Abstract
Ischemia-reperfusion (IR) injury is a major insult to postcapillary venules. We hypothesized that IR increases postcapillary venular hydraulic conductivity and that IR-mediated changes in hydraulic conductivity result from temporally and mechanistically separate processes. A microcannulation technique was used to determine hydraulic conductivity (Lp) in rat mesenteric postcapillary venules serially throughout ischemia (45 min) and reperfusion (5 h) induced by superior mesenteric artery occlusion and release. Mesenteric IR resulted in a biphasic increase in Lp. White blood cell (WBC) adhesion slowly increased with maximal adhesion corresponding to the second peak (P < 0.005). After IR, tissue was harvested for RT-PCR analysis of ICAM-1, E-selectin, and P-selectin mRNA. Intercellular adhesion molecule-1 (ICAM-1) mRNA in the gut showed the most significant upregulation. Quantitative real-time PCR revealed that ICAM-1 mRNA was upregulated 60-fold in the gut. An ICAM-1 antibody was therefore used to determine the effect of WBC adhesion on Lp during IR. ICAM-1 inhibition attenuated Lp during the first peak and completely blocked the second peak (P < 0.005). When rats were fed a tungsten diet to inhibit xanthine oxidase and then underwent IR, Lp was dramatically attenuated during the first peak and mildly decreased the second peak (P < 0.005). Inhibition of xanthine oxidase by oxypurinol decreased Lp during IR by over 60% (P < 0.002). Tempol, a superoxide dismutase mimetic, decreased Lp during IR by over 30% (P < 0.01). We conclude that IR induces a biphasic increase in postcapillary hydraulic conductivity. Reactive oxygen species impact both the first transient peak and the sustained second peak. However, the second peak is also dependent on WBC-endothelial cell adhesion. These serial measurements of postcapillary hydraulic conductivity may lead the way for optimal timing of pharmaceutical therapies in IR injury.
Keywords: microvascular permeability, reactive oxygen species, intercellular adhesion molecule-1
ischemia-reperfusion (IR) injury plays a pivotal role in cardiovascular disease, cerebrovascular disease, transplantation, trauma, shock, and sepsis. It is a complex insult that affects many physiological variables. Because the microvasculature is particularly vulnerable during IR, IR is often associated with microvascular dysfunction, including loss of endothelial barrier integrity. This loss of integrity leads to the loss of intravascular fluid into the interstitium and is a major cause of increased morbidity and mortality in critically ill patients (30, 37).
Endothelial cells are particularly vulnerable during IR, and endothelial cell dysfunction is an early and initiating event in the pathogenesis of IR. Derangements in endothelial cell function are a direct result of injury to endothelial cells and their intercellular junctions (3). Disturbed endothelial function leads to extravasation of fluid into the interstitium and to the clinical consequences of reperfusion injury that can plague critically ill patients, including acute respiratory distress syndrome, organ dysfunction, and the abdominal compartment syndrome (3). Such patients often develop an overwhelming microvascular leak that increases intravenous fluid requirements and contributes to cardiopulmonary difficulties and the development of systemic inflammatory response syndrome and multiple organ failure (29, 30, 37).
Of particular interest is the extravasation of fluid into the interstitium of the gut and mesentery, which leads to gut edema and the abdominal compartment syndrome. The abdominal compartment syndrome has been associated with delayed intestinal transit and altered gut barrier function (28), inadequate nutritional support due to protein/nitrogen loss (4), liver dysfunction (7), renal failure (8), a critical rise in intracranial pressure in patients with head trauma (11), and ultimately poor outcomes (1, 11). A better understanding of mesenteric IR injury and its sequelae is necessary to provide proper treatment for critically ill patients.
IR is a complex constellation of humoral and cellular components. The release of reactive oxygen species (ROS) is thought to occur early, whereas later on white blood cell (WBC) adhesion is thought to play a major role. The various molecular and subcellular mechanisms of IR, although independent of each other, augment each other and lead to increased endothelial dysfunction. However, the temporal relation and exact nature of these various mechanisms in IR have not been elucidated. We therefore sought to characterize the nature of the changes in hydraulic conductivity during mesenteric IR.
Since ROS release and WBC adhesion are separate yet interconnected processes, each may have a different impact on the loss of fluid from the intravascular space or hydraulic permeability. The majority of IR-induced barrier dysfunction occurs at the postcapillary venule (13, 14). And since the endothelial cells of the postcapillary venule account for most of the inflammatory responses observed during IR (3), this is where we focused our investigation. We hypothesized that IR increases postcapillary venular hydraulic conductivity and that IR-mediated changes in hydraulic conductivity result from processes that are temporally and mechanistically separate. Specifically, we hypothesized that ROS are responsible for the early changes in hydraulic conductivity and that WBC adhesion is responsible for the later changes.
MATERIALS AND METHODS
Animal and Solution Preparations
All studies were approved by an Institutional Committee for the use of animals in research and complied with institutional animal research protocols. Preparation of the animals and the mammalian Ringer solution has been described previously (35). They are briefly described below.
Red blood cells used as flow markers were harvested from female Golden Syrian hamsters (140–180 g; Harlan, Indianapolis, IN). The blood was centrifuged to remove the plasma and buffy coat and then washed three times in 15 ml of mammalian Ringer solution.
The Ringer solution was prepared daily in distilled deionized water and contained (in mM) 135 NaCl, 4.6 KCl, 2.0 CaCl, 2.46 MgS04, 5.0 NaHCO3, 5.5 dextrose, 9.03 HEPES salt (Research Organics, Cleveland, OH), and 11.04 HEPES acid (Research Organics). Bovine serum albumin (BSA) was added to the Ringer to prepare a 1% BSA Ringer solution for perfusion (BSA crystallized, Sigma, St. Louis, MO). In the ICAM-1 monoclonal antibody studies, the perfusate consisted of the 1% BSA Ringer solution plus the ICAM-1 monoclonal antibody (25 μg/ml). Tempol (4-hydroxy-2,2,6,6-tetramethyl piperidinoxyl, Aldrich-Milwaukee, WI) was administered as 100 mg/kg IV 10 min before the start of ischemia. In the oxypurinol studies (Sigma), the oxypurinol solution was made to a final concentration of 20 μM.
Animal Preparation
Adult female Sprague-Dawley rats (250–310 g; Hilltop Lab Animals, Scottsdale, PA) were anesthetized with subcutaneous pentobarbital sodium (60 mg/kg body wt). The bowel was exposed via a midline incision and retracted to the left upper quadrant of the abdominal cavity, exposing the celiac and superior mesenteric arteries (SMAs). The SMA was bluntly dissected and encircled with a snare consisting of 6-0 prolene suture passed through a 4-cm length of PE-60 tubing, which was placed loosely around the SMA. The bowel was positioned on an inverted microscope stage (Diaphot, Nikon; Melville, NY). The rat's body temperature was maintained at 37°C throughout the study. The mesentery was continuously bathed in Ringer solution.
Rat mesenteric postcapillary venules, 20–30 μm in diameter and at least 400 μm in length, were identified based on flow patterns. Vessels with no evidence of leukocyte adherence or side branches were chosen. The vessels were cannulated with micropipettes attached to a water manometer to control hydrostatic perfusion pressure.
IR Protocol
Study microvessels were perfused with 1% Ringer/BSA solution for 10 min before baseline Lp was assessed. After this assessment, the snare placed previously around the SMA was tightened for 45 min, constituting the gut ischemia phase of the procedure. Cessation of blood flow through the study vessel was confirmed by intravital microscopy. During this time, the bowel was covered with gauze and bathed with a warm Ringer solution. After 45 min of ischemia, the suture was released and reperfusion of the venule was confirmed. For the hydraulic conductivity studies, the reperfusion component of the study lasted 5 h. For the adhesion molecule, mRNA analysis, and ICAM-1 QRT-PCR analysis, tissue samples were obtained after 2 h of reperfusion. For control animals (sham animals), all steps were carried out in the same manner as the appropriate comparison experimental group, except the snare around the SMA was not tightened, thereby eliminating the ischemic and reperfusion components of the study. Measures of Lp and analysis of WBC adhesion were obtained in the same manner as the other study group animals and serve as time controls.
Measurement of Hydraulic Conductivity
Single vessel hydraulic conductivity (Lp) was determined using the modified Landis micro-occlusion technique. The assumptions and limitations of this model have been previously described (6). Initial cell velocity (dl/dt) was determined by recording marker cell position as a function of time. Transmural water flux per unit area (Jv/S) was calculated by the equation Jv/S = (dl/dt)(r/21), where r is the capillary radius and 1 is the initial distance between the marker cell and occluded site. Lp was determined using a modified version of Starling's equation of fluid filtration: Lp = (Jv/S)(1/Pc), where Pc is the capillary hydrostatic pressure. Lp was calculated from the slope of the regression of Jv/S on Pc derived from several occlusions at three different perfusion pressures. Control studies that document the stability of this model over time and after multiple cannulations of the vessels have been reported (6, 35).
WBC Adhesion
WBC adhesion was documented by intravital microscopy. Cells that adhered firmly to the wall of the study vessel were assessed during videotape replay. Cell adherence was defined as cells that remained motionless for >30 s and documented as the number of adherent WBC per 150 μM of study vessel.
Adhesion Molecule mRNA Analysis
To determine the impact on adhesion molecule mRNA due to IR, specimens from the bowel mesentery, liver, lung, kidney, and pancreas were harvested after the IR protocol and snap frozen with liquid nitrogen for later analysis. All reagents and solutions were purchased from Invitrogen (Carlsbad, CA).
mRNA extraction.
After RNAase was deactivated with trizole, the tissue was homogenized and centrifuged with chloroform at 4°C. The mRNA-containing supernatant was removed, isoproponol was added, and centrifugation was repeated. The mRNA pellet was resuspended in ice-cold 70% ethanol. After centrifugation, the pellet was resuspended in DEPC water. Buffer and DNAase were added to remove unwanted DNA, leaving behind purified mRNA.
First-strand cDNA synthesis.
Photometric analysis (Eppendorf Biophotometer) was used to determine mRNA concentration, and 5 μg were used for first-strand cDNA synthesis. The mRNA was added to a buffered random hexamer/dNTP mix solution, incubated at 65°C for 5 min, and incubated on ice for 1 min. A cDNA synthesis mix was prepared using reverse-transcription buffer, magnesium chloride, DTT, and RNaseOUT recombinant. The RNA/random hexamer/dNTP mixture was added to the cDNA synthesis mixture, centrifuged briefly, and incubated for 10 min at 25°C. SuperScript II RT was added to each tube followed by sequential incubation at 25°C for 10 min, 42°C for 50 min, and 17°C for 15 min. The reaction was collected by centrifugation, RNAse H was added, and the sample was incubated for 20 min at 37°C, thereby concluding first-strand cDNA synthesis.
Adhesion molecule gene amplification.
The control primer, gap dehydrogenase, and the adhesion molecule primers for ICAM-1, E-selectin and P-selectin, were added to the cDNA-buffered solution. PCR was performed for gene amplification. The samples were then used for agarose gel electrophoresis, and each band was evaluated with densitometry for quantification.
ICAM-1 quantitative real-time PCR analysis.
The ABI Prism 7000 Sequence Detection System (Applied Biosystems) was used for quantitative real-time PCR (qrt-PCR). Glyceraldehyde-3-phosphated dehydrogenase (GAPDH) was used as the endogenous/internal control for quantification/upregulation of ICAM-1. Reverse and forward primers were obtained from Genemed Synthesis (South San Francisco, CA) for GAPDH (forward: 5′-GGG/GTG/AGG/CCG/GTG/GTG/AGT/AT-3′, reverse: 5′-CAT/TGG/GGG/TAG/GAA/CAC/FFA/AGG-3′) and ICAM-1 (forward: 5′-TTC/AAG/CTG/AGC/GAC/ATT/GG-3′, reverse: 5′-CGC/TCT/GGG/AAC/GAA/TAC/ACA-3′). A buffered master mix was used containing the fluorescent intercalating agent SYBER-green (Applied Biosystems) for relative quantification of ICAM-1 upregulation. A standard curve was generated with each experiment for both GAPDH and ICAM-1. All samples were run in triplicate. Threshold cycle, mean fluorescence, standard deviation, and standard error of the mean were obtained for all samples. Dissociation curves were immediately performed on all samples to confirm specificity of primer binding. The relative fold-change was calculated by the relative standard method, and data are presented as a normalized ratio to GAPDH mRNA obtained without IR.
Experimental Design
Effect of IR on Lp.
Vessels were perfused with 1% Ringer/BSA solution for 10 min before baseline Lp was assessed. After this assessment, the IR procedure described above was initiated. Lp was measured after the ischemia period and then serially throughout the reperfusion period for up to 5 h. WBC adhesion was assessed at these same time points. The IR protocol was completed in nine rats. Three additional rats underwent a sham procedure in which the snare was placed around the SMA but was not tightened. Lp was measured using 1% Ringer/BSA solution, and WBC adhesion was assessed at the same time points as for the rats that underwent IR.
ICAM-1 Monoclonal Antibody
To assess the effect of WBC adhesion on IR and microvascular fluid leak, six rats that were maintained on a normal diet underwent the IR protocol as above. However, during the reperfusion period, 25 μg/ml of an ICAM-1 monoclonal antibody was perfused into the study venule. Lp and WBC adhesion were measured as above.
Inhibition of Xanthine Oxidase
To assess the effect of inhibiting xanthine oxidase (XOD) on IR and hydraulic conductivity, six rats were fed a tungsten-enriched diet (modified AIN-93M purified rodent diet without molybdenum with 0.7 g/kg sodium tungstate dihydrate; Dyets, Bethlehem, PA) ad libidum for 14 days before the IR study. When tungsten is added to a molybdenum-depleted diet, molybdenum is replaced as a coenzyme to XOD. The replacement of molybdenum with tungsten leads to the inhibition of XOD activity, which approaches zero after 14 days on this diet and effectively blocks production of superoxide and hydrogen peroxide in response to tissue ischemia and reperfusion (23). After being on the tungsten-enriched diet for 2 wk, rats then underwent the IR protocol. Lp and WBC adhesion were measured serially, as above.
Since the tungsten-enriched diet can influence other molybdenum-dependent enzymes, any potential nonspecific effects of the tungsten diet needs to be considered. Therefore, in another set of rats, we also inhibited XOD with oxypurinol (n = 4). Rats were pretreated with oxypurinol (20 μM) before undergoing the IR protocol. Lp and WBC adhesion were measured serially, as above.
Superoxide Dismutase Mimetic
The tungsten-enriched diet targets only one potential source of ROS, and leukocytes may not use molybdenum-dependent enzymes, including XOD, for ROS production. Therefore, in another set of rats, we assessed the effect of tempol, a cell-permeable superoxide dismutase mimetic, on IR (n = 4). Tempol was administered as 100 mg/kg IV 10 min before the start of ischemia. The rats then underwent the IR protocol. Lp and WBC adhesion were measured serially, as above.
Statistical Analysis
Group means of sequential measurements were analyzed by repeated-measures ANOVA with post hoc analysis. Measurements between different study groups were analyzed using unpaired Student's t-test and ANOVA with Bonferroni post hoc analysis. Statistical significance was set at an alpha error of 5%. All values for Lp are represented as means ± SE × 10−7 cm·s −1·cmH2O−1.
RESULTS
Effect of IR on Lp and WBC Adhesion
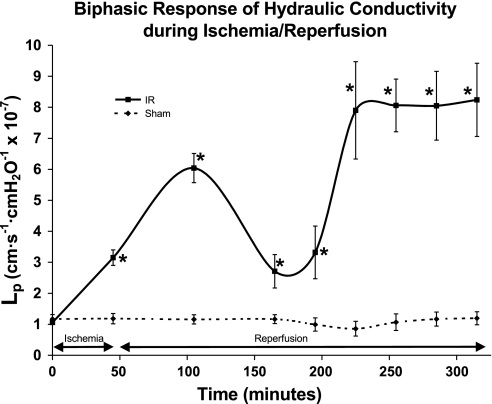
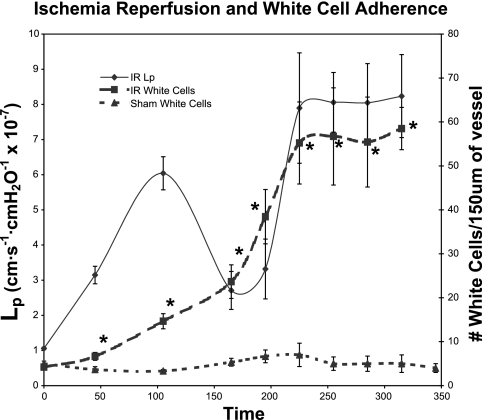
Ischemia for 45 min elevated Lp approximately threefold from baseline (Lp = 3.15 ± 0.10) (P ≤ 0.005). Hydraulic conductivity first peaked at 1 h of reperfusion, when Lp increased by sixfold (Lp = 6.04 ± 0.30) (P ≤ 0.001). At 2 h of reperfusion, Lp decreased to 2.1 ± 0.40. After this nadir, Lp again increased eightfold (Lp = 7.9 ± 1.20) (P ≤ 0.01), reaching a second peak at 3 h of reperfusion and remained at this level until the end of the experiment (Fig. 1). WBC adhesion slowly increased; maximal adhesion corresponded to the second peak of hydraulic conductivity at 3 h (P < 0.005) (Fig. 2). Sham animals showed no change in Lp (Fig. 1) and no change in WBC adhesion (Fig. 2) throughout the time period of the study protocol.
Fig. 1.
The biphasic increase in hydraulic conductivity in response to 45 min of ischemia and subsequent reperfusion shows a first peak of increased hydraulic conductivity (Lp) at 1 h of reperfusion and a second peak in at 3 h of reperfusion. Sham animals showed no change in Lp throughout the time period of the study protocol. The units for Lp are cm·s−1·cmH2O−1 × 10−7. *Significant difference from baseline values (P < 0.01).
Fig. 2.
Ischemia-reperfusion leads to an increase in white blood cell adhesion within the postcapillary venule. The increase in white blood cells slowly increases with the greatest increase and highest total number of white blood cells correlating with the second peak in hydraulic conductivity. Sham animals showed no change in white blood cell adhesion throughout the time period of the study protocol. The units for Lp are cm·s−1·cmH2O−1 × 10−7. *Significant difference from sham controls (P < 0.01).
Effect of IR on Adhesion Molecule mRNA
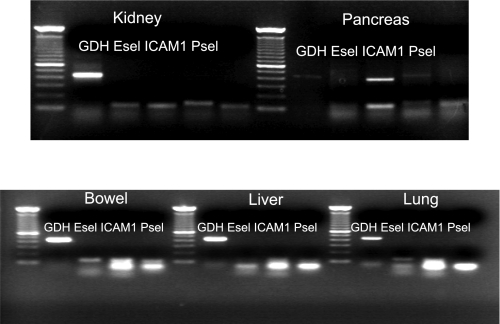
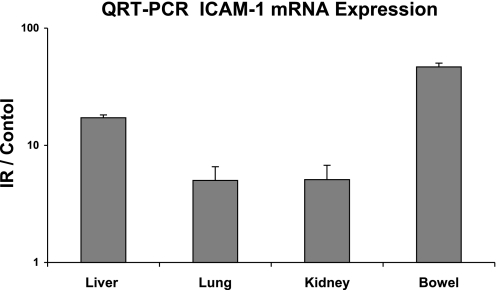
Because the second peak in hydraulic conductivity correlated with WBC adhesion, we investigated upregulation of adhesion molecules during IR. Specimens obtained during IR from liver, lung, kidney, pancreas, and bowel were analyzed for ICAM-1, E-selectin, and P-selectin mRNA (n = 6). After PCR for gene amplification, gel electrophoresis revealed that ICAM-1 showed the most pronounced upregulation (Fig. 3). When specimens were further analyzed by QRT-PCR (n = 6), ICAM-1 mRNA was upregulated 60-fold in the gut, 15-fold in the liver, and 5-fold in the lung and kidney (Fig. 4).
Fig. 3.
Because the ischemia-reperfusion-induced second peak in hydraulic conductivity correlated with an increase in white blood cell adhesion, we investigated adhesion molecule upregulation during ischemia-reperfusion by obtaining samples from liver, lung, kidney, pancreas, and bowel. Samples were analyzed for intercellular adhesion molecule-1 (ICAM-1), E-selectin, and P-selectin mRNA. Gel electrophoresis indicated that ICAM-1 showed the most pronounced upregulation.
Fig. 4.
Specimens obtained during ischemia-reperfusion were further analyzed by quantitative real-time polymerase chain reaction. Mesenteric ischemia-reperfusion injury markedly affects the gut because ICAM-1 mRNA was upregulated 60-fold in the gut, 15-fold in the liver, and 5-fold in the lung and kidney. Data are displayed as the standardized ratio to controls.
Inhibition of ICAM-1 during IR
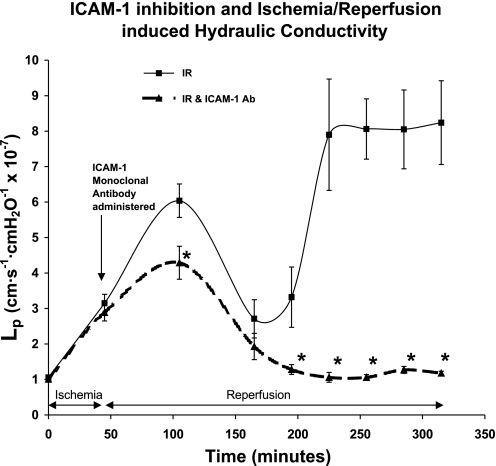
ICAM-1 inhibition mildly attenuated hydraulic conductivity during the first peak and completely blocked it during the second peak (Fig. 5). Blocking ICAM-1 mildly attenuated the first peak by 30% at 1 h of reperfusion, from an Lp of 6.04 ± 0.30 to 4.29 ± 0.30 (P < 0.01). However, at 3 h of reperfusion, ICAM-1 inhibition completely blocked the second peak of hydraulic conductivity; Lp was 1.06 ± 0.06, which was not different from baseline values (P = 0.2).
Fig. 5.
Treatment of rat postcapillary venules with anti-ICAM-1 antibody during reperfusion leads to a complete block of the second peak of microvascular conductivity. Lp values obtained during this time period of the study returned to baseline values. Treatment with anti-ICAM-1 antibody also attenuated the increase in hydraulic conductivity at the ischemia-reperfusion-induced first peak ∼30%. The units for Lp are cm·s −1·cmH2O−1 × 10−7. *Significant difference from rats that underwent ischemia-reperfusion alone (P < 0.01).
Inhibition of XOD during IR
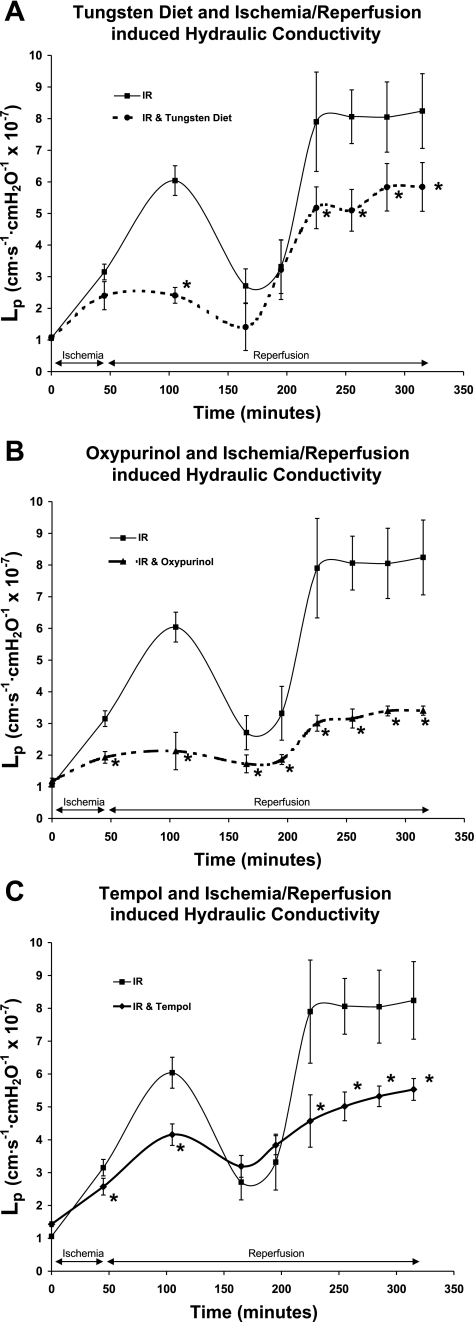
Inhibition of XOD by the tungsten-enriched diet dramatically decreased hydraulic conductivity during the first peak and mildly decreased it during the second peak (Fig. 6A). Hydraulic conductivity was decreased at the first peak by 60% at 1 h of reperfusion, from an Lp of 6.04 ± 0.30 to 2.41 ± 0.10 (P < 0.005). The effect of inhibition XOD by the tungsten-enriched diet was less striking during the second peak at 3 h of reperfusion, when it decreased hydraulic conductivity 35% from an Lp of 7.9 ± 1.20 to 5.18 ± 0.50 (P < 0.01).
Fig. 6.
A: pretreatment of rats with a tungsten-rich, molybdenum-depleted diet to inhibit xanthine oxidase suppressed hydraulic conductivity when pretreated rats were compared with rats that underwent ischemia-reperfusion alone. Inhibition of xanthine oxidase by the tungsten diet decreased the Lp at the first peak in hydraulic conductivity by ∼60% and decreased the Lp at the second peak by ∼35% (P < 0.01). B: inhibition of xanthine oxidase by oxypurinol dramatically decreased hydraulic conductivity throughout the ischemia-reperfusion study period. Hydraulic conductivity was decreased at the first peak by 65%, and at the second peak hydraulic conductivity was decreased 60% (P < 0.002). C: administration of tempol, a superoxide dismutase mimetic, decreased hydraulic conductivity 31% at the first peak and decreased hydraulic conductivity 36% during the second peak (P < 0.01). The units for Lp are cm·s −1·cmH2O−1 × 10−7. *Significant difference from rats that underwent ischemia-reperfusion alone.
Inhibition of XOD by oxypurinol dramatically decreased hydraulic conductivity throughout the IR study period (Fig. 6B). Hydraulic conductivity was decreased at the first peak by 65% at 1 h of reperfusion, from an Lp of 6.04 ± 0.30 to 2.12 ± 0.5 (P < 0.002), and at the second peak at 3 h of reperfusion, when hydraulic conductivity was decreased 60% from an Lp of 7.9 ± 1.20 to 3.15 ± 0.3 (P < 0.002).
Superoxide Dismutase Mimetic during IR
Administration of tempol, a superoxide dismutase mimetic, decreased hydraulic conductivity similarly during the first and second peaks (Fig. 6C). Hydraulic conductivity was decreased at the first peak by 31% at 1 h of reperfusion, from an Lp of 6.04 ± 0.30 to 4.16 ± 0.3 (P < 0.01), and it was decreased 36% from an Lp of 7.9 ± 1.20 to 5.02 ± 0.4 during the second peak at 3 h of reperfusion (P < 0.01).
WBC Adhesion during IR
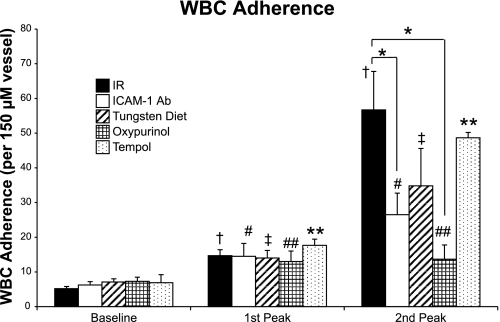
Comparison of WBC adherence between the different study groups is displayed in Fig. 7. WBC adherence increased during IR from a baseline of 5.2 ± 0.6 WBC/150 μM of vessel to 14.7 ± 1.7 at the first peak to 56.7 ± 11.1 at the second peak (P < 0.001). In all groups, WBC adherence was increased at the first and second peaks (P < 0.01). There were no differences in WBC adherence between groups at baseline and at the first peak, and there was no correlation at this time point between WBC adhesion and Lp (R2 = 0.19). At the second peak, IR alone showed the greatest increased in WBC adhesion to 56.7 ± 11.1 WBC/150 μM of vessel. Compared with IR alone, treatment with the ICAM-1 antibody attenuated WBC adhesion 53% to 26.5 ± 6.2 WBC/150 μM of vessel (P < 0.05), whereas treatment with oxypurinol attenuated WBC adhesion 76% to 13.7 ± 4.1 (P < 0.02). At the second peak, WBC adhesion varied directly with Lp (R2 = 0.63).
Fig. 7.
White blood cell adherence increased during ischemia-reperfusion. The first peak refers to the first peak of increased hydraulic conductivity that occurred at 45 min of ischemia and 1 h of reperfusion. The second peak refers to the second peak of increased hydraulic conductivity that occurred at 45 min of ischemia and 3 h of reperfusion. In all groups, white blood cell adherence was increased at the first and second peaks. There were no differences in white blood cell adherence between groups at baseline and at the first peak. At the second peak, ischemia-reperfusion alone showed the greatest increase in white blood cell adhesion. Treatment with either the ICAM-1 antibody or oxypurinol decreased white blood cell adhesion compared with ischemia-reperfusion alone at the second peak. †,#,‡,##,**Difference from each group's baseline values (P < 0.01). *Significant difference from ischemia-reperfusion alone at the second peak (P < 0.05).
DISCUSSION
Postcapillary venular endothelial cells are especially susceptible to the effects of IR and immediately alter in function (3). Most studies of endothelial cell changes during IR have been in cell culture and have used hypoxia and reoxygenation as a surrogate for IR (22). In vitro models do not account for the impact of the cessation and reestablishment of blood flow on endothelial cells, nor do they allow for the influences of other important factors such as paracrine functions of local auxiliary cells. Therefore, we sought to further define the endothelial changes that occur during IR by using an in vivo microcannulation model that allows serial measurements of microvascular hydraulic conductivity in postcapillary venules. This enabled us to examine the roles of ROS and WBC adhesion and their temporal relation with each other during IR. Since most barrier dysfunction induced by IR occurs at the postcapillary venule (13, 14), this is where we focused our investigation.
The major finding of this study is that the increase in microvascular hydraulic conductivity induced by IR is biphasic. In addition, it appears that the first peak of increased hydraulic conductivity is transient, ROS-dependent, and less influenced by WBC adhesion, whereas the second peak is sustained, also ROS-dependent, but more dependent on WBC-endothelial cell adhesion. A biphasic increase in WBC adhesion in response to hypoxia reoxygenation has been described in cell culture (16, 27), but to our knowledge we are the first to report a biphasic increase in microvascular hydraulic conductivity induced by IR. This biphasic increase in microvascular permeability suggests that there may be at least two mechanisms involved.
We found that WBC adhesion continually increased during IR until reaching a plateau that corresponded to the second sustained peak in hydraulic conductivity. WBC adhesion to the endothelium after IR injury releases potent inflammatory mediators such as platelet-activating factor, which play a powerful role in the development of microvascular dysfunction (2). A cell culture study of anoxia/reoxygenation found a biphasic peak in neutrophil-endothelial cell adhesion that consisted of an early 30-min ROS-mediated phase and a late 240-min peak in adhesion (22). Although we did not observe a biphasic response in WBC adhesion, the discrepancy may be explained by the difference between an in vitro anoxia/reoxygenation cell culture model and an in vivo IR model.
Because the second peak in hydraulic conductivity correlated with WBC adhesion, we investigated upregulation of adhesion molecules during IR. In specimens from several organs that were analyzed for ICAM-1, E-selectin, and P-selectin mRNA, ICAM-1 showed the most pronounced upregulation. Quantitative real-time PCR showed that ICAM-1 mRNA was upregulated 60-fold in the gut, 15-fold in the liver, and 5-fold in the lung and kidney. Taking into consideration the recent concern regarding the stability of GAPDH expression during IR and its use as a housekeeping gene, our findings agree with previous data suggesting that the increase and sustained elevation of WBC adhesion may be mediated by the transcriptional upregulation of adhesion molecules (22). In fact, IR is thought to stimulate a vigorous upregulation of adhesion molecules that leads to the more prolonged deleterious effects of ischemia (10). We therefore treated the postcapillary venules with a monoclonal antibody for ICAM-1, reasoning that this may prevent WBC adhesion and potentially affect the second peak in hydraulic conductivity induced by IR. Indeed, ICAM-1 inhibition completely blocked this second peak. Additionally, there was no association between WBC adhesion and hydraulic permeability at the first peak; however, at the second peak WBC adhesion varied directly with hydraulic permeability. These findings point to the important role of WBC adhesion and activation in modulating microvascular conductivity at the second peak. Others have previously documented the importance of WBC adhesion in IR injury (22). Furthermore, monoclonal antibodies against ICAM-1 or against CD11/18 on neutrophils have been shown to reduce WBC adhesion and attenuate the increased vascular permeability during IR (25, 36).
Our finding that blocking ICAM-1 also attenuated the first peak in microvascular conductivity during IR suggests that WBC adhesion and activation are important in the production of ROS after injury. WBCs are known to be a source of XOD and are therefore involved both in forming ROS and in the early response seen in IR (14, 26). Because the excess elaboration of ROS is a primary component of IR that damages numerous biological molecules, including amino acids, cytochrome enzymes, transport proteins, and nucleic acids (18, 19), it seemed likely that ROS were involved in the IR-induced biphasic increase we saw in microvascular permeability. Additional evidence for this likelihood is that, after an ischemia event, ROS are produced immediately during reperfusion (32, 33).
Because of the immediacy of ROS involvement in IR, we reasoned that if ROS were inhibited, that would affect the first peak of increased microvascular permeability during IR. Endothelial cells have an abundant supply of XOD, which is an important source of endothelial-derived superoxide and hydrogen peroxide (3). We inhibited XOD in two ways: 1) oxypurinol and 2) a tungsten diet. By placing rats on a diet that replaced molybdenum with tungsten as a coenzyme to XOD, XOD activity is inhibited, which blocks the production of superoxide and hydrogen peroxide in response to tissue ischemia and reperfusion (21, 23). Additionally, we also used the superoxide dismutase mimetic tempol to catalyze the removal of superoxide anions. Each of these methods of inhibiting ROS attenuated the first peak of increased hydraulic conductivity. Previous studies by different laboratories using in vitro cell monolayers have demonstrated the important role that oxygen radicals play in creating microvascular leak following hypoxia reoxygenation (15, 17, 20).
We found that the tungsten diet, oxypurinol, and tempol also led to a blunted increase in the second sustained peak of increased hydraulic conductivity. ROS are a powerful trigger for the formation of inflammatory mediators that correlate temporally with the later peak in microvascular leak (12). Furthermore, ROS stimulate WBC adhesion, and we found that the most prominent WBC adherence corresponded to the second peak. These data support a role for ROS during this second, WBC-dependent peak in hydraulic conductivity. In fact, extracellular XOD activity has been shown to promote WBC adhesion (15, 34). ROS are also a primary activator of the transcription factor nuclear-factor-κB, which upregulates several adhesion molecules, including selectins and ICAM-1 (5, 24). Although XOD derived from endothelial cells contributes to the initial oxidant stress, ROS derived from adherent leukocytes may provide the more substantial and prolonged oxidant burst that occurs in IR injury (14). There is also evidence that leukocyte-endothelial binding mediated by adhesion molecules and subsequent leukocyte emigration into tissues is proportional to the degree of endothelial-cell barrier dysfunction, further supporting a primary role for adhesion molecules as a central regulator of local and distant IR-related injury (25). Taken together, these findings add to the growing evidence suggesting that ROS production and WBC adhesion events are interconnected (15, 31).
Although the tungsten diet, oxypurinol, and tempol all attenuated the increase in hydraulic conductivity due to ischemia and reperfusion, the magnitudes of their effects were different. Oxypurinol appeared to have the greatest effect on hydraulic conductivity during IR, whereas tempol had the least effect. The observed differences in magnitude of each treatment may be due to their distinct mechanisms of action. For example, the replacement of molybdenum with tungsten leads to the inhibition of XOD activity; however, the tungsten-enriched diet can also influence other molybdenum-dependent enzymes (21, 23). Likewise, oxypurinol not only inhibits XOD, it may also directly scavenge ROS (9). Although both the tungsten diet and oxypurinol inhibit XOD, both treatments have other potential nonspecific effects that may also impact hydraulic conductivity and lead to the observed differences in the magnitude of their effects.
In summary, IR injury plays a pivotal role in many clinical scenarios and contributes to the morbidity and mortality of critically ill patients (29). IR is a complex constellation of humoral and cellular components. Endothelial cells are particularly vulnerable during IR, when many important pathophysiological changes occur in the endothelial cells of the postcapillary venules. We have demonstrated that IR induces a rapid increase in postcapillary venular hydraulic conductivity that occurs over two temporally independent peaks. Both peaks are influenced by the formation of ROS, whereas the second increase is dependent on WBC adhesion and activation. In a process as mechanistically complex as microvascular leak, there certainly is interplay between the many different cellular and subcellular mechanisms, which may augment or inhibit each other. Our findings suggest the presence of several potential therapeutic targets to help treat IR injury in critically ill patients.
GRANTS
Funding was partially provided by the American College of Surgeons Faculty Research Fellowship and the National Institute of General Medical Sciences.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA, Valdivia A, Ware DN, Moore FA. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma 54: 848–861, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg JS The biology of CD2: adhesion, transmembrane signal, and regulatory receptor of immunity. J Surg Res 54: 258–267, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255–266, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW. Nitrogen balance, protein loss, and the open abdomen. Crit Care Med 35: 127–131, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Christman JW, Blackwell TS, Juurlink BH. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol (Zurich) 10: 153–162, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curry FEHV, Sarelius IH. Measurement of Permeability, Pressure, and Flow. New York: Elsevier, 1983.
- 7.Dalfino L, Malcangi V, Cinnella G, Brienza N. Abdominal hypertension and liver dysfunction in intensive care unit patients: an “on-off” phenomenon? Transplant Proc 38: 838–840, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertensionand acute renal failurein critically ill patients. Int Care Med 34: 707–713, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun 148: 314–319, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Davies MGJT, Hagen PO. Endothelial Physiology. London: Blackwell Science, 1999.
- 11.Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med 28: 1747–1753, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med 22: 189–216, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Granger D Physiology and pathophysiology of the microcirculation. Prog Cardiovasc Med 3: 123–140, 1998. [Google Scholar]
- 14.Granger DN Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation 6: 167–178, 1999. [PubMed] [Google Scholar]
- 15.Granger DN Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 255: H1269–H1275, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 257: G683–G688, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med 25: 404–433, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett 486: 10–13, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 119: 598–620, 1992. [PubMed] [Google Scholar]
- 20.Harrison DG Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100: 2153–2157, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins ES, Richert DA, Westerfeld WW. Molybdenum deficiency and tungstate inhibition studies. J Nutr 59: 539–559, 1956. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ Res 81: 922–931, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JL, Rajagopalan KV, Cohen HJ. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem 249: 859–866, 1974. [PubMed] [Google Scholar]
- 24.Kokura S, Rhoads CA, Wolf RE, Yoshikawa T, Granger DN, Aw TY. NF kappa b signaling in posthypoxic endothelial cells: relevance to E-selectin expression and neutrophil adhesion. J Vasc Res 38: 47–58, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Kurose I, Anderson DC, Miyasaka M, Tamatani T, Paulson JC, Todd RF, Rusche JR, Granger DN. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ Res 74: 336–343, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Kurose I, Granger DN. Evidence implicating xanthine oxidase and neutrophils in reperfusion-induced microvascular dysfunction. Ann NY Acad Sci 723: 158–179, 1994. [PubMed] [Google Scholar]
- 27.Kvietys PR, Granger DN. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am J Physiol Gastrointest Liver Physiol 273: G1189–G1199, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Moore-Olufemi SD, Xue H, Attuwaybi BO, Fischer U, Harari Y, Oliver DH, Weisbrodt N, Allen SJ, Moore FA, Stewart R, Laine GA, Cox CS Jr. Resuscitation-induced gut edema and intestinal dysfunction. J Trauma 58: 264–270, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Neary PRH Ischaemia-reperfusion injury and the systemic inflammatory response syndrome. In: Ischemia-Reperfusion Injury, edited by Grace PA. London: Blackwell Science, 1999, p. 123–136.
- 30.Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL. Abdominal compartment syndrome. J Trauma 45: 597–609, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Salas A, Panes J, Elizalde JI, Granger DN, Pique JM. Reperfusion-induced oxidative stress in diabetes: cellular and enzymatic sources. J Leukoc Biol 66: 59–66, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Asako H, Kubes P, Jennings S, Grisham MB, Granger DN. Neutrophil-derived oxidants promote leukocyte adherence in postcapillary venules. Microvasc Res 42: 125–138, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Inauen W, Kvietys PR, Grisham MB, Meininger C, Schelling ME, Granger HJ, Granger DN. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 257: H1740–H1745, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Terada LS, Hybertson BM, Connelly KG, Weill D, Piermattei D, Repine JE. XO increases neutrophil adherence to endothelial cells by a dual ICAM-1 and P-selectin-mediated mechanism. J Appl Physiol 82: 866–873, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Victorino GP, Newton CR, Curran B. Effect of angiotensin II on microvascular permeability. J Surg Res 104: 77–81, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Vollmar B, Glasz J, Menger MD, Messmer K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery 117: 195–200, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Zikria BABJ, Oz MO, Carlson RW. Reperfusion Injuries and Clinical Capillary Leak Syndrome. Armonk, NY: Futura Publishing, 1994, p. 443–492.