 5.0 KCl, 2.4 CaCl2, 1.2 MgSO4, 0.027 EDTA, 11 glucose, and 25 NaHCO3]. The superior mesenteric artery was cannulated with a 20-gauge cannula, and 10% heparin solution was flushed through the mesenteric vascular bed. The four main branches of the mesenteric artery feeding the terminal ileum were located and left intact. The remaining branches were ligated and severed. The mesenteric vascular bed was carefully detached from the intestine. The preparation was placed in an organ bath maintained at 37°C and perfused with aerated Krebs buffer (pH 7.4) by a Gilson minipuls pump at a rate of 2.5 ml/min and superfused at a rate of 0.5 ml/min. PP was continuously monitored through a pressure transducer coupled with a Grass recorder. The tissue was equilibrated for 40 min with the perfusate before experimentation began. All drugs used were dissolved in Krebs buffer and delivered by continous infusion. Protease inhibitor cocktail (50 μl/1 g tissue) was added to Krebs buffer to ensure drug viability.
Periarterial NS.
Platinum ring electrodes were placed around the artery, and, when appropriate, the periarterial nerve was stimulated at 16 Hz for 30 s for catecholamine release and 1.5 min for NPY release using a Grass S-88 stimulator. Perfusate effluents were collected continuously in 1-min time frames before, during, and after periarterial NS into either 1 ml of cold 0.4 N perchloric acid with 0.1% cysteine for catecholamin experiments or 1% trifluoroacetic acid for NPY experiments. Collections were stored at −80°C for maximal stability until they were ready to be assayed.
NE Measurement
NE in acidified release medium, perfusate, and superperfusate samples was identified and quantified by HPLC with electrochemical detection (HPLC-EC). The system consisted of a Varian Pro-Star solvent delivery system and a model 9090 autosampler (Varian, Walnut Creek, CA) coupled to a C18 column and an ESA Coulochem II detector. Separations were performed isocratically using a filtered and degassed mobile phase consisting of 12% methanol, 0.1 M sodium phosphate, 0.2 mM sodium octyl sulfate, and 0.1 mM EDTA adjusted to pH 2.8 with phosphoric acid. The HPLC system was coupled to a computer, where the chromatograms were recorded and analyzed with Varian Star workstation software.
NPY Measurement
The samples collected were pooled in groups of two to facilitate concentration assessment. NPY amounts in the perfusate samples were purified with the use of C18 Sep-Pak columns and measured by a 96-well plate enzyme immunoassay kit (Peninsula Labs, San Carlos, CA). The 96-well plate was read by a Powerwave X plate reader (Biotek Instruments, Winooski, VT), and the calculations of sample value were analyzed by KC Junior Software (Biotek Instruments).
Statistical Analysis of Data
Data are expressed as means ± SE. Statistical analyses were carried out by Student's t-test or by one-way ANOVA followed by Neuman-Keuls multiple statistical comparison tests. Differences were accepted when P < 0.05.
RESULTS
NS Increases PP in Mesenteric Arterial Beds Obtained From WKY Rats and SHRs at 4–6, 10–12, and 18–20 wk of Age
Table 1 shows basal and NS-induced increases in PP in mesenteric arterial beds obtained from WKY rats and SHRs of 4–6, 8–10, and 18–20 wk of age. Values are presented as basal and NS measurements (in mmHg) and as increases from basal values (NS − basal value; in mmHg). We observed no differences in basal PP values among strains or as a function of age. NS resulted in a significant increase in PP in all mesenteric arterial bed preparations. WKY rats showed no difference in NS-induced increases in PP as a function of age.
Table 1.
Effect of periarterial NS on perfusion pressure of mesenteric arterial beds obtained from WKY rats and SHRs of 4–6, 10–12, and 18–20 wk of age
| Basal | NS | Increase (NS − Basal) | |
|---|---|---|---|
| WKY rats | |||
| 4–6 wk old | 32.4±5.1 | 91.2±4.7 | 59±5.8 |
| 10–12 wk old | 19.6±0.7 | 87.6±10.4 | 68±9.6 |
| 18–20 wk old | 24±0.6 | 85±11.2 | 61±11.5 |
| SHRs | |||
| 4–6 wk old | 24.8±2.7 | 137±7.3 | 112±9* |
| 10–12 wk old | 28±1.2 | 182±5.5 | 154±7* |
| 18–20 wk old | 29±2.1 | 204±7.5 | 178±6** |
Values are means ± SE of perfusion pressure (in mmHg). NS, nerve stimulation; WKY rats, Wistar-Kyoto rats; SHRs, spontaneously hypertensive rats.
P < 0.01 and
P < 0.001 compared with age-matched WKY rats.
We observed an age-related increase in the NS-induced PP change in SHR preparations. Moreover, the NS-induced increase in PP was greater in mesenteric beds obtained from SHRs of all three age groups compared with WKY controls. SHRs had significantly higher blood pressures at 10–12 and 18–20 wk of age compared with the normotensive strain despite similar values at 4–6 wk of age.
NS Increases the Overflow of NPY From Mesenteric Arterial Beds Obtained From SHRs Compared With WKY Rats at 10–12 and 18–20 wk of Age
Table 2 shows basal and NS-induced overflow of NPY from 4- to 6-wk-old, 10- to 12-wk-old, and 18- to 20-wk-old WKY and SHR preparations. Values are presented as nanograms per milliter of NPY. Basal NPY overflow was not different between strains or as a function of age. NS resulted in a significant increase in NPY overflow from both strains at all ages studied. The NS-induced overflow of NPY was similar for WKY rats and SHR at 4–6 wk of age (Table 2). However, the NS-induced NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old SHR preparations was greater than from age-matched WKY controls.
Table 2.
Effect of periarterial NS on neuropeptide Y overflow (ng/ml) from mesenteric arterial beds obtained from WKY rats or SHRs of 4–6, 10–12, or 18–20 wk of age
|
WKY |
SHR
|
|||||
|---|---|---|---|---|---|---|
| Basal | NS | Increase | Basal | NS | Increase | |
| 4–6 wk old | 0.02±0.03 | 1.09±0.15 | 1.07 | 0.03±0.01 | 1.14±0.15 | 1.11 |
| 10–12 wk old | 0.03±0.004 | 0.85±0.09 | 0.82 | 0.06±0.02 | 1.19±0.09* | 1.13 |
| 18–20 wk old | 0.07±0.02 | 1.22±0.18 | 1.15 | 0.08±0.02 | 1.76±0.09*† | 1.68 |
Results are expressed as means ± SE of neuropeptide Y overflow (in ng/ml); n = 5–7 preparations.
P < 0.05 compared with WKY rats;
P < 0.05 compared with SHRs of 4–6 and 10–12 wk of age.
NS-Induced Increase in PP Is Reduced by an NPY-Y1 Antagonist and an α1-Adrenergic Receptor Antagonist
We observed that both the NPY-Y1 antagonist BIBO-3304 and the α1-adrenergic antagonist prazosin produced a significant reduction in the increase in PP due to NS of mesenteric arterial beds obtained from 10- to 12-wk-old SHRs. The NS-induced increase in PP was 160 ± 8 mmHg (n = 6) in the absence of drugs and 18 ± 5 mmHg (n = 6) in the presence of prazosin and 65 ± 6 mmHg (n = 6) in the presence of BIBO-3304. Prazosin (30 nM) produced a 88% reduction, whereas BIBO-3304 (100 nM) produced a 59% reduction, of the increase in PP.
ANG II Enhances NS-Induced Increases in PP in Mesenteric Arterial Beds Obtained SHRs at 4–6, 10–12, and 18–20 wk of Age More Than Age-Matched WKY Rats
ANG II failed to alter the NS-induced increase in PP of mesenteric arterial beds from WKY rats at any age (Table 3). In contrast, ANG II at both 0.01 and 0.1 μM significantly enhanced the NS-induced increase in PP of mesenteric beds obtained from 4- to 6-wk-old and 10- to 12-wk-old SHRs compared with WKY rats. In addition, ANG II (0.1 μM) significantly increased the NS-induced change in PP of 18- to 20-wk-old SHR preparations to a greater extent than 0.01 μM (Table 3). The effect of ANG II on the NS-induced increase in PP was greater in mesenteric beds obtained from SHRs than age-matched WKY rats.
Table 3.
Effect of ANG II on NS-induced increases in perfusion pressure (increase from basal in mmHg) from mesenteric bed preparations of WKY rats and SHRs at 4–6, 10–12, and 18–20 wk of age
| WKY Rats | SHRs | |
|---|---|---|
| 0 μM ANG II | ||
| 4–6 wk old | 59±5.8 | 112±9* |
| 10–12 wk old | 68±9.6 | 154±7* |
| 18–20 wk old | 61±11 | 178±7* |
| 0.01 μM ANG II | ||
| 4–6 wk old | 66±15 | 156±16*† |
| 10–12 wk old | 60±12 | 190±7*† |
| 18–20 wk old | 74±14 | 175±24* |
| 0.1 μM ANG II | ||
| 4–6 wk old | 60±10 | 169±10*† |
| 10–12 wk old | 76±11 | 203±12*† |
| 18–20 wk old | 44±7 | 251±9.8*‡ |
Results are expressed as means ± SE of increases of perfusion pressure from basal (in mmHg); n = 5-7 preparations.
P < 0.001 compared to WKY rats;
P < 0.05 compared with SHR of the same age with no ANG II;
P < .05, 0.1 μM ANG II compared with 0.01 μM ANG II in 18- to 20-wk-old SHR.
ANG II Increases Basal NPY Overflow From Preparations From SHRs of 10–12 and 18–20 wk in Age More Than WKY Rats
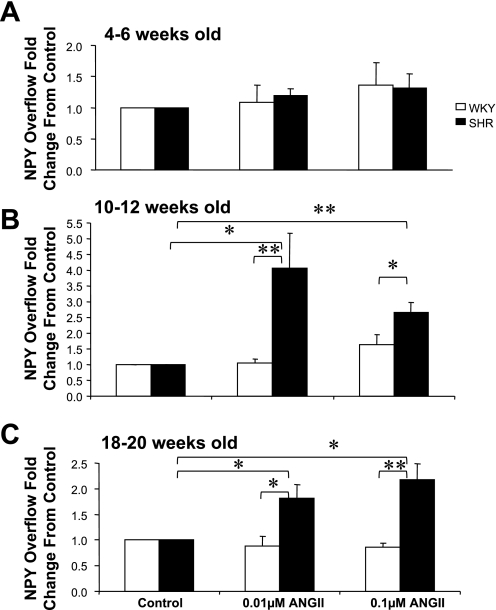
Figure 1 shows the effect of ANG II (0.01 and 0.1 μM) on the basal NPY overflow from mesenteric beds obtained from WKY rats and SHRs at 4–6, 10–12, and 18–20 wk of age. Data are plotted as fold changes from control (with control being NPY overflow values before ANG II administration). Each bar in the graph represents the mean ± SE of 5–7 preparations. ANG II administration at either concentration did not alter the basal NPY overflow from preparations obtained from 4- to 6-wk-old rats (Fig. 1A). In contrast, ANG II at both concentrations produced a significant increase in basal NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old SHR mesenteric beds compared with age-matched WKY controls (Fig. 1, B and C). ANG II at 0.01 μM produced 4.1 ± 1.1- and 1.82 ± 2.5-fold increases in NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old SHR mesenteric bed preparations, respectively. NPY overflow increases in response to 0.1 μM ANG II were 2.66 ± 0.3- and 2.17 ± 0.3-fold from 10- to 12-wk-old and 18- to 20-wk-old SHR preparations, respectively. Both concentrations of ANG II failed to alter the basal NPY overflow from mesenteric arterial beds obtained from WKY rats at all ages.
Fig. 1.
Effects of ANG II (0.1 and 0.01 μM) on basal neuropeptide Y (NPY) overflow from mesenteric beds of Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) of 4–6 (A), 10–12 (B), and 18–20 (C) wk of age. Data are plotted as fold changes from basal overflow before the administration of ANG II. Each bar is the mean ± SE of 5–7 preparations. *P < 0.05 and **P < 0.01 compared with control or WKY rats.
ANG II Increases the NS-Induced Overflow of NPY From Preparations From SHRs at 4–6, 10–12, and 18–20 wk in Age More Than WKY Rats
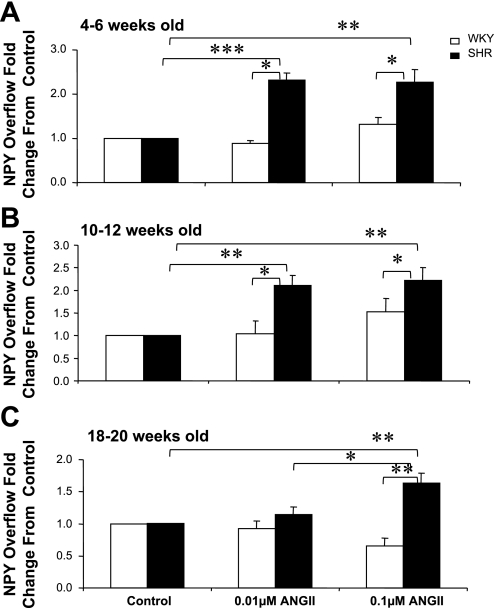
ANG II produced a significant increase in the NS-induced overflow of NPY from mesenteric beds obtained from 4- to 6-wk-old SHRs but not age-matched WKY rats (Fig. 2A). ANG II at 0.01 μM produced a 2.26 ± 0.28-fold increase and a similar increase (2.3 ± 0.3-fold) at 0.1 μM. ANG II had similar effects in 10- to 12-wk-old SHR preparations, with a 2.1 ± 0.2-fold increase at 0.01 μM ANG II and 2.7 ± 0.3-fold increase at 0.1 μM ANG II (Fig. 2B). In 18- to 20-wk-old SHRs, ANG II at 0.1 μM resulted in a 1.6 ± 0.2-fold increase in the nerve-stimulated NPY overflow (Fig. 2C).
Fig. 2.
Effects of ANG II (0.1 and 0.01 μM) on nerve stimulation (NS)-induced NPY overflow from mesenteric beds of WKY and SHR of 4–6 (A), 10–12 (B), and 18–20 (C) wk of age. Data are plotted in a similar way as in Fig. 1. Each bar is the mean ± SE of 5–7 preparations. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control, WKY rats, or SHRs treated with 0.1 μM ANG II.
ANG II Failed to Produce an Increase in NS-Induced Overflow of NPY in Mesenteric Arterial Beds Obtained From WKY Rats at Any Age
In summary, the increase in the NS-induced overflow of NPY from mesenteric beds of SHRs by ANG II at both concentrations was greater than from WKY rats at all ages.
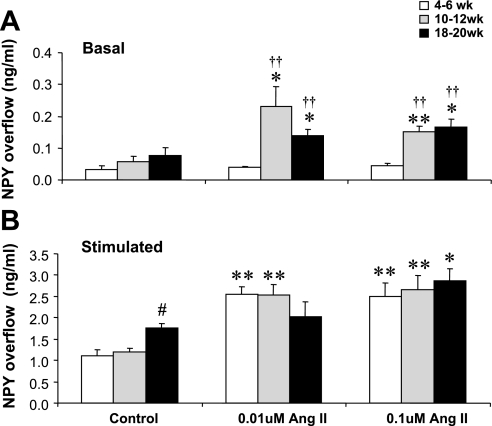
ANG II Modulation of NPY Overflow Changes with Age in SHRs
In addition to comparing the effect of ANG II-induced NPY overflow from SHR preparations with that from WKY controls, we also compared the effects of ANG II on basal and NS-induced NPY overflow in mesenteric arteries obtained from SHRs as a function of age. This is shown in Fig. 3. Data are plotted as means ± SE in nanograms per milliliter of NPY for 5–7 preparations. At the prehypertensive age of 4–6 wk, neither 0.01 nor 0.1 μM ANG II altered basal NPY overflow. In contrast, both 0.01 and 0.1 μM ANG II significantly increased basal NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old SHR mesenteric arterial beds (Fig. 3A).
Fig. 3.
Effects of ANG II (0.1 and 0.01 μM) on basal (A) and NS-induced (B) NPY overflow from mesenteric arterial beds of 4- to 6-wk-old, 10- to 12-wk-old, and 18- to 20-wk-old SHR. Data are plotted as NPY overflow (in ng/ml). Each bar represents mean ± SE of 5–7 preparations. *P < 0.05 and **P < 0.01 compared with the same age control SHRs; ††P < 0.01 compared with 4- to 6-wk-old SHRs; #P < 0.05 compared with 4- to 6-wk-old and 10- to 12-wk-old control SHRs (pre-ANG II).
As already noted, NS itself increased the NPY overflow from 18- to 20-wk-old SHR preparations more than from 4- to 6-wk-old or 10- to 12-wk-old SHR preparations (Fig. 3B). Moreover, both 0.01 and 0.1 μM ANG II significantly enhanced the NS-induced NPY overflow from SHR preparations at all three ages. It is of interest that 0.01 μM ANG II resulted in a smaller increase in the NPY overflow from 18- to 20-wk-old SHRs compared with 4- to 6-wk-old or 10- to 12-wk-old SHR mesenteric beds (Fig. 3B).
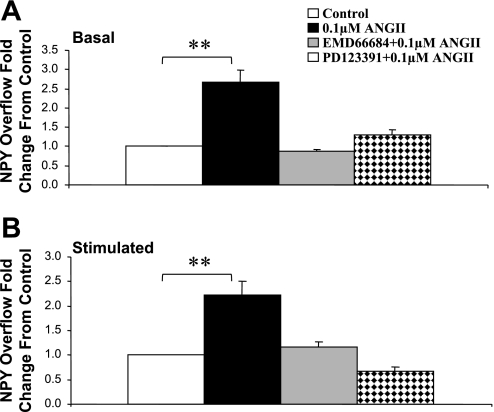
AT1 Receptor Antagonist EMD-66684 and AT2 Receptor Antagonist PD-123319 Attenuated ANG II-Induced Increases in NPY Overflow From Mesenteric Arterial Beds From SHRs of 10–12 wk of Age
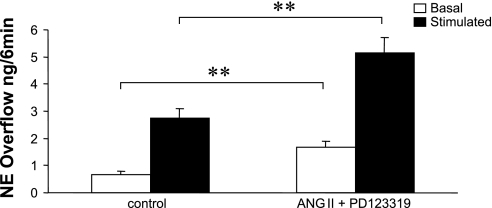
ANG II is known to activate at least two receptor subtypes, identified as AT1 and AT2 receptors. Figure 4 shows that the presence of either the AT1 receptor antagonist EMD-66684 (0.1 μM) or the presence of the AT2 receptor antagonist PD-123319 attenuated the ANG II (0.1 μM)-induced increase in basal (Fig. 4A) and nerve-stimulated NPY overflow (Fig. 4B). In contrast, in the presence of the AT2 receptor antagonist PD-123319, ANG II greatly enhance basal and NS-induced NE overflow (Fig. 5). The effect of ANG II on PP changes was attenuated in the presence of the AT1 receptor antagonist (control = 153 ± 13.5 mmHg and ANG II + EMD-66684 = 164 ± 12.5 mmHg), whereas in the presence of the AT2 receptor antagonist ANG II significantly enhanced the NS-induced increase in PP (control = 121 ± 5.3 mmHg and ANG II + PD-123319 = 165 ± 7.8 mmHg).
Fig. 4.
Effects of ANG II (0.1 μM), ANG II (0.1 μM) + EMD-66684 (0.1 μM), and ANG II (0.1 μM) + PD-123391 (0.1 μM) on basal (A) and NS-induced (B) NPY overflow from mesenteric beds of 10- to 12-wk-old SHRs. Data are plotted as fold changes from control (pre-ANG II values). Each bar is the mean ± SE of 5–7 preparations. **P < 0.01 compared with control.
Fig. 5.
ANG II (0.1 μM) greatly enhanced both basal (2.6-fold of control) and stimulated (2-fold of control) norepinephrine (NE) overflow from mesenteric bed preparations (n = 6) obtained from 10- to 12-wk-old SHRs in the presence of PD-123391 (AT2 receptor antagonist). **P < 0.01 compared with control.
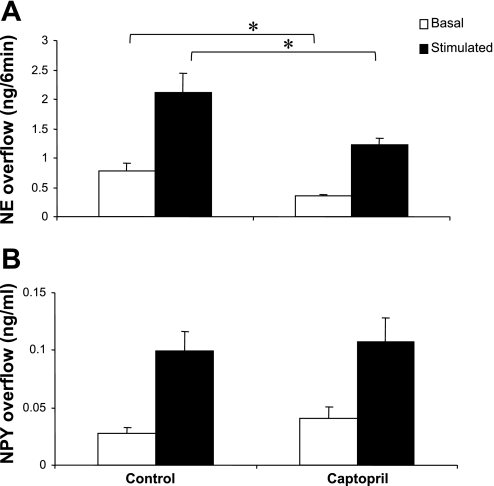
Captopril Decreased Basal and NS-Induced Overflow of NE From Mesenteric Arterial Beds Obtained From SHRs at 10–12 wk of Age
As shown above, ANG II enhanced the basal overflow of NPY from SHR mesenteric beds, suggesting that a local RAS might be active in this preparation. To investigate such a possibility, we examined the effect of an angiotensin-converting enzyme (ACE) inhibitor, captopril, on basal and NS-induced increases in NE and NPY overflow.
The addition of captopril to the buffer perfusing mesenteric arterial beds from 10- to 12-wk-old SHR for 45 min decreased basal and NS-induced NE overflow (Fig. 6A). In contrast, captopril had no effect on either the basal or NS-induced overflow of NPY (Fig. 6B). In addition, captopril did not significantly alter the NS-induced PP increase (NS − basal) (control = 122.4 ± 21.7 mmHg and captopril = 120 ± 29.5 mmHg).
Fig. 6.
Basal and nerve-stimulated overflow of NE (A) and NPY (B) in the absence (control) and presence of captopril (0.1 μM). Data are plotted as NE overflow (in ng/min) or NPY overflow (in ng/ml). Each bar is the mean ± SE of 5 preparations obtained from 10- to 12-wk-old SHRs. *P < 0.05 compared with control.
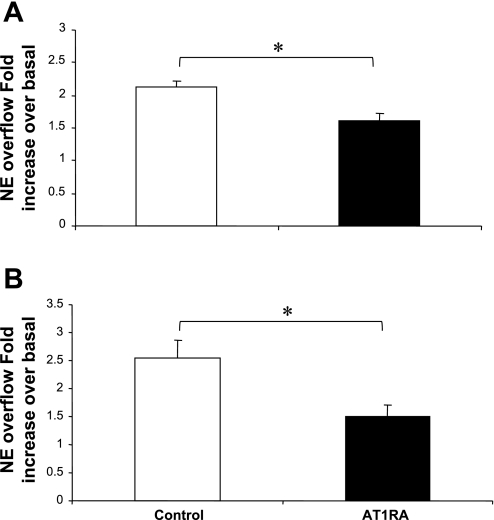
AT1 Receptor Antagonist EMD-66684 Decreased the NS-Induced Overflow of NE and NPY From Preparations From SHRs at 10–12 wk of Age
To further investigate whether or not an endogenous RAS is active in perfused mesenteric arterial beds obtained from 10- to 12-wk-old SHRs, we examined the effect of the AT1 receptor antagonist EMD-66684 on the basal and NS-induced overflow of NE and NPY. EMD-66684 (0.1 μM for 45 min) did not alter the basal overflow of either NE or NPY; however, it attenuated the NS-induced stimulation of both NE and NPY overflow (Fig. 7, A and B). In addition, EMD-66684 also attenuated the NS-induced PP increase (control = 161.2 ± 11.8 mmHg and EMD-66684 = 139.2 ± 7.0 mmHg).
Fig. 7.
Nerve-stimulated overflow of NE (A) and NPY (B) from mesenteric beds of SHRs in the absence (control) or presence of EMD-66684 [0.1 μM; AT1 receptor antagonist (AT1RA)]. Data are plotted as fold increases in NE or NPY overflow. Each bar is the mean ± SE of 5 preparations obtained from 10- to-12-wk-old SHRs. *P < 0.05 compared with control.
DISCUSSION
We observed that NS significantly increases PP along with NPY overflow in the mesenteric arterial beds of both SHR and normotensive WKY rats at all ages. However, SHR preparations showed a stepwise increase in nerve-stimulated PP with increasing ages, whereas in WKY preparations, the NS-induced increase in PP remained constant. In addition, the degree of PP increases in mesenteric beds from SHRs was greater than WKY rats across all ages. The nerve-stimulated NPY overflow did not show the same age-dependent incremental increase as PP in SHR preparations. However, the increase in NPY overflow was greater in 18- to 20-wk-old SHR preparations than in both 4- to 6-wk-old and 10- to 12-wk-old SHR preparations. In comparison, the NS-induced increase in NPY overflow from arteries of 10- to 12-wk-old and 18- to 20-wk-old SHRs was greater compared with those from age-matched WKY rats. The NS-induced increase in PP was greatly attenuated in the presence of either a NPY-Y1 antagonist or an α1-adrenergic receptor antagonist, suggesting a role for both NE and NPY in contributing to the increase in PP.
These findings are consistent with the large body of evidence implicating the SNS as a contributor to the initiation and maintenance of hypertension in a significant portion of the hypertensive population (16, 23–25, 31) and various experimental models such as the SHR (20, 45, 69). There is evidence that increased sympathetic nerve density (28, 33), altered neuronal NE reuptake (37), diminished arterial baroreflex buffering of sympathetic nerve traffic (41), alterations in modulation by prejunctional receptors, and facilitation of NE release by neurohumoral factors such as ANG II (74) all contribute to the development and maintenance of hypertension. However, the precise causal mechanisms leading to the increased sympathetic activity are poorly understood.
The increase in NPY overflow and PP from SHR preparations upon NS is consistent with our previous observations that nerve-stimulated NE overflow is also increased from SHR blood vessels compared with normotensive controls (68). The difference in the increase in PP and NPY overflow from mesenteric arterial beds of 10- to 12-wk-old and 18- to 20-wk-old SHRs compared with WKY rats is consistent with NPY playing a role in the increase in PP. The fact that ANG II did not produce an increase in the NS-induced change in PP or NPY release from mesenteric arterial beds of WKY rats is in contrast to what we observed from another normotensive strain, the Sprague-Dawley rat. In Sprague-Dawley rats, there was an increase in both PP and NPY release, but it was significantly less than from SHRs. There seems to be a spectrum of effects, therefore, going from no response in WKY rats to some response in Sprague-Dawley and the greatest increase in PP and NPY release from mesenteric beds obtained from SHRs.
In addition, we observed that ANG II produced a significant increase in basal NPY overflow from mesenteric arteries of hypertensive SHRs (10–12 and 18–20 wk old) and nerve-stimulated NPY overflow from SHR arteries of all ages. In contrast, ANG II had no effect on NPY overflow in WKY preparations of any age group. ANG II had a greater effect on NPY overflow from SHR arteries than WKY arteries at all ages and concentrations. However, the effect of ANG II on NPY overflow from SHR preparations did not appear to be concentration dependent. This could be a result of the breakdown of ANG II by ACE-2. We included protease inhibitor cocktail in the perfusion buffer to prevent the breakdown of ANG II; however, there was no specific ACE-2 inhibitor present in this cocktail. This newly discovered enzyme is reported to have a high affinity for ANG II and results in its rapid breakdown (7, 9, 54), which could explain the lack of additional effect by the higher concentration of ANG II. The effect of ANG II on NPY release is consistent with results reported by Pernow and Lundberg (56), who observed an enhancement of NPY release from the pig kidney upon stimulation of ANG II receptors, as well as results of Cavadas et al. (10) and Grouzmann et al. (34), who demonstrated that ANG II enhances NPY release from models of sympathetic nerve cultures. It is also consistent with observations on plasma and tissue levels of NPY after the administration of ACE inhibitors (13, 73). These results support numerous other reports of the facilitatory effect of ANG II on sympathetic neurotransmission and stimulation-induced NE release in other tissues such as the dog hindlimb (74), rat caudal artery (14), rat and human kidneys (60, 61), rat and guinea pig atria (6), rat and rabbit mesenteric arteries (2, 3), rabbit thoracic aorta (52), rat and rabbit vas deferens (14, 63), human atria (21), rat prostate (26), and pithed rat (4, 5).
To our knowledge, this is the first report demonstrating that ANG II significantly enhances both basal and evoked overflow of NPY from blood vessels of SHRs compared with normotensive control rats. We conclude that ANG II can increase the overflow of NPY in a manner similar to NE in SHR blood vessels. This increased sensitivity to the modulatory effect of ANG II on the release of these sympathetic cotransmitters could contribute to their elevated circulating level, which has been repetitively demonstrated in vivo in the SHR as well as in human hypertensive patients.
We expected that ANG II would significantly enhance the NS-induced NPY overflow from SHR preparations compared with WKY preparations. However, we were surprised that ANG II had such a pronounced effect on the basal NPY overflow from 10- to 12-wk-old and 18- to 20-wk-old preparations (Fig. 2, B and C). This effect was clearly greater than that in age-matched WKY rats. It is also of interest that ANG II did not have an effect on basal NPY overflow from mesenteric beds of 4- to 6-wk-old SHR. However, upon NS, ANG II facilitated NPY overflow from prehypertensive SHR preparations in a manner similar to those of hypertensive 10- to 12-wk-old and 18- to 20-wk-old SHR (Fig. 3B). This suggests that under resting conditions, prehypertensive animals behave similarly to their normotensive controls; however, upon intense stimulation (such as stress, etc.), their response is exaggerated and could contribute to the cascade of events that lead to hypertensive disease.
ANG II also enhanced the NS-induced increase in PP to a greater degree in arterial beds of SHRs than age-matched WKY controls (Table 3). We conclude that SHRs display an increased sensitivity to the modulatory effects of ANG II on NPY overflow compared with age-matched normotensive controls (this study) and NE overflow (69), which is translated to greater increases in PP and consequently increases in blood pressure in vivo.
The present findings add to the body of literature reporting that there are dysfunctions or alterations in the modulation of sympathetic neurotransmitter release from blood vessels obtained from the SHR (3, 36, 66, 67, 73). For example, there are reports from our laboratory and others demonstrating that the prejunctional inhibition of NE release by activation of α2-, adenosine, and NPY receptors is attenuated in mesenteric preparations from SHRs, whereas prejunctional β2-receptor facilitatory mechanisms are enhanced, resulting in a net increase in neurotransmitter release (37, 64, 65, 70).
ANG II can activate two separate receptors: namely, AT1 and AT2, which have been further subdivided into AT1A, AT1B, AT2A, and AT2B receptors by molecular cloning studies. It is well established that the direct effect of ANG II on vascular smooth muscle contraction and the increased release of aldosterone from the adrenal cortex is via the activation of the AT1 receptor. There is controversy regarding the receptor mediating the enhancing effect of ANG II on sympathetic neurotransmission and NE release. The majority of studies implicate the AT1 receptor. For instance, the enhancement of NE overflow by ANG II was blocked by AT1 receptor antagonists but not by AT2 receptor antagonists in several preparations, such as rat and guinea pig atria (6, 29), the isolated rat mesenteric artery (3), the pithed rat (4, 5, 53), the mesenteric artery of the rabbit (2), etc. However, other studies are at variance with the above data and implicate both AT1 and AT2 receptors. For instance, in the rat caudal artery, both the AT1 receptor antagonist losartan and the AT2 receptor antagonist PD-123319 blocked the enhancing effect of ANG II on NE overflow (14, 15). Moreover, the enhancement of the twitch response to sympathetic NS by ANG II in the rat vas deferens was blocked by both antagonists (14). Similar evidence for the involvement of both receptor subtypes was obtained in human atria (21) and the rat prostate (26). Finally, Trachte et al. (63) reported that both losartan and PD-123319 failed to abolish the enhancement of noradrenergic transmission by ANG II in the isolated rabbit vas deferens. These studies suggest that the ANG II receptor mediating the facilitatory effect on the release of NE varies in different preparations, tissues, and species.
In the present study, we examined the effect of the AT1 receptor antagonist EMD-66684 and AT2 receptor antagonist PD-123319 on ANG II-induced increases in basal and nerve-stimulated NPY overflow from mesenteric arterial beds of 10- to 12-wk-old SHRs. The addition of either antagonist completely attenuated the ANG II-induced increase in the overflow of NPY (P < 0.01). EMD-66684 also attenuated the effect of ANG II on PP change (P < 0.01); however, PD-123319 did not. As mentioned above, the effects of ANG II on NE overflow from the rat mesenteric artery seem to be mediated by the AT1 receptor. In addition, Diniz et al. (19) reported that mesenteric arterial sections obtained from SHRs display a vast reduction in AT2 receptor labeling compared with WKY controls. AT2 receptor activation is thought to result in the stimulation of nitric oxide (NO) release (1, 18), and we have previously shown that NO can deactivate NE, resulting in alterations in PP and neurotransmitter overflow (38, 39). Therefore, the antagonism of AT2 receptors should result in a decrease in NO, which, when coupled with the unopposed activation of AT1 receptors by ANG II, results in a net increase in NE overflow. Therefore, this increased NE inhibits the release of NPY by acting on prejunctional α2-receptors, explaining the inhibition of ANG II-induced NPY release by the AT2 receptor antagonist. This is supported by our results showing that ANG II enhanced the release of NE in the presence of the AT2 receptor antagonist (Fig. 5).
Components of the RAS have been identified in blood vessels and other tissues (9, 27, 54). In the present study, we observed that ANG II increased basal NPY overflow from SHR mesenteric arterial beds. Therefore, we investigated the presence of an active local RAS at the sympathetic vascular neuroeffector junction. The strategies used were to prevent the endogenous formation of ANG II using an ACE inhibitor and to block the effect of any endogenously formed ANG II on its receptor with EMD-66684 (an AT1 receptor antagonist). We reasoned that if endogenous ANG II were exerting a facilitatory effect on sympathetic neurotransmission, these treatments should inhibit or attenuate these effects. The AT1 receptor antagonist EMD-66684 was shown to attenuate NS-induced increases in the overflow of NE and NPY as well as PP. In addition, captopril decreased basal and NS-induced increases in NE but not NPY overflow. This is consistent with the presence of an active endogenous RAS in the perfused mesenteric arterial bed.
We believe that these results significantly add to the evidence that NPY released from sympathetic nerves, and the actions of ANG II on sympathetic neurotransmission, play a role in the development and maintenance of hypertension. While the data in support of the involvement of NPY in hypertension is extensive (11, 12, 32, 35, 48, 50, 55), questions still remain. To date, standard antihypertensive therapy does not specifically target NPY. ACE inhibitors and AT1 receptor antagonists are currently widely used treatments for essential hypertension. However, the exact mechanism behind their blood pressure-lowering abilities remains poorly understood and even controversial. Our data suggest that if ANG II can enhance NPY overflow vasoconstriction in hypertensive humans like it does in SHRs, interfering with this effect of ANG II (either by altering its synthesis or blocking the receptor mediating its function) could be one mechanism resulting in the blood pressure-lowering ability of ACE inhibitors and AT1 receptor antagonists. In addition, we believe that regulating NPY activity in hypertensive subjects could be of benefit in preventing the progression of the end-organ damage caused by high blood pressure and, as a result, reduce the morbidity and mortality of hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-60260 (to T. C. Westfall) and by the American Heart Association (to M. Byku).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension 42: 600–604, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Balt JC, Belterman CN, Mathy MJ, Nap A, Baartscheer A, Pfaffendorf M, Van Zwieten PA. Decreased facilitation by angiotensin II of noradrenergic neurotransmission in isolated mesenteric artery of rabbits with chronic heart failure. J Cardiovasc Pharmacol 41: 356–362, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Balt JC, Mathy MJ, Nap A, Pfaffendorf M, van Zwieten PA. Effect of the AT1-receptor antagonists losartan, irbesartan, and telmisartan on angiotensin II-induced facilitation of sympathetic neurotransmission in the rat mesenteric artery. J Cardiovasc Pharmacol 38: 141–148, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Balt JC, Mathy MJ, Nap A, Pfaffendorf M, van Zwieten PA. Involvement of the AT2-receptor in angiotensin II-induced facilitation of sympathetic neurotransmission. J Renin Angiotensin Aldosterone Syst 3: 181–187, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Balt JC, Mathy MJ, Pfaffendorf M, van Zwieten PA. Inhibition of angiotensin II-induced facilitation of sympathetic neurotransmission in the pithed rat: a comparison between losartan, irbesartan, telmisartan, and captopril. J Hypertens 19: 465–473, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brasch H, Sieroslawski L, Dominiak P. Angiotensin II increases norepinephrine release from atria by acting on angiotensin subtype 1 receptors. Hypertension 22: 699–704, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 15: 166–169, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 39: 519–524, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24: 261–271, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cavadas C, Grand D, Mosimann F, Cotrim MD, Fontes Ribeiro CA, Brunner HR, Grouzmann E. Angiotensin II mediates catecholamine and neuropeptide Y secretion in human adrenal chromaffin cells through the AT1 receptor. Regul Pept 111: 61–65, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Henderson K, Beinfeld MC, Westfall TC. Alterations in blood pressure of normotensive and hypertensive rats following intrathecal injections of neuropeptide Y. J Cardiovasc Pharmacol 12: 473–478, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Chen XL, Knuepfer MM, Westfall TC. Hemodynamic and sympathetic effects of spinal administration of neuropeptide Y in rats. Am J Physiol Heart Circ Physiol 259: H1674–H1680, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Corder R Effect of lisinopril on tissue levels of neuropeptide Y in normotensive and spontaneously hypertensive rats. J Hum Hypertens 14: 381–384, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Cox SL, Ben A, Story DF, Ziogas J. Evidence for the involvement of different receptor subtypes in the pre- and postjunctional actions of angiotensin II at rat sympathetic neuroeffector sites. Br J Pharmacol 114: 1057–1063, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox SL, Story DF, Ziogas J. Angiotensin II receptors involved in the enhancement of noradrenergic transmission in the caudal artery of the spontaneously hypertensive rat. Br J Pharmacol 119: 965–975, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Champlain J Pre- and postsynaptic adrenergic dysfunctions in hypertension. J Hypertens Suppl 8: S77–S85, 1990. [PubMed] [Google Scholar]
- 17.de Champlain J, Karas M, Toal C, Nadeau R, Larochelle P. Effects of antihypertensive therapies on the sympathetic nervous system. Can J Cardiol 15, Suppl A: 8A–14A, 1999. [PubMed] [Google Scholar]
- 18.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000. [PubMed] [Google Scholar]
- 19.Diniz C, Leal S, Logan K, Rocha-Pereira C, Soares AS, Rocha E, Goncalves J, Fresco P. Immunohistochemical localization of angiotensin II receptor types 1 and 2 in the mesenteric artery from spontaneously hypertensive rats. Microsc Res Tech 70: 677–681, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Dubinion JH, Mi Z, Jackson EK. Role of renal sympathetic nerves in regulating renovascular responses to angiotensin II in spontaneously hypertensive rats. J Pharmacol Exp Ther 317: 1330–1336, 2006. [DOI] [PubMed] [Google Scholar]
- 21.El Muayed M, Stegbauer J, Oberhauser V, Vonend O, Rump LC. AT1 and AT2-receptor antagonists inhibit Ang II-mediated facilitation of noradrenaline release in human atria. J Cardiovasc Pharmacol 43: 318–324, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Esler M Sympathetic nervous system: contribution to human hypertension and related cardiovascular disease. J Cardiovasc Pharmacol 26: S24–S28, 1995. [PubMed] [Google Scholar]
- 23.Esler M Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol 26, Suppl 2: S24–S28, 1995. [PubMed] [Google Scholar]
- 24.Esler MD, Lambert GW, Ferrier C, Kaye DM, Wallin BG, Kalff V, Kelly MJ, Jennings GL. Central nervous system noradrenergic control of sympathetic outflow in normotensive and hypertensive humans. Clin Exp Hypertens 17: 409–423, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol Regul Integr Comp Physiol 268: R278–R285, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Fabiani ME, Sourial M, Thomas WG, Johnston CI, Johnston CI, Frauman AG. Angiotensin II enhances noradrenaline release from sympathetic nerves of the rat prostate via a novel angiotensin receptor: implications for the pathophysiology of benign prostatic hyperplasia. J Endocrinol 171: 97–108, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira JC, Bacurau AV, Evangelista FS, Coelho MA, Oliveira EM, Casarini DE, Krieger JE, Brum PC. The role of local and systemic renin angiotensin system activation in a genetic model of sympathetic hyperactivity-induced heart failure in mice. Am J Physiol Regul Integr Comp Physiol 294: R26–R32, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto C, Ito M, Sekine I. Noradrenergic and neuropeptide Y-immunoreactive nerves in the pancreatic islets of spontaneously hypertensive rats. Regul Pept 47: 171–178, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Gironacci MM, Adler-Graschinsky E, Pena C, Enero MA. Effects of angiotensin II and angiotensin-(1–7) on the release of [3H]norepinephrine from rat atria. Hypertension 24: 457–460, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS Plasma norepinephrine in essential hypertension. A study of the studies. Hypertension 3: 48–52, 1981. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DS, Lake CR, Chernow B, Ziegler MG, Coleman MD, Taylor AA, Mitchell JR, Kopin IJ, Keiser HR. Age-dependence of hypertensive-normotensive differences in plasma norepinephrine. Hypertension 5: 100–104, 1983. [DOI] [PubMed] [Google Scholar]
- 32.Gradin K, Li JY, Anderson O, Simonsen U. Enhanced neuropeptide Y immunoreactivity and vasoconstriction in mesenteric small arteries from spontaneously hypertensive rats. J Vasc Res 40: 252–265, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Gradin KA, Li JY, Andersson O, Simonsen U. Enhanced neuropeptide Y immunoreactivity and vasoconstriction in mesenteric small arteries from spontaneously hypertensive rats. J Vasc Res 40: 252–265, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Grouzmann E, Werffeli-George P, Fathi M, Burnier M, Waeber B, Waeber G. Angiotensin-II mediates norepinephrine and neuropeptide-Y secretion in a human pheochromocytoma. J Clin Endocrinol Metab 79: 1852–1856, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Chen X, Cox B, Yang CL, Wu YM, Naes L, Westfall T. Role of neuropeptide Y in cold stress-induced hypertension. Peptides 19: 351–358, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Kawasaki H, Cline WH Jr, Su C. Enhanced angiotensin-mediated facilitation of adrenergic neurotransmission in spontaneously hypertensive rats. J Pharmacol Exp Ther 221: 112–116, 1982. [PubMed] [Google Scholar]
- 37.Kawasaki H, Cline WH Jr, Su C. Enhanced presynaptic beta adrenoceptor-mediated modulation of vascular adrenergic neurotransmission in spontaneously hypertensive rats. J Pharmacol Exp Ther 223: 721–728, 1982. [PubMed] [Google Scholar]
- 38.Kolo LL, Westfall TC, Macarthur H. Modulation of neurotransmitter release by NO is altered in mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 287: H1842–H1847, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 286: H296–H303, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol 290: H1706–H1712, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res 71: 129–138, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JM Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 48: 113–178, 1996. [PubMed] [Google Scholar]
- 43.Majewski H, Hedler L, Schurr C, Starke K. Modulation of noradrenaline release in the pithed rabbit: a role for angiotensin II. J Cardiovasc Pharmacol 6: 888–896, 1984. [DOI] [PubMed] [Google Scholar]
- 44.Meldrum MJ, Westfall TC. Angiotensin facilitation of 3H-norepinephrine release in central tissue of spontaneously hypertensive rats but not in Wistar-Kyoto rats: effects of sodium depletion. J Cardiovasc Pharmacol 8: 582–587, 1986. [PubMed] [Google Scholar]
- 45.Meldrum MJ, Westfall TC. Comparison of norepinephrine release in hypertensive rats: I. Hypothalamic and brainstem tissues. Clin Exp Hypertens A 8: 201–219, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Meldrum MJ, Xue CS, Badino L, Westfall TC. Angiotensin facilitation of noradrenergic neurotransmission in central tissues of the rat: effects of sodium restriction. J Cardiovasc Pharmacol 6: 989–995, 1984. [PubMed] [Google Scholar]
- 47.Meldrum MJ, Xue CS, Badino L, Westfall TC. Effect of sodium depletion on the release of [3H]norepinephrine from central and peripheral tissue of Wistar-Kyoto and spontaneously hypertensive rats. J Cardiovasc Pharmacol 7: 59–65, 1985. [DOI] [PubMed] [Google Scholar]
- 48.Mezzano V, Donoso V, Capurro D, Huidobro-Toro JP. Increased neuropeptide Y pressor activity in Goldblatt hypertensive rats: in vivo studies with BIBP 3226. Peptides 19: 1227–1232, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Miller DW, Tessel RE. Age-dependent hyperresponsiveness of spontaneously hypertensive rats to the pressor effects of intravenous neuropeptide Y (NPY): role of mode of peptide administration and plasma NPY-like immunoreactivity. J Cardiovasc Pharmacol 18: 647–656, 1991. [DOI] [PubMed] [Google Scholar]
- 50.Moreau P, de Champlain J, Yamaguchi N. Alterations in circulating levels and cardiovascular tissue content of neuropeptide Y-like immunoreactivity during the development of deoxycorticosterone acetate-salt hypertension in the rat. J Hypertens 10: 773–780, 1992. [PubMed] [Google Scholar]
- 51.Morris J, Gibbins IL. Co-Transmission and Neuromodulation. Reading: Hardwood Academic, 1992.
- 52.Nap A, Balt JC, Pfaffendorf M, Van Zwieten PA. Sympatholytic properties of several AT1-receptor antagonists in the isolated rabbit thoracic aorta. J Hypertens 20: 1821–1828, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Nap A, Balt JC, Pfaffendorf M, Zwieten PA. No involvement of the AT2-receptor in angiotensin II-enhanced sympathetic transmission in vitro. J Renin Angiotensin Aldosterone Syst 4: 100–105, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell Mol Life Sci 60: 350–377, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pernow J, Lundberg JM. Modulation of noradrenaline and neuropeptide Y (NPY) release in the pig kidney in vivo: involvement of alpha 2, NPY and angiotensin II receptors. Naunyn Schmiedebergs Arch Pharmacol 340: 379–385, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Potter E Neuropeptide Y as an autonomic transmitter. In: Novel Peripheral Neurotransmitters, edited by Bell C. New York: Pergamon, 1991.
- 58.Reinhart GA, Lohmeier TE, Hord CE Jr. Hypertension induced by chronic renal adrenergic stimulation is angiotensin dependent. Hypertension 25: 940–949, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Roizen MF, Weise V, Grobecker H, Kopin IJ. Plasma catecholamines and dopamine-beta-hydroxylase activity in spontaneously hypertensive rats. Life Sci 17: 283–288, 1975. [DOI] [PubMed] [Google Scholar]
- 60.Rump LC, Bohmann C, Schaible U, Schultze-Seemann W, Schollmeyer PJ. Beta-adrenergic, angiotensin II, and bradykinin receptors enhance neurotransmission in human kidney. Hypertension 26: 445–451, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Rump LC, Schuster MJ, Wilde K, Schollmeyer P. Modulation of noradrenaline release from rat cortical kidney slices: effects of angiotensin I and II. Br J Clin Pharmacol 30, Suppl 1: 168S–170S, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69: 864–989, 1989. [DOI] [PubMed] [Google Scholar]
- 63.Trachte GJ, Heller LJ. Adenosine receptor antagonism attenuates angiotensin II effects on adrenergic neurotransmission in the rabbit isolated vas deferens. J Pharmacol Exp Ther 253: 490–496, 1990. [PubMed] [Google Scholar]
- 64.Tsuda K, Kimura K, Shima H, Nishio I, Masuyama Y. Presynaptic alpha 2-adrenoceptor-mediated modulation of norepinephrine release from vascular adrenergic neurons in reduced renal mass salt hypertensive rats. Clin Exp Pharmacol Physiol 19: 531–535, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Tsuda K, Kuchii M, Nishio I, Masuyama Y. Neurotransmitter release, vascular responsiveness and their suppression by Ca-antagonist in perfused mesenteric vasculature of DOCA-salt hypertensive rats. Clin Exp Hypertens A 8: 259–275, 1986. [DOI] [PubMed] [Google Scholar]
- 66.Westfall TC, Carpentier S, Chen X, Beinfeld MC, Naes L, Meldrum MJ. Prejunctional and postjunctional effects of neuropeptide Y at the noradrenergic neuroeffector junction of the perfused mesenteric arterial bed of the rat. J Cardiovasc Pharmacol 10: 716–722, 1987. [DOI] [PubMed] [Google Scholar]
- 67.Westfall TC, Han SP, Chen XL, Del Valle K, Curfman M, Ciarleglio A, Naes L. Presynaptic peptide receptors and hypertension. Ann NY Acad Sci 604: 372–388, 1990. [DOI] [PubMed] [Google Scholar]
- 68.Westfall TC, Meldrum MJ. Alterations in the release of norepinephrine at the vascular neuroeffector junction in hypertension. Annu Rev Pharmacol Toxicol 25: 621–641, 1985. [DOI] [PubMed] [Google Scholar]
- 69.Westfall TC, Meldrum MJ, Carpentier S, Naes L, Zhang SQ. Alterations in the release of norepinephrine at the vascular neuroeffector junction in hypertension. Blood Vessels 24: 94–99, 1987. [DOI] [PubMed] [Google Scholar]
- 70.Westfall TC, Xue CY, Carpentier S, Meldrum MJ. Modulation of Noradrenaline Release by Presynaptic Adrenoceptors in Experimental Hypertension. London: MacMillan, 1985.
- 71.Yanagawa T, Yamamoto N, Suzuki A. The modification of the responses to noradrenaline and acetylcholine by aging and the effects of some smooth muscle relaxants on the smooth muscle contractile drugs. Experiment in the isolated vas deferens of SHRSP and Wistar-Kyoto rats. Jpn Heart J 19: 642–643, 1978. [DOI] [PubMed] [Google Scholar]
- 72.Ye S, Zhong H, Yanamadala V, Campese VM. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens 15: 717–724, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Zeng C, Wang X, Liu G, Yang C. Effects of ACE inhibitor and beta-adrenergic blocker on plasma NPY and NPY receptors in aortic vascular smooth muscle cells from SHR and WKY rats. Neuropeptides 36: 353–361, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerman BG, Gomer SK, Liao JC. Action of angiotensin on vascular adrenergic nerve endings: facilitation of norepinephrine release. Fed Proc 31: 1344–1350, 1972. [PubMed] [Google Scholar]
- 75.Zimmerman BG, Gomez J. Increased response to sympathetic stimulation in the cutaneous vasculature in presence of angiotensin. Int J Neuropharmacol 4: 185–193, 1965. [DOI] [PubMed] [Google Scholar]
- 76.Zukowska-Grojec Z, Golczynska M, Shen GH, Torres-Duarte A, Haass M, Wahlestedt C, Myers AK. Modulation of vascular function by neuropeptide Y during development of hypertension in spontaneously hypertensive rats. Pediatr Nephrol 7: 845–852, 1993. [DOI] [PubMed] [Google Scholar]