Abstract
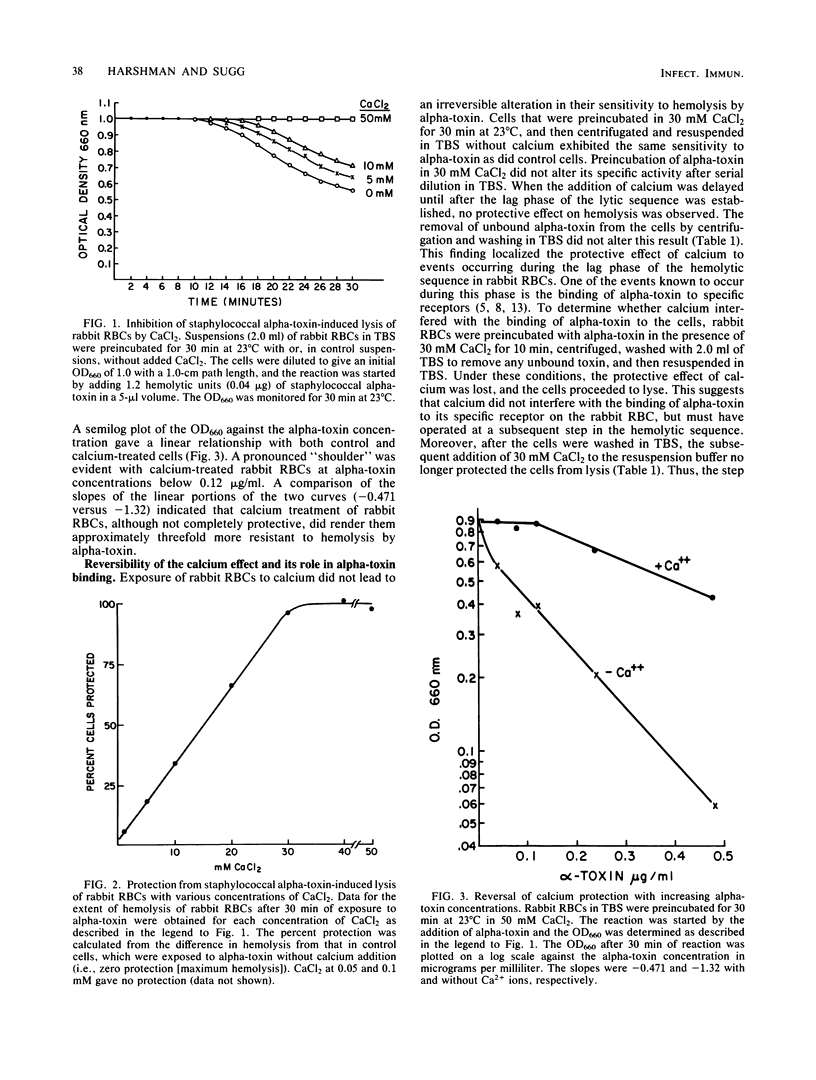
Calcium in millimolar concentrations protected rabbit erythrocytes from hemolysis caused by staphylococcal alpha-toxin. This effect was maximal at 30 mM CaCl2 and required the continued presence of calcium. The protection was not absolute and could be overcome by increased concentrations of alpha-toxin. Calcium did not block the binding of alpha-toxin to erythrocytes but inhibited the alpha-toxin-induced release of small ions from the cell as measured by 86Rb release. The transient removal of calcium was sufficient to abrogate its protective effect, suggesting that its action involves a reversible alteration in the state of the membrane. The three steps of the alpha-toxin-induced hemolytic sequence are: (i) binding to specific receptors, (ii) formation of transmembrane pores, and (iii) cell lysis. We concluded that calcium acted at step ii by impeding the lateral movement of alpha-toxin necessary to form the transmembrane hexamer pores.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. The relationship between Ca2+-mediated polyphosphoinositide phosphodiesterase activity, 1, 2-diacylglycerol accumulation, and microvesiculation in erythrocytes. Prog Clin Biol Res. 1979;30:523–529. [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immun. 1976 May;13(5):1378–1381. doi: 10.1128/iai.13.5.1378-1381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition of hemolysis by zinc and its reversal by L-histidine. Infect Immun. 1978 Mar;19(3):1101–1103. doi: 10.1128/iai.19.3.1101-1103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Barei G. M., Fackrell H. B. The binding of fluorescein-labelled stapbylococcal alpha toxoid to erythrocytes. Can J Microbiol. 1979 Nov;25(11):1219–1226. doi: 10.1139/m79-192. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Characterization of detergent-solubilized iodine-125-labeled alpha-toxin bound to rabbit erythrocytes and mouse diaphragm muscle. Biochemistry. 1979 Jan 9;18(1):232–236. doi: 10.1021/bi00568a036. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Purification of staphylococcal alpha-toxin by adsorption chromatography on glass. Infect Immun. 1976 Mar;13(3):982–986. doi: 10.1128/iai.13.3.982-986.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Faulstich H., Bühring H. J., Seitz J. Physical properties and function of phallolysin. Biochemistry. 1983 Sep 13;22(19):4574–4580. doi: 10.1021/bi00288a035. [DOI] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S. Action of staphylococcal alpha-toxin on membranes: some recent advances. Mol Cell Biochem. 1979 Feb 9;23(3):143–152. doi: 10.1007/BF00219453. [DOI] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry. 1982 Mar 30;21(7):1662–1667. doi: 10.1021/bi00536a029. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Enzymatic basis of membrane stiffening in human erythroyctes. Semin Hematol. 1979 Jan;16(1):65–74. [PubMed] [Google Scholar]
- Lucy J. A. Fusogenic mechanisms. Ciba Found Symp. 1984;103:28–44. doi: 10.1002/9780470720844.ch3. [DOI] [PubMed] [Google Scholar]
- MADOFF M. A., COOPER L. Z., WEINSTEIN L. HEMOLYSIS OF RABBIT ERYTHROCYTES BY PURIFIED STAPHYLOCOCCAL ALPHA-TOXIN. III. POTASSIUM RELEASE. J Bacteriol. 1964 Jan;87:145–149. doi: 10.1128/jb.87.1.145-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Hatanaka M., Murachi T. The cytosol of human erythrocytes contains a highly Ca2+-sensitive thiol protease (calpain I) and its specific inhibitor protein (calpastatin). J Biochem. 1981 Dec;90(6):1809–1816. doi: 10.1093/oxfordjournals.jbchem.a133659. [DOI] [PubMed] [Google Scholar]
- Parker J. C. Effects of drugs on calcium-related phenomena in red blood cells. Fed Proc. 1981 Dec;40(14):2872–2876. [PubMed] [Google Scholar]
- Reed P. W. Effects of divalent cation ionophore A23187 on potassium permeability of rat erythrocytes. J Biol Chem. 1976 Jun 10;251(11):3489–3494. [PubMed] [Google Scholar]
- Takeda Y., Ogiso Y., Miwatani T. Effect of zinc ion on the hemolytic activity of thermostable direct hemolysin from Vibrio parahaemolyticus, streptolysin O, and Triton X-100. Infect Immun. 1977 Aug;17(2):239–243. doi: 10.1128/iai.17.2.239-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


