Abstract
To determine whether conduit artery size affects functional responses, we compared the magnitude, time course, and eliciting shear rate stimulus for flow-mediated dilation (FMD) in healthy men (n = 20; 31 ± 7 yr). Upper limb (brachial and radial) and lower limb (common and superficial femoral) FMD responses were simultaneously assessed, whereas popliteal responses were measured in the same subjects during a separate visit. Glyceryl trinitrate (GTN)-mediated responses were similarly examined. Edge detection and wall tracking of high-resolution B-mode arterial ultrasound images, combined with synchronized Doppler waveform envelope analysis, were used to calculate conduit artery diameter, blood flow, and shear rate continuously across the cardiac cycle. Baseline artery size correlated inversely with the FMD response (r = −0.57, P < 0.001). Within-artery comparisons revealed a significant inverse correlation between artery size and FMD% for the radial (r = −0.66, P = 0.001), brachial (r = −0.55, P = 0.01), and popliteal artery (r = −0.48, P = 0.03), but not for the superficial and common femoral artery. Normalization of FMD responses for differences in eliciting shear rate did not abolish the between-artery relationship for artery function and size (r = −0.48, P < 0.001), suggesting that differences between artery function responses were not entirely due to size-related differences in shear rate. This was reinforced by a significant between-artery correlation for GTN responses and baseline artery size (r = −0.74, P < 0.001). In summary, systematic differences exist in vascular function responses of conduit arteries that differ in size. This raises the possibility that differences in artery size within or between individuals may influence functional responses.
Keywords: flow-mediated dilation, nitroglycerine, arterial diameter, high-resolution ultrasound, Doppler
measures of arterial function have increasingly been adopted as surrogate markers of cardiovascular risk (31). For example, conduit artery vasodilation to a 5-min ischemic period, commonly referred to as flow-mediated dilation (FMD), reflects bioactivity of endothelium-derived nitric oxide (NO) (14, 17), which possesses myriad antiatherogenic effects (31). Endothelial dysfunction can be considered an early and integral manifestation of vascular disease that predicts cardiovascular events (8, 15, 16, 18, 21), whereas improvement in endothelial function impacts significantly on cardiovascular risk (10, 11, 16).
For many years, it has been recognized that baseline artery diameter correlates strongly with the magnitude of the FMD response. It was recently proposed that this relationship might be explained as a consequence of smaller arteries being exposed, by virtue of their size, to a greater shear stress stimulus during reactive hyperemia. In relative terms, this results in enhanced dilator responses compared with arteries of larger dimension (25, 27, 28). Correcting the FMD response to the magnitude of the shear stress stimulus may eliminate the between-subject variability (23). To our knowledge, this well-founded hypothesis has not been systematically examined in humans. In addition, studies on which the relationship between artery size and FMD response are based have primarily been derived from correlations between baseline diameter and FMD responses in cross-sectional studies of single arteries (3, 12, 13, 26).
An alternate explanation for the association between baseline artery diameter and FMD responses may relate to the impact of artery structure per se on dilator responses. It is well established that regional heterogeneity exists between, and within, arterial beds in terms of wall architecture and thickness. This, in turn, may influence functional responses (4). Indeed, Folkow and colleagues (6, 7) suggested as far back as the 1950s that differences in resistance vessel wall-to-lumen ratios might result in nonspecific exaggeration of responses to vasoactive stimuli. These findings raise the possibility that conduit arteries of different size and structure may exhibit differences in function that are not specific to particular signal transduction pathways.
The aim of the present study was to examine the impact of artery size on functional responses in healthy subjects. To this end, we simultaneously examined arteries of different size, within subjects, to the same FMD and glyceryl trinitrate (GTN) stimuli. We hypothesized that diameter size might correlate significantly with FMD responses for reasons other than diameter-related differences in the eliciting shear stress stimulus. We also hypothesized that these correlations would be present using between-artery comparison (i.e., all 5 arteries), but also when analyzing each individual artery using a within-artery comparisons.
METHODS
Subjects
Twenty healthy, recreationally active volunteers (31.4 ± 6.5 yr, 25.2 ± 3.2 kg/m2) were recruited from the community. No subject reported having been diagnosed with cardiovascular disease, diabetes, insulin resistance, or cardiovascular risk factors such as hypercholesterolemia or hypertension. Subjects who smoked or were on medications of any type were excluded. The study procedures were approved by the Ethics Committee of Liverpool John Moores University and adhered to the Declaration of Helsinki, and all subjects gave prior written consent.
Experimental Design
Participants reported three times to the laboratory. During one of these visits, brachial and radial artery FMD responses were examined simultaneously (Fig. 1A). After a resting period of at least 30 min, endothelium-independent dilation of these arteries was examined simultaneously using the sublingual administration of GTN. On a second occasion, popliteal artery FMD and GTN responses were assessed. The third visit involved simultaneous assessment of common and superficial femoral artery diameter and shear rate changes during the FMD response and GTN administration (Fig. 1B). The order of the three measuring days was randomized.
Fig. 1.
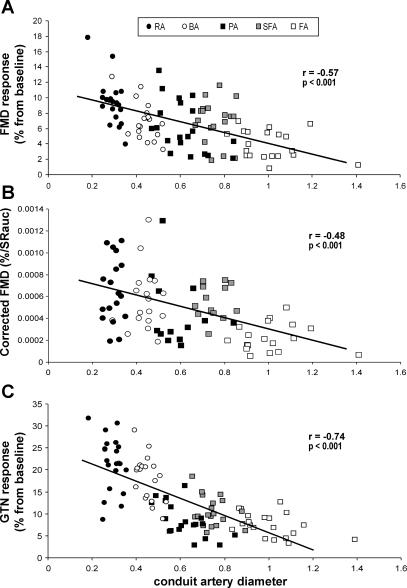
Scatter plot for all 5 artery diameters (RA, radial artery; BA, brachial artery; PA, popliteal artery; SFA, superficial femoral artery; FA, common femoral artery) vs. the relative increase from baseline diameter during flow-mediated dilation (FMD; A), the corrected FMD for shear rate area under the curve (SRAUC; B), and the relative change during glyceryl trinitrate (GTN) response (C) in 20 healthy young subjects.
All measurements were performed under standardized conditions. Diameter and shear rate changes to a 5-min ischemic period were continuously recorded for at least 6 min, whereas at least 10 min were used to record diameter and shear rate following GTN (1). Conduit arteries were chosen based on their differences in dimension and their frequent use to assess conduit artery FMD responses in human studies. All measures were performed at the same time of day, following a ≥6-h fast, ≥8-h abstinence from caffeine, and at least 24 h after strenuous physical activity.
Experimental Procedures
Vascular measurements.
RADIAL AND BRACHIAL ARTERIES.
Patients rested supine with the right arm extended and immobilized with foam, supported at an angle of ∼80° from the torso. Heart rate and mean arterial pressure were determined from an automated sphygmomanometer (Dinamap GE Pro 300V2; Tampa, FL) on the contralateral arm. For assessment of the FMD response, a rapid inflation/deflation pneumatic cuff was positioned on the imaged arm distal to the olecranon process to provide a stimulus to forearm ischemia. A 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (T3000; Terason, Burlington, MA) was used to image the brachial artery in the distal third of the upper arm. Simultaneously, the radial artery was imaged in the upper part of the forearm, above the occluding cuff (Fig. 1A). Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen/arterial wall interface. Continuous Doppler velocity assessment also was also obtained using the Terason and was collected using the lowest possible insonation angle (always <60°), which did not vary during each study. Dynamic range and Doppler gain settings were similar between the machines (22), and simultaneous imaging of two conduit arteries did not lead to interference of the signals in any subject.
COMMON FEMORAL AND SUPERFICIAL FEMORAL ARTERIES.
Patients rested supine with the lower leg slightly elevated while resting on ∼15-cm-thick foam. The rapid inflation/deflation pneumatic cuff was positioned ∼15 cm below the inguinal ligament to induce the 5-min ischemic stimulus. Right common femoral artery images were recorded ∼3 cm proximal to the femoral bifurcation into the deep and superficial femoral artery. Simultaneously, a second Terason was used to image the superficial femoral artery in the proximal third of the thigh, at least 5 cm distal from the bifurcation and above the cuff position (Fig. 1B). Ultrasound B-mode images were used to continuously assess the lumen/arterial wall interface while Doppler velocity assessment was obtained to examine blood flow and calculate shear rate during the 6-min postdeflation period.
POPLITEAL ARTERY.
Patients rested prone with the right knee at an angle of ∼20°. The rapid inflation/deflation pneumatic cuff was positioned on the imaged leg immediately distal to the popliteal fossa to provide the 5-min ischemic stimulus. The popliteal artery was imaged in the popliteal fossa. Ultrasound B-mode images were used to assess the lumen/arterial wall interface while continuous Doppler velocity assessment was obtained to examine blood flow and calculate shear rate.
Endothelium-dependent and -independent dilation.
To examine conduit artery endothelium-dependent dilation via the FMD response, we observed an initial 30-min resting period, with baseline scans assessing resting vessel diameter and flow recorded over the final minute. The occluding cuff was then inflated to >200 mmHg for 5 min. Diameter and flow recordings resumed 30 s before cuff deflation and continued for 6 min thereafter (1). Peak artery diameter, and the time taken to reach this peak, following the release of the occlusion were recorded (see below). After a resting period of 30 min, to allow arterial diameter to return to baseline, another 1-min baseline recording of the conduit artery was made. Subsequently, maximal endothelium-independent dilation was examined using a single spray of sublingual GTN (400 μg), a NO donor, followed by at least a 10-min recording of the diameter images.
Conduit artery diameter and blood flow analysis.
Posttest analysis of the diameter of the five conduit arteries was performed using custom-designed edge-detection and wall-tracking software that is independent of investigator bias (32). Briefly, the echo Doppler signal was real-time encoded and stored as a digital file. Subsequent software analysis of these data was performed at 30 Hz using an icon-based graphic programming language and toolkit (LabView 6.02; National Instruments, Austin, TX). The initial phase of image analysis involved the identification of regions of interest (ROI) on the first frame of every individual study. These ROIs allowed automated calibration for diameters on the B-mode image and velocities on the Doppler strip. A ROI was then drawn around the optimal area of the B-mode image, and within this ROI, a pixel-density algorithm automatically identified the angle-corrected near and far wall e-lines for every pixel column within the ROI. The algorithm begins by dividing the ROI into an upper half, containing the near wall lumen-intima interface, and a lower half, containing the far wall interfaces. The near wall intimal edge is identified by a Rake routine that scans from the bottom to the top of the upper half of the ROI. The position of the edge is established by determining the point where the pixel intensity changes most rapidly. Typical B-mode ROIs therefore contained ∼200–300 diameter measures per frame, the average of which was calculated and stored. This process occurred at 30 frames/s.
A final ROI was drawn around the Doppler waveform and automatically detected the peak of the waveform, and the mean velocity across the cardiac cycle was calculated. The mean diameter measure derived from within the B-mode ROI (above) was synchronized with the velocity measure derived from the Doppler ROI at 30 Hz. Ultimately, from these synchronized diameter and velocity data, blood flow [the product of cross-sectional area (CSA) and mean Doppler velocity (υ)] and shear rate (4 times velocity divided by diameter) were calculated at 30 Hz (32). All data were written to file and retrieved for analysis in a custom-designed analysis package. We have shown that reproducibility of diameter measurements using this semiautomated software is significantly better than manual methods, reduces observer error significantly, and possesses an intraobserver coefficient of variance of 6.7%. Furthermore, our method of blood flow assessment is closely correlated with actual flow through a “phantom” arterial flow system (9).
Data Analysis
Baseline diameter, flow, and shear rate were calculated as the means of data acquired across the 1 min preceding the cuff inflation period. Peak diameter following cuff deflation was automatically detected according to an algorithm that identified the maximum bracket of data subsequent to performance of a moving window smoothing function. This smoothing routine calculates the median value from 100 consecutive samples, before the window shifts to the next bracket of data, which shares 20% overlap with the preceding bracket. The maximum value of all the calculated median values is then automatically detected and chosen to represent the peak of the diameter curve. FMD% was calculated as the percentage rise of this peak diameter from the preceding baseline diameter. The time to peak diameter (in seconds) was calculated from the point of cuff deflation to the maximum postdeflation diameter. Calculation of FMD% and time to peak were therefore observer independent and based on standardized algorithms applied to data that had undergone automated edge detection and wall tracking.
In accordance with recent findings (24), we expressed FMD data normalized to the shear rate stimulus responsible for endothelium-dependent FMD. The postdeflation shear rate data, derived from simultaneously acquired velocity and diameter measures at 30 Hz, were exported to a spreadsheet, and the area under the shear rate curve (AUC) was calculated for data up to the point of maximal postdeflation diameter (FMD) for each individual. In this way, an individual's FMD was normalized to the area under the shear rate curve between the point of deflation and maximal dilation for that individual.
Statistics
Statistical analyses were performed using SPSS 14.0 software (SPSS, Chicago, IL). All data are reported as means (SD), and statistical significance was assumed at P < 0.05. Pearson's correlation coefficients, repeated-measures ANOVA, and post hoc paired t-tests were used to assess the impact of artery size on functional responses (between and within different arteries) during the FMD response and after sublingual GTN administration. Cook's distance test was used to identify potential statistical outliers that influence the linear regression coefficient analysis. A linear regression model was used to examine the contribution of baseline diameter and the shear rate AUC (independent variables) to the magnitude of the FMD response (dependent variable).
RESULTS
Blood pressure and heart rate were not significantly affected by inflating the occlusion cuff (Table 1). Baseline resting diameter and blood flow differed between arteries (P < 0.001), whereas shear rate demonstrated a post hoc difference between the femoral and popliteal artery (Table 2). Administration of GTN induced a small but significant decrease in diastolic blood pressure, compensated by a small increase in heart rate (Table 1). Cook's distance test indicated no data points in any of the parameters in this study, which significantly influenced regression coefficients.
Table 1.
Subject characteristics of participants and effects of flow- and glyceryl trinitrate-mediated dilation on hemodynamic variables
| Baseline | FMD | P Value | Baseline | GTN | P Value | |
|---|---|---|---|---|---|---|
| Systolic blood pressure, mmHg | 117±10 | 117±9 | NS | 117±9 | 117±10 | NS |
| Diastolic blood pressure, mmHg | 67±8 | 68±8 | NS | 68±8 | 64±9 | <0.001 |
| Heart rate, beats/min | 52±10 | 51±9 | NS | 51±9 | 54±9 | <0.001 |
Values are means (SD) of subject characteristics of the participants (n = 20) and effect of flow (FMD)- and glyceryl trinitrate (GTN)-mediated dilation on hemodynamic variables. NS, no significant difference.
Table 2.
Effects of FMD and GTN administration on conduit artery responses in healthy young individuals
| FA | SFA | PA | BA | RA | P Value | |
|---|---|---|---|---|---|---|
| Baseline characteristics | ||||||
| Diameter, mm | 10.1±1.4f | 7.7±0.7f | 6.2±1.0f | 4.4±0.5f | 2.9±0.4f | <0.001 |
| Blood flow, ml/min | 618±394f | 184±78f | 82±66a,b,e | 61±37a,b,e | 17±14f | <0.001 |
| Shear rate, s | 48±30c | 36±15 | 29±23a | 64±57 | 52±44 | 0.03 |
| Flow-mediated dilation | ||||||
| SRAUC, s | 19489±10040 | 14541±7004 | 16132±8743 | 14687±7383 | 15974±7654 | 0.01 |
| Peak diameter, mm | 10.5±1.4f | 8.1±0.7f | 6.6±1.0f | 4.7±0.5f | 3.2±0.4f | <0.001 |
| Absolute diameter change, mm | 0.4±0.2 | 0.5±0.2d,e | 0.4±0.2 | 0.3±0.1b | 0.3±0.1b | <0.001 |
| Time to peak FMD, s | 243±79b,d,e | 172±80a,d | 181±85d | 85±28a,b,c | 107±64a | <0.001 |
| FMD, % | 3.9±1.9b,d,e | 6.9±2.7a,e | 6.1±3.3e | 7.0±2.4a,e | 9.4±3.0f | <0.001 |
| Corrected FMD, %/SRAUC × 10−4 | 2.2±1.3b | 5.5±1.5a | 4.5±3.2 | 5.7±2.8 | 6.5±3.0 | 0.004 |
| GTN-mediated dilation | ||||||
| Time to peak GTN dilation, s | 431±145 | 499±99 | 416±158 | 509±101 | 463±115 | 0.03 |
| GTN-induced peak dilation, mm | 10.9±1.3f | 8.3±0.7f | 6.7±0.9f | 5.2±0.5f | 3.5±0.5f | <0.001 |
| Absolute diameter change, mm | 0.7±0.3c | 0.8±0.2c | 0.5±0.2b,d | 0.8±0.2c,e | 0.6±0.2d | <0.001 |
| GTN-induced peak dilation, % | 7.5±2.7b,d,e | 10.5±3.3a,d,e | 8.5±3.8d,e | 18.4±5.0a,b,c | 21.5±6.3a,b,c | <0.001 |
Values are means (SD) of FMD and GTN responses in radial (RA), brachial (BA), popliteal (PA), superficial femoral (SFA), and common femoral (FA) conduit arteries in healthy young individuals (n = 20). Post hoc tests (with Bonferroni correction for multiple comparisons) indicated significant differences from FA (
P < 0.05), SFA (
P < 0.05), PA (
P < 0.05), BA (
P < 0.05), RA (
P < 0.05), or all other arteries (
P < 0.05). Postocclusion shear rate area under the curve (AUC) data from 15 scans were excluded due to the inability to adequately track velocity responses through the entire postischemic period.
Impact of Baseline Diameter on FMD: Comparison of Different Arteries Within Subjects
Endothelium-dependent vasodilation (FMD).
UPPER LIMB.
Forearm occlusion for 5 min resulted in simultaneous vasodilation of the brachial as well as the radial artery. The radial artery showed a significantly larger FMD response (FMD%) than the brachial artery but also compared with the lower limb conduit arteries (post hoc, P < 0.05; Table 2).
LOWER LIMB.
Thigh occlusion for 5 min resulted in simultaneous vasodilation of the common and superficial femoral arteries. In keeping with the upper limb responses, the smaller artery (i.e., superficial femoral) showed a significantly larger FMD% (post hoc, P < 0.05; Table 2). The popliteal artery FMD response was not different from that of the common or superficial femoral artery (Table 2).
Endothelium-independent vasodilation (GTN).
UPPER LIMB.
The GTN-induced dilation differed significantly among the five arteries. GTN induced a larger dilation of the smaller radial artery compared with the brachial artery, but this did not reach statistical significance (post hoc, P = 0.054; Table 2). The dilation in the arteries in the upper limb were significantly larger than that observed in the lower limb (Table 2).
LOWER LIMB.
Administration of GTN induced larger dilation of the superficial femoral artery relative to the larger common femoral vessel (post hoc, P < 0.05; Table 2). Popliteal artery dilation to GTN was not different from the response reported for the femoral and superficial femoral artery (Table 2).
Between-artery correlations.
ENDOTHELIUM-DEPENDENT VASODILATION (FMD).
When FMD responses were plotted against baseline diameter across all arteries, a significant inverse correlation was evident (r = −0.57, r2 = 0.33, P < 0.001; Fig. 1A). Also, when the absolute diameter change during the FMD response was plotted against baseline artery diameter, a significant correlation was found (r = 0.29, r2 = 0.09, P < 0.01). To correct the FMD response for the eliciting stimulus, shear rate AUC from cuff deflation to the point of peak dilation was calculated (Table 2) (24). The shear rate AUC-corrected FMD responses remained significantly inversely correlated with baseline diameter (r = −0.48, r2 = 0.23, P < 0.001; Fig. 1B). A significant correlation also was found between resting artery diameter and time to peak dilation during the FMD response (see Fig. 3). The linear regression revealed a significant β-coefficient for baseline diameter (−0.61, P < 0.001), whereas the shear rate AUC was not significant (0.10, P = 0.29) in relation to the magnitude of FMD% across arteries.
ENDOTHELIUM-INDEPENDENT VASODILATION (GTN).
A strong and significant inverse correlation was found between artery size and magnitude of GTN response (Fig. 1C), whereas no correlation was found between baseline artery size and time of peak dilation (r = −0.16, r2 = 0.03, P = 0.12).
Within-artery correlations.
ENDOTHELIUM-DEPENDENT VASODILATION (FMD).
Comparison between baseline artery size and magnitude of the FMD response within arteries revealed a significant correlation for the radial, brachial, and popliteal arteries, whereas the superficial and common femoral arteries did not exhibit a significant correlation (Fig. 2A). In addition, the slope of the linear regression line demonstrated a gradual decline as baseline artery size increased (Fig. 2A). Correcting the FMD response for differences in the eliciting shear rate AUC abolished the correlation with the baseline diameter in all arteries (Fig. 2B). Although this might suggest that differences in the shear rate AUC explain the correlation observed between baseline diameter and FMD%, we further examined this by correlating FMD% and the shear rate AUC. A significant correlation was reported for the superficial femoral artery only (Fig. 2C).
Fig. 2.
Individual correlations for all 5 artery diameters (designated with the same symbols as in Fig. 1) regarding the comparisons between baseline artery size vs. FMD (A), baseline artery size vs. the corrected FMD (SRAUC from cuff deflation until peak diameter; B), and FMD vs. the SRAUC until peak diameter (C) in 20 healthy young subjects.
ENDOTHELIUM-INDEPENDENT VASODILATION (GTN).
A significant inverse correlation between artery size and magnitude of GTN response was found for the brachial (r = −0.53, r2 = 0.28, P = 0.02) and popliteal arteries (r = −0.62, r2 = 0.39, P = 0.003), but not in the radial (r = −0.24, r2 = 0.06, P = 0.31), superficial (r = −0.31, r2 = 0.09, P = 0.20), or common femoral arteries (r = −0.41, r2 = 0.17, P = 0.07). The slope of the linear regression differed markedly between individual arteries.
Because variation exists in time to brachial artery peak diameter between and within subjects (1), we documented the time to peak diameter during the FMD or sublingual GTN administration for all arteries. We compared these with typically used time windows adopted in the literature (Fig. 3).
Fig. 3.
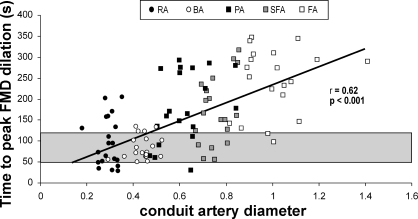
Scatter plot for all 5 artery diameters vs. the time to peak dilation during the FMD response in 20 healthy young subjects. The shaded area represents the time frame (50–120 s) commonly adopted in the current literature to capture the peak diameter during the FMD response.
DISCUSSION
The results of this study indicate that baseline artery size is inversely correlated with the FMD response, a finding that essentially confirms various previous reports (3, 12, 13, 26). However, we used a within-subjects approach and simultaneous recording of different-sized arteries in response to the same stimulus. We demonstrated that the relationship between FMD and baseline diameter across the five arteries studied remains significant despite normalization for shear rate using the most appropriate contemporary method (24). Also, the response to sublingual GTN, a shear rate-independent response, was strongly correlated with baseline artery size. Within- artery correlations between baseline artery size and the FMD and GTN responses revealed that the impact of artery size on the magnitude of functional stimuli is larger in smaller arteries than in larger vessels. Together with the observation that time to peak dilation following cuff deflation strongly correlates with baseline artery size, these data confirm the impact of artery size on the magnitude and timing of functional responses. Furthermore, our data suggest that shear stress-independent factors contribute to differences in vasodilation between arteries of varying dimension. Our findings may have implications for the interpretation of studies examining conduit artery responses to functional stimuli in humans, where between- or within-subject comparisons are undertaken that involve arteries of different baseline size.
Between-Artery Comparisons
A relationship between artery diameter and FMD has been suggested previously in studies performed using between-subjects comparisons of brachial (3, 12, 13, 25–27) or femoral (3, 28) responses. In the present study we confirmed this finding, using a novel approach of simultaneous assessments of different artery responses to the same stimulus within individuals. The larger dilation in the radial and superficial femoral arteries compared with the brachial and common femoral arteries, respectively, indicates that artery size impacts the magnitude of functional responses. A common explanation relates to the suggestion that smaller arteries are subject to a larger shear stress stimulus and exaggerated “flow-mediated” dilation as a consequence (25, 27, 28). Although some previous studies have normalized FMD for peak shear, it was recently suggested that the postdeflation shear rate AUC until the peak diameter is more appropriate to quantify the stimulus that elicits FMD (24). Using this approach, we found that the relationship between baseline artery diameter and the FMD response is slightly weaker but still persists. This suggests that, in addition to the shear rate AUC stimulus, other factors might be responsible for the relationship between baseline diameter and the magnitude of FMD. This proposition is reinforced by the observation that the magnitude of conduit artery responses to GTN, a shear stress-independent stimulus, is strongly and inversely correlated with artery size. Because GTN is highly diffusible and its concentration should be higher in more proximal vessels, a larger response in smaller arteries cannot be explained on the basis of differential pharmacokinetics. In addition, we observed no correlation between shear rate AUC stimulus and FMD% (r = 0.07, r2 < 0.01, P = 0.54) or between the shear rate AUC stimulus and the time to peak dilation of the FMD response between arteries (r = 0.16, r2 = 0.02, P = 0.17). These observations also argue against a primarily role for shear rate in explaining the relationship between artery size and FMD.
Within-Artery Comparisons
In this study we were able to assess the relationship between baseline artery size and the magnitude of functional responses between different arteries. Pyke and Tschakovsky (24) examined the relationship between brachial artery size and the magnitude of the FMD response and found that 64% of the FMD response was explained by the artery size (r2 = 0.64). Although our r2 values between brachial artery size and the FMD response (r2 = 0.33) are lower, other studies report comparable (27) or even lower values (23, 27) than those we observed. Nonetheless, all of these studies demonstrate a significant relationship between baseline artery size and the magnitude of the FMD response. Whereas other studies commonly assessed the brachial artery, we examined five different vessels. Interestingly, the strength and slope of correlation between artery size and functional responses differed markedly between arteries, demonstrating an attenuation of the slope with an increase in artery diameter (Fig. 2). These differences among arteries indicate that baseline artery size in smaller arteries has a much greater impact on the magnitude of FMD and GTN responses than the baseline size effect for larger vessels. Our results therefore suggest that previously identified effects of baseline artery size on functional responses in the brachial artery may not hold true for other arteries. This will be of special importance for conduit arteries in the lower limb, which recently have become a popular site of interest for functional assessments (14, 19, 34).
As discussed above, Pyke and Tschakovsky (23–25) provided evidence that the larger dilation in the smaller-sized brachial artery can be explained by individual differences in the postdeflation shear rate stimulus. These findings are reinforced by our observation that baseline diameter did not correlate with the FMD corrected for the shear rate AUC (Fig. 2B). This finding, however, does not necessarily indicate that shear rate accounts for differences observed in the magnitude of FMD. In fact, we found no correlation between the shear rate AUC stimulus and magnitude of the FMD responses (Fig. 2C). In addition, the linear regression model revealed a significant β-coefficient for artery size, but not for the shear rate AUC stimulus, in relation to the magnitude of the FMD response. Hence, this analysis suggests that baseline diameter might have a more important impact on FMD than the shear rate AUC. It is conceivable that dividing a ratio (FMD) by another number multiplies the error associated with the corrected FMD measures. Finally, the strong correlation we found between baseline artery diameter and the magnitude of the GTN response, which is shear independent, suggests that factors in addition to the shear rate contribute to differences in arterial dilation among vessel of different size.
Because shear alone cannot explain our findings, we propose, in line with Folkow (6, 7), that differences in the architecture or structure of the arteries, particularly the wall-to-lumen ratio, exert a generalized impact on vasomotor responsiveness. He provided evidence that subjects with enlarged wall-to-lumen ratios exhibit generalized hyperresponsiveness to a variety of vasoactive agents that worked through distinct biochemical pathways. Extrapolating this concept to our data, the differences in artery responsiveness not only between arteries but also for within-artery comparisons may relate to differences in wall morphology. As arteries become smaller, they possess more smooth muscle relative to elastic laminae and therefore have enlarged wall-to-lumen ratios. This may, in part, explain that smaller vessels react with a relatively larger dilation during functional stimulation, a finding that is consistent with the evidence provided by Folkow and others. We therefore suggest that baseline diameter may be acting as a surrogate measure of vessel wall composition or structure or other intrinsic factors that affect FMD% measurements independently of the shear stimulus.
Clinical Relevance
One implication of the present findings is that potential differences in artery size and wall structure should be considered when comparisons are made between arteries, either within or between subjects. To illustrate, recent studies that assessed FMD in the arm and leg (5, 19, 33, 34) have concluded that limb differences exist in FMD, possibly as a consequence of differences in activity level and/or arterial pressure. However, limb differences may, in part, also relate to baseline artery size, since arteries in the leg and arm differ in dimension. This is supported by our finding that differences exist in simultaneously measured FMD in arteries of different size, within each limb. Our novel observation that artery size and the time to peak dilation during the FMD correlate has important consequences for future FMD studies. To support this, we recently reported (1) that the brachial artery peak dilation often occurs outside the commonly used time points (2, 3) or time windows (20, 29, 30, 33), leading to underestimation of the true FMD response and incorrect calculation of shear rate AUC. In this report, we extend these observations, since smaller arteries reach a peak FMD faster than larger vessels. This indicates that a longer time window must be used when examining larger arteries. Regarding the GTN measurement, if the traditional 5-min window had been adopted, 86% of true peak diameters would have been missed in this study (Table 3). Therefore, the use of arbitrary methodological approaches for detection of peak FMD or GTN diameters may generate misleading findings, especially when examining different-sized arteries.
Table 3.
Proportion of subjects in whom FMD and GTN responses were correctly identified using typical assessment methods from the literature
| RA | BA | PA | SFA | FA | All Arteries | |
|---|---|---|---|---|---|---|
| FMD responses | ||||||
| Traditional 60 s | 0 | 0 | 5 | 0 | 0 | 1 |
| 50–90 s | 25 | 65 | 10 | 16 | 0 | 23 |
| 0–90 s | 50 | 65 | 15 | 16 | 0 | 29 |
| 0–2 min | 65 | 80 | 25 | 26 | 10 | 41 |
| 0–3 min | 90 | 100 | 60 | 58 | 25 | 67 |
| 0–4 min | 100 | 100 | 65 | 79 | 40 | 77 |
| 0–5 min | 100 | 100 | 100 | 95 | 75 | 94 |
| 0–6 min | 100 | 100 | 100 | 100 | 100 | 100 |
| GTN responses | ||||||
| 0–4 min | 5 | 0 | 10 | 5 | 10 | 6 |
| 0–5 min | 10 | 0 | 30 | 5 | 25 | 14 |
| 0–6 min | 15 | 10 | 35 | 11 | 25 | 19 |
| 0–8 min | 45 | 40 | 55 | 32 | 55 | 45 |
| 0–11 min | 100 | 100 | 100 | 100 | 100 | 100 |
Values are percentages representing the proportion of subjects (n = 20) in whom FMD and GTN responses were correctly identified using typical assessment methods from the literature.
Limitations
The shear rate AUC from the point of cuff deflation to the peak diameter was recently suggested as the most appropriate method for quantification of the FMD stimulus (24). Nonetheless, the correlation between baseline artery size and the magnitude of the corrected FMD remained significant. Although marked differences in time to peak dilation, as evident in our study, will influence the quantification of the shear rate stimulus, we believe that we have adopted the most appropriate contemporary approach to shear assessment (1). Nonetheless, given the lack of correlation observed between shear rate AUC and FMD across arteries in the present study, we support future studies to further optimize technique aimed at determining the most appropriate eliciting shear rate stimulus for use in FMD normalization. Another limitation relates to the fact that we cannot rule out the possibility that factors other than artery size influence FMD and GTN responses and contribute to potential differences in these functional responses between or within groups or individuals. Although artery size may act as a surrogate for wall composition or thickness (6, 7), we did not directly assess these factors in the present experiment. Finally, our results are limited to the impact of artery size on FMD in healthy subjects. Subjects with preexisting vascular disease or risk factors, which may have an impact on both artery structure and function, may exhibit different relationships.
In summary, our results demonstrate that magnitude and timing of the FMD response across five different conduit arteries is significantly correlated with baseline artery size. Moreover, the within-artery analysis reveals that the inverse relation between artery size and the magnitude of the functional response differs in vessels of different baseline diameter. After FMD was corrected for its eliciting shear rate stimulus, baseline artery size remained strongly correlated with the magnitude of these functional responses between arteries. Shear rate-independent GTN responses also were strongly correlated with baseline diameter. Consequently, we suggest that systematic differences exist in the magnitude and time course of vascular dilation responses of conduit arteries that vary in size. This raises the possibility that differences in artery size within individuals or between groups may influence the magnitude of functional responses and that baseline diameter may be serving as a surrogate measure of vessel wall composition or structure that affect FMD% measurements, independently of shear. Therefore, we suggest that future studies examining functional responses (FMD and GTN) should, in addition to the relative change from baseline (FMD%) and the shear responses, also present baseline and absolute changes in arterial diameter. Differences in artery diameter may help for the interpretation of the FMD, particularly when analyzing groups that differ in baseline artery size or different arteries within subjects.
GRANTS
D. H. J. Thijssen is financially supported by The Netherlands Organization for Scientific Research Grant 82507010. M. A. Black is supported by a British Heart Foundation Grant FS/05/117/19971.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, Deanfield JE. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 22: 854–858, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Daemen MJ, De Mey JG. Regional heterogeneity of arterial structural changes. Hypertension 25: 464–473, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Folkow B The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl 4: 3s–22s, 1978. [DOI] [PubMed] [Google Scholar]
- 7.Folkow B, Grimby G, Thulesius O. Adaptive structural changes in the vascular walls in hypertension and their relation to the control of peripheral resistance. Acta Physiol Scand 44: 255–272, 1958. [DOI] [PubMed] [Google Scholar]
- 8.Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105: 766–768, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington DM, Fan L, Drum M, Riley WA, Pusser BE, Crouse JR, Burke GL, McBurnie MA, Morgan TM, Espeland MA. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk 8: 319–328, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Joannides R, Costentin A, Iacob M, Compagnon P, Lahary A, Thuillez C. Influence of vascular dimension on gender difference in flow-dependent dilatation of peripheral conduit arteries. Am J Physiol Heart Circ Physiol 282: H1262–H1269, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol 38: 1843–1849, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogeneous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ou P, Celermajer DS, Mousseaux E, Giron A, Aggoun Y, Szezepanski I, Sidi D, Bonnet D. Vascular remodeling after “successful” repair of coarctation: impact of aortic arch geometry. J Am Coll Cardiol 49: 883–890, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Potter K, Reed CJ, Green DJ, Hankey GJ, Arnolda LF. Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound 6: 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder S, Enderle MD, Baumbach A, Ossen R, Herdeg C, Kuettner A, Karsch KR. Influence of vessel size, age and body mass index on the flow-mediated dilatation (FMD%) of the brachial artery. Int J Cardiol 76: 219–225, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol 288: H822–H828, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med 356: 911–920, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 358: 1400–1404, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol 99: 81–86, 2005. [DOI] [PubMed] [Google Scholar]