Abstract
The cardiotoxic effects of doxorubicin, a potent chemotherapeutic agent, have been linked to DNA damage, oxidative mitochondrial damage, and nuclear translocation of p53, but the exact molecular mechanisms causing p53 transactivation and doxorubicin-induced cardiomyopathy are not clear. The present study was carried out to determine whether extracellular signal-regulated kinases (ERKs), which are known to be activated by DNA damaging agents, are responsible for doxorubicin-induced p53 activation and oxidative mitochondrial damage in H9c2 cells. Cell death was measured by terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling, annexin V-fluorescein isothiocyanate, activation of caspase-9 and -3, and cleavage of poly(ADP-ribose) polymerase (PARP). We found that doxorubicin produced cell death in H9c2 cells in a time-dependent manner, beginning at 6 h, and these changes are associated decreased expression of Bcl-2, increases in Bax and p53 upregulated modulator of apoptosis-α expression, and collapse of mitochondria membrane potential. The changes in cell death and Bcl-2 family proteins, however, were preceded by earlier activation and nuclear translocation of ERKs, followed by increased phosphorylation at Ser15 and nuclear translocation of the phosphorylated p53. The functional importance of ERK1/2 and p53 in doxorubicin-induced toxicity was further demonstrated by the specific ERK inhibitor U-0126 and p53 inhibitor pifithrin (PFT)-α, which abrogated the changes in Bcl-2 family proteins and cell death produced by doxorubicin. U-0126 blocked the phosphorylation and nuclear translocation of both ERK1/2 and p53, whereas PFT-α blocked only the changes in p53. Doxorubicin and ERK inhibitors produced similar changes in ERK1/2-p53, PARP, and caspase-3 in neonatal rat cultured cardiomyocytes. Thus we conclude that ERK1/2 are functionally linked to p53 and that the ERK1/2-p53 cascade is the upstream signaling pathway responsible for doxorubicin-induced cardiac cell apoptosis. ERKs and p53 may be considered as novel therapeutic targets for the treatment of doxorubicin-induced cardiotoxicity.
Keywords: oxidative stress, mitochondrial death pathway
doxorubicin is one of the effective, commonly used chemotherapeutic agents in the treatment of a variety of solid and hematologic malignancies (17). Its antitumor efficacy appears to be dose dependent, but its clinical use is greatly restricted by the development of cardiomyopathy and clinical congestive failure (5, 14, 23). Studies have shown that the doxorubicin-induced cardiomyopathy is caused by increased oxidant production (11, 23, 38) and activation of the p53 tumor suppressor protein (7) after DNA damage in the heart (22). Upon activation, p53 is phosphorylated at Ser15 and transactivated (36, 41) into the nucleus to induce the expression of genes associated with cell arrest, DNA repair, and apoptosis. However, the molecular mechanism by which doxorubicin activates p53 has been not well elucidated. Recently, the activation of extracellular signal-regulated kinases (ERK)1/2 has been implicated for the proapoptotic action of another DNA-damaging chemotherapeutic agent cisplatin (21, 34). Furthermore, since the mitogen-activated protein kinase (MAPK) cascade can both positively and negatively influence p53-dependent apoptosis (2, 6, 32, 33), we sought to study whether ERK1/2 are responsible for the activation of p53 and cell death induced by doxorubicin in H9c2 cells. In addition to the measurement of cell apoptosis and the phosphorylated states of ERK1/2 and p53, experiments were also carried out to study nuclear translocation of phosphorylated ERK1/2 and p53(Ser15), p53 upregulated modulator of apoptosis (PUMA)-α, Bcl-2, Bax, mitochondrial membrane potential (ΔΨm), activations of caspase-9 and -3, and cleavage of poly(ADP-ribose) polymerase (PARP). The functional importance of ERK1/2 and p53 in doxorubicin-induced cell apoptosis was further studied by pretreating the cells with U-0126, a selective inhibitor for MEK1/2-mediated ERK activation (31, 43) or cyclic pifithrin (PFT)-α, a cell permeable and potent p53 transactivation inhibitor (7, 24). Finally, to study whether this ERK/p53 signaling pathway is also involved in the cytotoxic effect of doxorubicin in cardiomyocytes, we carried out similar experiments using doxorubicin and U-0126 in neonatal rat cultured cardiomyocytes. To confirm the role on ERK1/2 inhibition by U-0126, we also included in the latter studies another selective MEK-ERK inhibitor, PD-98059 (12, 35), which is chemically dissimilar to U-0126.
MATERIALS AND METHODS
Cell culture and study design.
H9c2 cells from rat embryonic ventricular myocardium and neonatal rat cultured cardiomyocytes were used in the study. H9c2 cells, obtained from American Type Culture Collection (CRL-1446; Manassas, VA), were cultured in DMEM (Invitrogen GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum in a humidified atmosphere of 95% air-5% CO2 at 37°C. Before reaching confluence, the cells were split and plated at low density in culture medium containing 10% fetal bovine serum. Doxorubicin (1 μM; Sigma Aldrich, St. Louis, MO) was added to complete medium and incubated for various intervals (1, 3, 6, 12, 24, and 48 h). This dose of doxorubicin was chosen from preliminary studies showing significant activation of ERK1/2 and cell death by the terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay over 48 h. This concentration of doxorubicin is also clinically relevant, since it falls well within the plasma concentrations of doxorubicin in patients receiving doxorubicin therapy (16, 27). In addition to cell death and ERK1/2 and p53 phosphorylation, we also measured the effects of doxorubicin on the activation of caspase-3 and -9 and PARP, and changes in Bax, Bcl-2, and PUMA-α, over the 48-h period. Additional experiments were performed to determine whether 1) doxorubicin induced nuclear translocation of phospho-ERK1/2 and phospho-p53 by immunohistochemical nuclear staining and Western blot analysis of relative abundance of the proteins in cytosolic and nuclear fractions and 2) the cytotoxic effects of doxorubicin were associated with collapse of ΔΨm, using a mitochondrial membrane potential-sensitive fluorescent dye. To study whether the toxic effects of doxorubicin were mediated via the ERKs or p53 signaling pathways, we employed U-0126 (20 μM; Promega, Madison, WI), a highly selective inhibitor of MEK1/2, to block the formation of ERK1/2 and PFT-α (20 μM; Sigma Aldrich) to inhibit p53 phosphorylation. The inhibitors were added to the culture medium 60 min before the addition of doxorubicin. For experiments involving immunohistochemistry, cells were plated on precoated 8-well culture slides.
To study whether the effects of doxorubicin in H9c2 cells were applicable to cardiomyocytes, we performed additional investigations in neonatal rat cultured cardiomyocytes. Cardiac ventricles, taken from 1- to 2-day-old Sprague-Dawley rat neonates, were gently minced and enzymatically dissociated using collagenase H (Worthington Biochemical, Lakewood, NJ) in Ca- and Mg-free HBSS medium at 37°C. Dissociated cells were then filtered through 200-μm mesh and collected by centrifugation and plated at a density of 5 × 104/cm2 on a 60-mm dish in DMEM (Cellgro; Mediatech, Herndon, VA) containing 10% (vol/vol) fetal bovine serum and 1% penicillin-streptomycin for 24 h. Cytosine 1-β-D-arabinofuranoside (10 μM; Sigma Aldrich) was added to retard the growth of contaminating fibroblasts. On day 5, the cultured cardiomyocytes were added to fresh culture medium and then pretreated with either U-0126 (20 μM) or PD-98059 (50 μM; Calbiochem), before exposed to doxorubicin (1 μM). We measured the effects of doxorubicin and MEK inhibitors on the phosphorylation of ERK1/2 and p53 and the activation of PARP and caspase-3. Animal protocol studies were approved by the University of Rochester Committee on Animal Resources, and animal handling and disposal were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, Revised 1996).
TUNEL assay.
The TUNEL assay was performed on slides of H9c2 cell using an apoptosis detection kit (Promega), according to the manufacturer's instructions. In brief, control H9c2 cells and cells treated with doxorubicin were fixed in 4% (wt/vol) paraformaldehyde and permeabilized in 0.3% Triton X-100 (vol/vol). The cells were washed and then incubated with TUNEL. TUNEL-positive cells exhibit green fluorescence. Cell nuclei were identified by propidium iodide. The slides were then examined under an Olympus BX-FLA reflected light fluorescence microscope (Olympus Imaging America, Melville, NY); more than 500 nuclei were counted in random fields per slide. Cell apoptosis was determined by counting the number of TUNEL-positive nuclei and expressed as a percentage of total H9c2 cells counted.
Flow cytometric detection of apoptosis.
The TUNEL assay detects the cells at a late stage of apoptosis. However, because it does not discriminate against necrotic cells, we also carried out experiments using the annexin V-fluorescein isothiocyanate (FITC)/propidium iodide apoptosis detection kit (Sigma Aldrich) to detect the early apoptotic cells in the studies of U-0126 and PFT-α. Briefly, cells were trypsinized in phosphate-buffered saline (PBS). After cells were washed twice with PBS, 1 × 105 cells were resuspended in 1× binding buffer containing (in mM) 10 HEPES/NaOH (pH 7.4), 140 NaCl, and 2.5 CaCl2. Annexin V-FITC and propidium iodide were then added to produce a final concentration of propidium iodide of 1 μg/ml cell suspension. The mixture was incubated for 15 min at room temperature in the dark and then analyzed for the percentages of early (annexin V-FITC positive and propidium iodide negative) and late (annexin V-FITC positive and propidium iodide positive) apoptotic cells using a FACSCalibur flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ).
Western blot analysis.
Total cell extracts were prepared by collecting attached and floating cells in radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet (N)P-40, 1% sodium deoxycholate, and 0.1% SDS with phosphatase inhibitor cocktail and protease inhibitor cocktail kit (Pierce Biotechnology, Rockford, IL). After being vortexed, the lysates were clarified by centrifugation, and supernatants were collected.
To prepare the nuclear fractions, cells were suspended in sucrose extraction buffer containing (in mM) 250 sucrose, 1 DTT, 80 KCl, 15 NaCl, 5 EDTA, 15 PIPES (pH 7.4) and 1 PMSF and 0.1 NP-40 at 4°C, homogenized, and centrifuged at 1,000 g for 10 min to separate the nuclei. The supernatant was recentrifuged at 13,000 g for 15 min three times to yield the cytosolic fraction. The nuclear fraction was lysed in Tris buffer containing 50 mM Tris·HCl (pH 7.5), 1 mM EDTA, 15 mM β-mercaptoethylamine, 0.1% Triton X-100, and 0.5 mM PMSF and stored in −80°C before use.
Protein concentration of the cell samples was determined using the bicinchoninic acid assay reagent kit (Pierce Biotechnology) with bovine serum albumin as standard. For Western blot analysis, 10–20 μg of protein was denatured by heating 95°C for 5 min in SDS sample buffer (Cell Signaling Technology), loaded onto 10∼12% SDS-polyacrylamide gels, and then transferred electrically to a polyvinylidene fluoride membrane. The membrane was blocked in 5% (wt/vol) nonfat milk 0.1% Tween TBS buffer for 1 h and was then incubated overnight with the following primary antibodies: anti-Bcl-2 and anti-Bax (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-p53(Ser15), anti-p53, anti-procaspase-9, anti-active caspase-3, anti-PARP, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-ERK1/2, anti-phospho-ERK1/2, anti-c-Jun-NH2-terminal kinase (JNK), anti-phospho-JNK, anti-p38 MAPK, anti-phospho-p38 MAPK (Cell Signaling Technology), anti-β actin, and anti-PUMA-α (Abcam, Cambridge, MA).
Anti-β actin antibody was used to show equal loading of the protein in the Western blot analyses. The membranes were incubated with horseradish peroxidase-linked anti-mouse or anti-rabbit secondary antibody at 1:1,000 dilutions and, after washes, visualized for immunoreactivity using an enhanced chemiluminescence system (Cell Signaling Technology). The optical density was determined using NIH 1.6 Gel Image Software, and the readings were normalized to a control sample in an arbitrary densitometry unit.
Assessment of mitochondrial membrane potential.
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1; R&D Systems, Minneapolis, MN), a fluorescent dye that exhibits potential-dependent accumulation in mitochondria, is commonly employed to detect the change in mitochondrial membrane potential (ΔΨm). It has been shown to be more specific for mitochondria than other fluorescent dyes such as dihexaoxacarbocyanine iodide (DiOC6[3]) or rhodamin 123. JC-1 selectively enters mitochondria and spontaneously forms complexes known as J-aggregates with intense red-orange fluorescence in normal cells with high mitochondrial membrane potential. If the membrane potential is disturbed as in apoptotic or unhealthy cells, the dye remains in the monomeric form, emitting only green fluorescence. In our experiments, cells exposed to various stimuli were incubated with 5 μl/ml JC-1 for 15 min at 37°C. The medium was then removed, and the cells were washed three times with PBS. The cells were then mounted on slides and examined immediately under a fluorescence microscope at excitation/emission of either 590/610 nm for JC-1 red fluorescence or 485/535 nm for JC-1 green fluorescence. At least 200 cells were examined. The ratio of JC-1 red and JC-1 green cells was taken as index of mitochondrial membrane potential.
Immunofluorescence and nuclear staining.
Control cells and cells treated with doxorubicin were rinsed with PBS and fixed in 4% (wt/vol) paraformaldehyde in PBS for 15 min at room temperature. After being washed with PBS, the cells were permeabilized in 0.3% Triton X-100 (vol/vol) in PBS for 5 min at room temperature. The cells were washed, blocked, and then incubated with primary antibodies for 2 h at room temperature. Secondary goat anti-rabbit or goat anti-mouse conjugated to Alexa fluor 488 or Alexa fluor 594 (Invitrogen Molecular Probes, Carlsbad, CA) were used at 1:250 dilution. To study nuclear translocation, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA) was used to identify the nucleus. Coverslips were washed three times in PBS, mounted, and viewed under a fluorescence microscope. A combination of Alexa fluor 488 and Alexa fluor 594 probes, which have a low degree of spectral overlap, was used in colocalization experiments.
Statistics.
Experimental data were analyzed using SPSS 16 for Windows (SPSS, Chicago, IL), and results are expressed as means ± SE. One-way ANOVA was used to determine the significance of differences among the groups. The statistical significance of differences between two groups was determined using Tukey post hoc test. For a comparison between two groups, Student's t-test for unpaired data was used. A P value of <0.05 was considered to be significant.
RESULTS
Induction of apoptosis by doxorubicin in H9c2 cells.
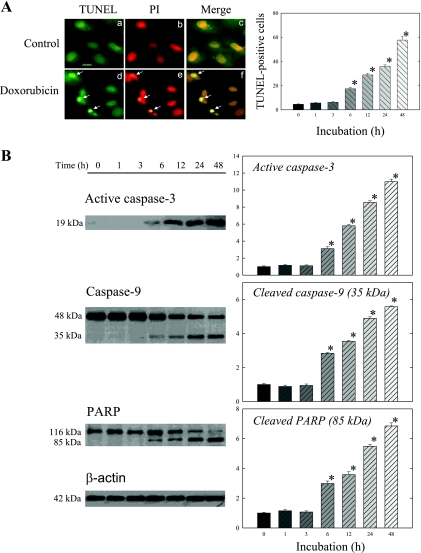
Doxorubicin caused time-dependent cell apoptosis in H9c2 cells. As shown in Fig. 1A, doxorubicin induced TUNEL-positive apoptotic changes in the cells, beginning at 6 h of incubation. The number of TUNEL-positive cells increased with the duration of incubation; by 48 h apoptotic changes occurred in about 60% of the cells examined.
Fig. 1.
Doxorubicin induces cell apoptosis in a time-dependent manner, beginning at 6 h of incubation. A: H9c2 cells were incubated in medium with or without 1 μM doxorubicin for the indicated times. Representative (24-h incubation) immunofluorescence images are shown. Arrows identify apoptotic cells with condensed nuclei and positive terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining as visualized by green fluorescence. Cell nuclei were counterstained red with propidium iodide (PI). Magnification scale, 20 μm. TUNEL-positive cells were counted as described in materials and methods. B: whole cell lysates were processed for Western blot analysis to detect active caspase-3, caspase-9, and poly(ADP-ribose) polymerase (PARP). β-actin was used as an equal loading control. Representative Western blots are shown at left. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with control (0 h).
Similarly, doxorubicin incubation significantly increased active caspase-3 and cleavage of caspase-9 and PARP at 6 h (Fig. 1B). The increases in cleaved caspase-9 and cleaved PARP were associated with decreases in procaspase-9 and uncleaved PARP. The magnitude of changes also paralleled that of doxorubicin on cell apoptosis. By 48 h, active caspase-3, cleaved caspase-9, and cleaved PARP increased 11-, six-, and sevenfold, respectively.
Phosphorylation and nuclear translocation of ERK1/2 and p53 in H9c2 cells.
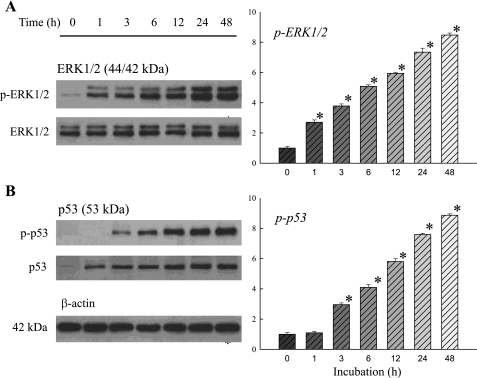
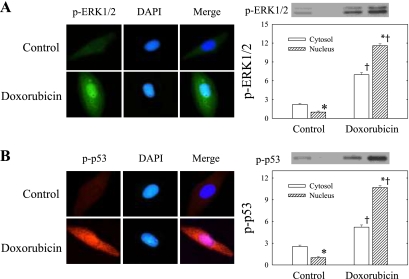
The effects of doxorubicin on ERK1/2 and p53 phosphorylation and nuclear translocation are shown on Figs. 2 and 3. Like the effects on cell apoptosis and caspase activation, doxorubicin also produced time-dependent increases of phospho-ERK1/2, p53, and phospho-p53, up to ninefold the control level at 48 h of incubation (Fig. 2). Total ERK1/2 showed no significant changes over time. In addition, the figure shows that the effect on phospho-ERK1/2 occurred very early within 1 h of incubation, and this was followed by an increase in phospho-p53(Ser15) within 3 h of incubation. The activation and nuclear translocation of phospho-ERK1/2 was also demonstrated by immunohistochemistry (Fig. 3A). Phospho-ERK1/2, which was very low in amount in control untreated cells, increased conspicuously in doxorubicin-treated cells. In this figure the colocalization of phospho-ERK1/2 and DAPI is also shown, indicating the apparent translocation of phospho-ERK1/2 to the nucleus after the activation by doxorubicin. The findings were confirmed by the Western blot analysis of phospho-ERK1/2 in the cytosolic and nuclear fractions. Figure 3A shows that although phospho-ERK1/2 was increased in both the cytosolic and nuclear compartments after doxorubicin treatment, it increased 11- to 12-fold in the nuclear fraction, far exceeding that in the cytosolic fraction. The positive cytosol-to-nucleus ratio seen in the control H9c2 cells was reversed after doxorubicin treatment.
Fig. 2.
Doxorubicin induces early activation of ERK1/2 (A) and p53 (B) in H9c2 cells, beginning at 1 and 3 h of incubation, respectively. Cells were treated with 1 μM doxorubicin for the indicated times. Whole cell lysates were processed for Western blot analysis to detect changes in ERK1/2, phospho (p)-ERK1/2, p53, and p-p53. β-actin was used as an equal loading control. Representative Western blots are shown at left. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with control (0 h).
Fig. 3.
Phosphorylation and nuclear translocation of ERK1/2 and p53 following doxorubicin treatment in H9c2 cells. Cells were maintained in medium with or without 1 μM doxorubicin for 24 h. p-ERK1/2 (A) and p-p53 (B) proteins were measured by indirect immunofluorescence using fluorescein-conjugated secondary antibodies (p-ERK1/2, green; and p-p53, red). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Experiments were carried out at least 3 times, and the representative pictures are shown. The merge images demonstrate nuclear translocation of p-ERK1/2 and p-p53 after phosphorylation by doxorubicin. Cytosolic and nuclear proteins were processed for Western blot analysis to detect p-ERK1/2 and p-p53. Representative Western blots are shown at top right. The optical density is expressed in arbitrary units normalized against a control nuclear sample. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with cytosol of the same treatment group; †P < 0.05, compared with the same cell compartment of the untreated control.
Similarly, in examining the cellular distribution of phospho-p53(Ser15) in H9c2 cells by fluorescence microscope (Fig. 3B), we found that in control cells there was only little phospho-p53(Ser15), which is mainly distributed in the cytoplasm. Whereas when the cells were exposed to doxorubicin for 24 h, phospho-p53(Ser15) was upregulated and translocated to the nucleus, as evidenced by both the immunofluorescence and Western blot analysis. Nuclear phospho-p53 protein increased 10- to 11-fold after doxorubicin treatment.
Bcl-2 family proteins in H9c2 cells.
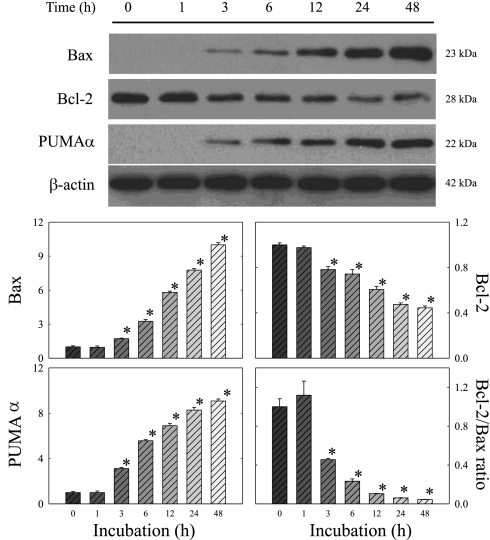
To gain information on the relationship between doxorubicin-induced apoptosis and Bcl-2 family protein, we measured the changes of Bax, Bcl-2, and PUMA-α over the 48 h of incubation by Western blot. As shown in Fig. 4, Bax and PUMA-α were upregulated following doxorubicin treatment, whereas Bcl-2 was suppressed. As a result, the ratio of Bcl-2 to Bax fell drastically, beginning at 3 h of incubation. In contrast, β-actin was unchanged, indicating that the changes in the Bcl-2 family proteins could not be explained by the unequal loading of the proteins.
Fig. 4.
Effects of doxorubicin on Bcl-2 family proteins in H9c2 cells. Cells were treated with 1 μM doxorubicin for the indicated times. Whole cell lysates were processed for Western blot analysis to detect changes in Bax, Bcl-2, and p53 upregulated modulator of apoptosis (PUMA)-α. β-actin was used as an equal loading control. Representative Western blots are shown. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with control (0 h).
Collapse of mitochondria membrane potential in H9c2 cells.
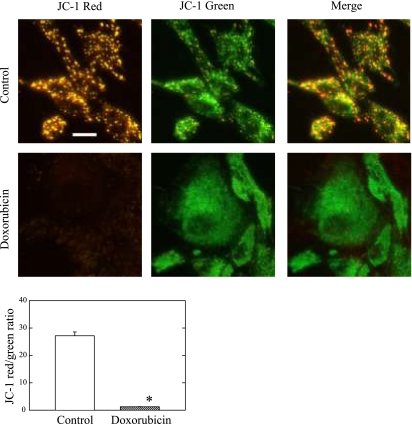
JC-1 dye was used to study mitochondrial membrane potential (ΔΨm) in H9c2 cells. Figure 5 shows that the control untreated cells exhibited mostly brightly stained mitochondria that emitted red/orange fluorescence, whereas many H9c2 cells exposed to doxorubicin exhibited green fluorescence, which reflects mitochondria depolarization or ΔΨm collapse. The ratio of cells exhibiting red/orange and cells exhibiting green fluorescence was 27.2 ± 1.4 in the control, which was greatly reduced to 1.3 ± 0.1 in the doxorubicin-treated cells (n = 10 in each group; P < 0.001).
Fig. 5.
Collapse of mitochondrial membrane potential (ΔΨm) after doxorubicin treatment in H9c2 cells. Cells plated on coverslides were maintained in medium with or without 1 μM doxorubicin for 24 h. The cells were stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocar bocyanine iodide (JC-1) and examined under fluorescence microscopy for red JC-1 aggregates or green JC-1 monomers, as described in materials and methods. Typical images are shown. Magnification scale, 20 μm. Ratio of normal ΔΨm and collapsed ΔΨm is presented at bottom. Data in histograms represent means ± SE from 10 experiments in each group. *P < 0.001, compared with control.
Effects of U-0126 and PFT-α on doxorubicin-induced apoptotic pathway in H9c2 cells.
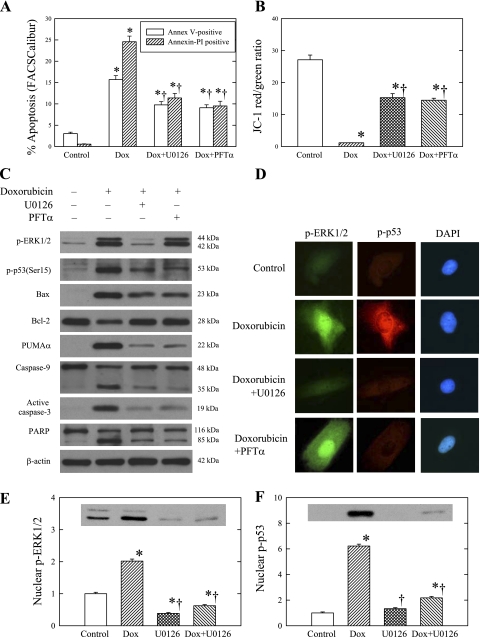
To determine whether the doxorubicin induction of apoptosis was affected by ERK1/2 or p53 inhibition, we pretreated H9c2 cells with either U-0126 or PFT-α for 1 h and then added doxorubicin to the culture medium for 24 h. In these experiments, cell apoptosis was measured by both annexin V-FITC binding (Fig. 6A) and TUNEL staining (Table 1). As shown previously, doxorubicin increased the number of TUNEL-positive cells. Doxorubicin treatment also increased the numbers of early and late apoptotic cells. The figure and table also show that the numbers of apoptotic cells produced by doxorubicin were reduced significantly by U-0126 and PFT-α. Further, the reduction of cell apoptosis was associated with the preservation of mitochondrial membrane potential in the U-0126 or PFT-α-treated cells (Fig. 6B). In addition, Table 1 shows that U-0126 and PFT-α also reduced cleavage of caspase-9 and the activation of caspase-3 produced by doxorubicin.
Fig. 6.
Effects of U-0126 and pifithrin (PFT)-α on doxorubicin (Dox)-induced apoptosis pathway in H9c2 cells. Cells were pretreated with or without 20 μM U-0126 or PFT-α for 1 h and then cultured in the presence or absence of 1 μM doxorubicin for 24 h. A: cell apoptosis was measured by annexin V-fluorescein isothiocyanate (FITC) and PI using FACSCalibur flow cytometry. Histograms show the percentages of early (annexin V-FITC positive and PI negative) and late (annexin V-FITC positive and PI positive) apoptotic cells in control and doxorubicin-treated cells with and without U-0126 and PFT-α treatment. B: mitochondrial damage was measured by changes in mitochondrial membrane potentials (ΔΨm) using fluorescent JC-1 under fluorescence microscopy. Histograms show the ratios of normal mitochondrial membrane potential (ΔΨm) and collapsed ΔΨm in control and doxorubicin-treated cells with and without U-0126 and PFT-α pretreatment. C: whole cell lysates were used to measure p-ERK1/2 (p-ERK), p-p53, Bax, Bcl-2, PUMA-α, caspase-9, cleaved caspase-3, and PARP by Western blot analysis. β-actin was used as an equal loading control. D: nuclear translocation of p-ERK1/2 and p-p53 was studied by indirect immunofluorescence using fluorescein-conjugated secondary antibodies (p-ERK1/2, green; and p-p53, red). Cell nuclei were counterstained with DAPI. Representative images are shown from 3 experiments. E and F: nuclear proteins were processed for Western blot analysis to detect p-ERK1/2 and p-p53. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with control; †P < 0.05, compared with doxorubicin.
Table 1.
Effects of U-0126 and PFT-α on cell apoptosis and activation of caspases and PARP
| Control |
Doxorubicin |
|||
|---|---|---|---|---|
| None | U-0126 | PFT-α | ||
| Apoptosis, % | 4.64±0.30 | 36.10±1.75* | 9.67±0.41*† | 10.58±0.64*† |
| Active caspase-3 | 1.00±0.11 | 8.40±0.15* | 2.54±0.14*† | 2.78±0.13*† |
| Cleaved caspase-9 | 1.00±0.10 | 3.74±0.07* | 1.71±0.09*† | 1.58±0.09*† |
| Cleaved PARP | 1.00±0.09 | 4.93±0.11* | 2.13±0.10*† | 2.14±0.11*† |
Values are means ± SE; n = 6 experiments. Apoptosis denotes the percentage of terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL)-positive apoptotic cells in each experimental condition. Values of active caspase-3, cleaved caspase-9, and cleaved poly(ADP-ribose) polymerase (PARP) were derived from optical density readings of the Western blots normalized to a control sample for each of the proteins.
P < 0.05, compared to control;
P < 0.05, compared to doxorubicin alone. PFT, pifithrin.
We also studied the effects of U-0126 and PFT-α on phospho-ERK1/2 and phospho-p53. Table 2 and Fig. 6C show that the increases of phospho-ERK1/2 and phospho-p53 produced by doxorubicin were markedly attenuated by U-0126, whereas PFT-α inhibited only the increase in phospho-p53(Ser15). Using tissue immunostaining, we also found that U-0126 pretreatment blocked the increases in protein and nuclear translocation of phospho-ERK1/2 and phospho-p53 produced by doxorubicin (Fig. 6D). PFT-α also reduced the increased protein and nuclear translocation of phospho-p53 but had no effect on the nuclear translocation of phospho-ERK1/2. The effects of U-0126 on nuclear translocation of phospho-ERK1/2 and phospho-p53 were further demonstrated by Western blot analyses of the proteins in the nuclear fraction (Fig. 6, E and F). Figure 6E also shows that U-0126 reduced the basal level of nuclear phospho-ERK1/2 to 39 ± 3% of the control.
Table 2.
Effects of U-0126 and PFT-α on phospho-ERK1/2, phospho-p53, and Bcl-2 family proteins
| Control |
Doxorubicin |
|||
|---|---|---|---|---|
| None | U-0126 | PFT-α | ||
| Phospho-ERK1/2 | 1.00±0.05 | 7.9±0.32* | 2.50±0.22*† | 6.86±0.22* |
| Phospho-p53 | 1.00±0.07 | 4.56±0.11* | 2.39±0.10*† | 2.10±0.07*† |
| Bax | 1.00±0.07 | 8.62±0.28* | 4.87±0.19*† | 4.59±0.24*† |
| Bcl-2 | 1.00±0.02 | 0.51±0.02* | 0.86±0.02*† | 0.90±0.03*† |
| PUMA-α | 1.00±0.13 | 9.56±0.10* | 2.74±0.35*† | 2.78±0.22*† |
Values are means ± SE; n = 6 experiments. Optical density for the Western blots was normalized to a control sample for each of the proteins.
P < 0.05, compared to control;
P < 0.05, compared to doxorubicin alone. PUMA, p53 upregulated modulator of apoptosis.
The influences of U-0126 and PFT-α on the effects of doxorubicin on the Bcl-2 family proteins were also examined. Figure 6C and Table 2 show that both inhibitors attenuated the doxorubicin-induced increases of Bax, PUMA-α, cleaved caspase-9, cleaved PARP, and active caspase-3. They also blocked the decrease in the Bcl-2 protein produced by doxorubicin.
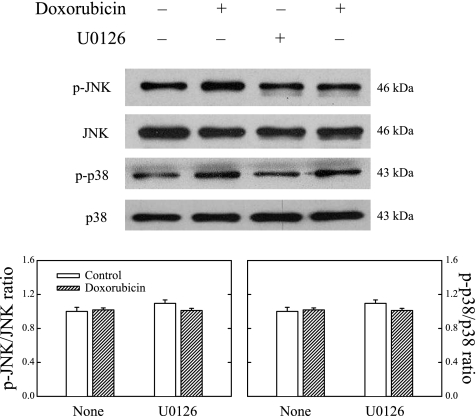
To study whether the cytoprotective effect of U-0126 was specific to its inhibitory action on ERK1/2, we also measured the effects of doxorubicin and U-0126 on p38 MAPK and JNK. Figure 7 shows that unlike its effects on phospho-ERK1/2, doxorubicin affected neither phospho-p38 MAPK nor phospho-JNK. U-0126 pretreatment also had no effect on the phosphorylation of p38 MAPK or JNK either alone or in combination with doxorubicin treatment. These findings suggest that the cardiotoxic effects of doxorubicin were not mediated via either the p38 MAPK or the JNK pathway.
Fig. 7.
Neither doxorubicin nor U-0126 produced significant changes on JNK and p38 MAPK proteins in H9c2 cells. Cells were pretreated with or without 20 μM U-0126 for 1 h and then in the presence or absence of 1 μM doxorubicin for 24 h. Whole cell lysates were processed for Western blot analysis of p-JNK, JNK, p-p38, and p38 MAPK. Representative Western blots are shown. Data in histograms represent means ± SE from 6 experiments.
Effects of doxorubicin on neonatal rat cardiomyocytes.
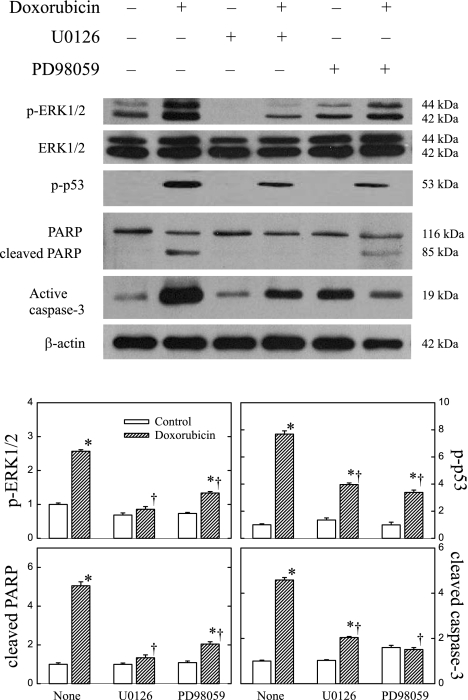
We studied the effects of doxorubicin in rat cultured cardiomyocytes. Two structurally different MEK inhibitors, U-0126 and PD-98059, were used to study whether the toxic effects of doxorubicin were mediated via the ERK1/2 phosphorylation. Figure 8 shows that similar to the effects in H9c2 cells, doxorubicin increased the phosphorylation of ERK1/2 and p53 and cleavage of PARP and caspase-3 in neonatal cardiac myocytes. The figure also shows that U-0126 and PD-98059 attenuated the increased phosphorylation of ERK1/2 and p53. The inhibitors also reduced the doxorubicin-induced activation of PARP and caspase-3, confirming the results of doxorubicin in H9c2 cells. The effects of U-0126 and PD-98059 were qualitatively similar.
Fig. 8.
Effects of MEK inhibitors U-0126 and PD-98059 on p-ERK1/2 and p-p53 expression, PARP activation, and cleaved caspase-3 in neonatal rat cardiomyocytes. Cells were pretreated with or without 20 μM U-0126 or 50 μM PD-98059 for 1 h and then in the presence or absence of 1 μM doxorubicin for 24 h. Whole cell lysates were processed for Western blot analysis of p-ERK1/2, ERK1/2, p-p53, PARP, and active caspase-3. β-actin was used as an equal loading control. Representative Western blots are shown. Data in histograms represent means ± SE from 6 experiments. *P < 0.05, compared with control of the same treatment group; †P < 0.05, compared with doxorubicin treatment alone without MEK inhibitors.
DISCUSSION
We report in the present study that at a clinically relevant concentration, doxorubicin produced a cytotoxic effect in H9c2 cells and neonatal rat cultured cardiomyocytes, which was associated with the increased activation and nuclear translocation of ERK1/2 and p53. We also demonstrated that the cell death caused by doxorubicin was associated with the downregulation of Bcl-2, upregulation of Bax and PUMA-α, collapse of ΔΨm, and activation of caspases-9 and -3 and PARP. PARP is a substrate for caspase-3, and cleaved PARP has been shown to be an important marker for apoptosis. The functional importance of ERK1/2 and p53 phosphorylation was further demonstrated in our present study by U-0126 and PFT-α pretreatment, which significantly attenuated the loss of mitochondrial membrane potential and cell apoptosis induced by doxorubicin in H9c2 cells. Studies were also presented in neonatal rat cardiomyocytes with U-0126 and PD-98059 showing that ERK1/2 is an important early mediator for the doxorubicin-induced cell apoptosis. We conclude that ERK1/2 functions upstream to p53 and that ERK1/2 activation is responsible for the phosphorylation of p53(Ser15) and the subsequent Bcl-2 family-mediated cell apoptosis.
The effect of doxorubicin on the MEK-ERK cascade has been studied previously, but the results are conflicting. Lou et al. (26) reported that doxorubicin caused an early increase of ERK1/2 phosphorylation in the rat heart, with a maximum rise to 513% seen at 4 h, which was followed by the progressive decline of phospho-ERK1/2 to 66.8% of the control at 3 wk after the last injection of doxorubicin. On the other hand, Spallarossa et al. (40) observed no effect of doxorubicin on ERK phosphorylation in H9c2 cells. Although these findings contradict our present study, our results are consistent with more recent findings that doxorubicin activated the MEK1/2-ERK1/2 cascade in the mouse failing heart (3) and increased phospho-ERK1/2 in H9c2 cells (7). In addition, unbeknown heretofore, we report herein a functional linkage between ERK activation and p53 phosphorylation in mediating the apoptotic effects of doxorubicin in cardiac myocytes.
The p53 protein, a transcription factor, has a short half-life and is present at a low level in normal cells. When exposed to DNA damage stress, p53 is phosphorylated on multiple residues in both the amino- and carboxy-termial domains by several different protein kinases. Among serine residues, the phosphorylation at Ser15 by ERKs has been shown to interfere with binding of p53 to murine double minute 2; this leads to the stabilization and accumulation of p53 in the cytoplasm and subsequent transactivation of p53 (4, 31, 36). The nuclear localization of p53 is critical for its transcriptional activity as well as the apoptosis-inducing function of p53 (9, 44, 45). The functional importance of p53 in mediating the cardiac toxicity of doxorubicin was demonstrated previously in transgenic mice in which p53 knockout attenuated the decline of left ventricular systolic function and myocyte apoptosis produced by doxorubicin (37). Increased p53 protein expression has also been found in human failing ventricular myocardium (39).
Although the mechanism of p53-dependent apoptosis is still not fully understood, it appears that this effect involves the transcriptional activation of many target genes including MAPK family and Bcl-2 family proteins (20, 28, 42, 44). The Bcl-2 family consists of both proapoptotic and antiapoptotic proteins. Their functions are determined by the presence and organization of their Bcl-2 homology (BH) domains (1, 10, 15). Antiapoptotic proteins such as Bcl-2 and Bcl-xL contain BH1-4 domains, whereas the proapoptotic members (Bax, Bak, Bid, and Bad) contain BH1-3 domains. The increase in p53, as shown in our experiments, was associated with the downregulation of Bcl-2 and upregulation of Bax (29). The ratio of Bcl-2 and Bax decreased markedly. In our study, PUMA-α, a newly identified BH3-only protein, was also increased in H9c2 cells after doxorubicin treatment. PUMA has been shown to be induced by p53 and implicated in p53-mediated apoptosis in vitro as well as in vivo gene knockout studies (18, 29, 44). Numerous studies have shown that the shift of the Bcl-2 family protein to apoptotic Bax and PUMA would lead to the collapse of mitochondrial membrane potential (ΔΨm), cytochorme c release, and the formation of the apoptosome with Apaf-1 and procaspase-9, which in turn activates caspases-9 and -3 and PARP (8). In addition, it has been speculated that p53 may bind to Bcl-xL protein, causing conformational alterations and mitochondrial translocations of p53 and Bcl-2 proteins and leading to the release of cytochrome c and cell apoptosis (13). Indeed, doxorubicin has been shown to increase mitochondrial p53 (30) and reduce mitochondrial membrane potential (22). However, the precise mechanism by which doxorubicin causes the mitochondrial translocation of p53 and Bcl-2 proteins has not been fully elucidated. Further studies, which are beyond the scope of our present study, are needed.
U-0126 and PFT-α are effective inhibitors for ERK and p53 transactivation. Our studies showed U-0126 inhibited the activation and nuclear translocation of not only ERK1/2 but also p53 in H9c2 cells after doxorubicin. Our study confirms that U-0126 is a MEK1/2-specific inhibitor. It reduced the production of ERK1/2 and phospho-ERK1/2 (Fig. 6), without affecting the p38 MAPK or the JNK pathway (Fig. 7). In contrast, PFT-α abrogated the activation and nuclear translocation of only p53 (Fig. 6). The findings suggest that p53 activation acts downstream of the ERK signaling pathway. To confirm that the changes produced by U-0126 are mediated via its inhibitory action on MEK1/2, we also used PD-98059 in rat cardiomyocytes. Since the two MEK inhibitors produced qualitatively similar changes in the cultured rat cardiomyocyte (Fig. 8), we conclude that the effects of doxorubicin on ERK1/2 and p53 phosphorylation and activation of PARP and caspase-3 were mediated by the MEK1/2-ERK1/2 cascade. However, because the MEK-ERK inhibitors did not completely inhibit the accumulation of p53 protein, additional upstream signaling pathways may be present and contribute to the phosphorylation of p53 following doxorubicin treatment.
In the present study, we showed that at the dose administered, doxorubicin treatment produced no significant increases of p-38 MAPK and JNK proteins in H9c2 cells (Fig. 7). However, when the dose of doxorubicin was increased fivefold (5 μM), significant increases in p-38 MAPK and JNK phosphorylation were reported in H9c2 cells (7). Activation of p-38 MAPK and JNK has also been observed in the mouse heart following doxorubicin administration (20, 26). We suspect that the discrepancies in p38 MAPK and JNK activation among the studies are related, at least in part, to the differences in the doses of doxorubicin and experimental conditions used. The increases of myocardial p38 MAPK and JNK in animals could also be caused by an enhanced stimulation of multiple signal-transduction pathways secondary to the state of heart failure, instead of the direct effects of doxorubicin on the heart.
In conclusion, our present investigation is the first to demonstrate the involvement of the ERKs/p53 signal transduction pathway in the doxorubicin-induced apoptosis in H9c2 cells and neonatal cardiomyocytes. We showed that ERKs and p53 were both phosphorylated and translocated into the nucleus before the development of changes in Bcl-2 family proteins, ΔΨm, or cell apoptosis following doxorubicin treatment. These changes were markedly reduced when the cells were pretreated by the MEK/ERK and p53 inhibitors. PFT-α has also been shown to inhibit doxorubicin-induced cardiomyocyte apoptosis in mice without interfering the antitumor potency of doxorubicin (25). Our results contribute to our insights of doxorubicin-induced cardiac toxicity and identify new targets for strategies to treat and prevent doxorubicin-induced cardiomyopathy.
GRANTS
The study was supported in part by National Heart, Lung, and Blood Institute Grant HL-68151.
Acknowledgments
We thank Megan Simeone for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 19: 488–496, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhalaf M, Jaffal S. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med 41: 318–325, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bien S, Riad A, Ritter CA, Gratz M, Olshausen F, Westermann D, Grube M, Krieg T, Ciecholewski S, Felix SB, Staudt A, Schultheiss HP, Ewert R, Völker U, Tschöpe C, Kroemer HK. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res 67: 10428–10435, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Thompson PD, Martin RP, Mason JW, Billinghan ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med 65: 823–832, 1978. [DOI] [PubMed] [Google Scholar]
- 6.Brown L, Benchimol S. The involvement of MAPK signaling pathways in determining the cellular response to p53 activation: cell cycle arrest or apoptosis. J Biol Chem 281: 3832–3840, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chua CC, Liu X, Gao J, Hamdy RC, Chua BHL. Multiple actions of pifithrin-α on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol 290: H2606–H2613, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Schroering A, Ding HF. P53 mediates DNA damaging drug-induced apoptosis through a caspase-9-dependent pathway in SH-SY5Y neuroblastoma cells. Mol Cancer Ther 1: 679–686, 2002. [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: critical control point. Cell 116: 205–219, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Doroshow JH Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43: 4543–4551, 1983. [PubMed] [Google Scholar]
- 12.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92: 7686–7689, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erster S, Moll UM. Stress-induced p53 runs a transcription-independent death program. Biochem Biophys Res Commun 331: 843–850, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gilladoga AC, Manuel C, Tan CTC, Wollner N, Sternberg SS, Murphy ML. The cardiotoxicity of adriamycin and daunomycin in children. Cancer 37, Suppl 2: 1070–1078, 1976. [DOI] [PubMed] [Google Scholar]
- 15.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hempel G, Flege S, Würthwein G, Boos J. Peak plasma concentrations of doxorubicin in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Cancer Chemother Pharmacol 49: 133–141, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hortobagyi GN Anthracyclines in the treatment of cancer. An overview. Drugs 54, Suppl 4: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-α by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kang YJ, Zhou ZX, Wang GW, Buridi A, Klein JB. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibitor of p38 mitogen-activated protein kinases. J Biol Chem 275: 13690–13698, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, Kim JM. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol 25: 374–382, 2005. [DOI] [PubMed] [Google Scholar]
- 22.L’Ecuyer T, Sanjeev S, Thomas R, Novak R, Das L, Campbell W, Heide RV. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol 291: H1273–H1280, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32: 302–314, 1973. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Waldman BC, Waldman AS. Suppression of high-fidelity double-strand break repair in mammalian chromosones by PFT-α, a chemical inhibitor of p53. DNA Repair 2: 1–11, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Chua CC, Gao J, Chen Z, Landy CLC, Hamdy R, Chua BHL. Pifithrin-α protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. Am J Physiol Heart Circ Physiol 286: H933–H939, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lou H, Danelisen I, Singal PK. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 288: H1925–H1930, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Menna P, Salvatorelli E, Minotti G. Doxorubicin degradation in cardiomyocytes. J Pharmacol Exp Ther 322: 408–419, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7: 683–694, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nithipongvanitch R, Ittarat W, Cole MA, Tangpong J, St. Clair DK, Oberley TD. Mitochondrial and nuclear p53 localization in cardiomyocytes: redox modulation by doxorubicin (adriamycin)? Antioxid Redox Signal 9: 1001–1008, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Okuno T, Matsuoka M, Sumizawa T, Igisu H. Involvement of the extracellular signal-regulated protein kinase pathway in phosphorylation of p53 protein and exerting cytotoxicity in human neuroblastoma cells (SH-SY5Y) exposed to acrylamide. Arch Toxicol 80: 146–153, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem 275: 35778–35785, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, Thelen P, Schlott T, Radzun HJ, Fayyazi A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer 91: 589–598, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Servant MJ, Glasson E, Meloche S. Inhibition of growth factor-induced protein synthesis by a selective MEK inhibitor in aortic smooth muscle cells. J Biol Chem 271: 16047–16052, 1996. [DOI] [PubMed] [Google Scholar]
- 36.She QB, Chen N, Dong Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV Radiation. J Biol Chem 275: 20444–20449, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Target disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem 273: 25–32, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207: 77–86, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Song H, Conte JV Jr, Foster AH, McLaughlin JS, Wei C. Increased p53 protein expression in human failing myocardium. J Heart Lung Transplant 18: 744–749, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: the role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res 69: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki K, Inageda K, Nishitai G, Matsuoka M. Phosphorylation of p53 at serine 15 in A549 pulmonary epithelial cells exposed to vanadate: involvement of ATM pathway. Toxicol Appl Pharmacol 220: 83–91, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem 275: 39435–39443, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331: 851–858, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Zhou BBS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439, 2000. [DOI] [PubMed] [Google Scholar]