Abstract
Soluble epoxide hydrolase (sEH) metabolizes epoxyeicosatrienoic acids (EETs) to dihydroxyeicosatrienoic acids. EETs are formed from arachidonic acid during myocardial ischemia and play a protective role against ischemic cell death. Deletion of sEH has been shown to be protective against myocardial ischemia in the isolated heart preparation. We tested the hypothesis that sEH inactivation by targeted gene deletion or pharmacological inhibition reduces infarct size (I) after regional myocardial ischemia-reperfusion injury in vivo. Male C57BL\6J wild-type or sEH knockout mice were subjected to 40 min of left coronary artery (LCA) occlusion and 2 h of reperfusion. Wild-type mice were injected intraperitoneally with 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE), a sEH inhibitor, 30 min before LCA occlusion or during ischemia 10 min before reperfusion. 14,15-EET, the main substrate for sEH, was administered intravenously 15 min before LCA occlusion or during ischemia 5 min before reperfusion. The EET antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE) was given intravenously 15 min before reperfusion. Area at risk (AAR) and I were assessed using fluorescent microspheres and triphenyltetrazolium chloride, and I was expressed as I/AAR. I was significantly reduced in animals treated with AUDA-BE or 14,15-EET, independent of the time of administration. The cardioprotective effect of AUDA-BE was abolished by the EET antagonist 14,15-EEZE. Immunohistochemistry revealed abundant sEH protein expression in left ventricular tissue. Strategies to increase 14,15-EET, including sEH inactivation, may represent a novel therapeutic approach for cardioprotection against myocardial ischemia-reperfusion injury.
Keywords: 14,15-epoxyeicosatrienoic acids; 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester
the p-450 epoxygenase pathway metabolizes arachidonic acid into four biologically active eicosanoids, referred to as epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EET) (10). EETs play an important role in regulating tissue perfusion in both cardiac and extracardiac organs. The actions of EETs are terminated by conversion to dihydroxyeicosatrienoic acids (DHETs) by epoxide hydrolases (11). Two major epoxide hydrolases are found in mammalian tissues, the microsomal (mEH) and soluble epoxide hydrolases (sEH) (2). However, sEH is the primary enzyme involved in the in vivo metabolism of EETs (19). In addition to their vascular effects, EETs exhibit a cardioprotective effect, which has been linked to activation of the reperfusion injury salvage kinase pathway (18) and mediated in part through activation of the phosphatidylinositol 3-kinase/Akt pathway and the mitochondrial ATP-sensitive K+ channels (5, 15). Augmenting endogenously released EETs by inhibiting the converting enzyme sEH represents an attractive strategy to increase ischemic tolerance. Our laboratory has recently used this strategy to show that pharmacological inhibition (20) and gene deletion (21) are protective against experimental stroke in vivo. Similarly, using the isolated heart preparation, Seubert et al. (16) demonstrated that sEH knockout (sEHKO) mouse hearts display improved postischemic functional recovery after transient global ischemia-reperfusion (I/R). In addition, Gross et al. (4) recently showed that sEH inhibition via intracoronary injection reduced infarct size (I) when given before ischemia in dogs, and this effect was abolished by the EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE).
In the present studies, we expand on these reports by examining the effects of both sEH inhibition and gene deletion on cardiac injury using a clinically relevant in vivo model of regional myocardial I/R. The long-term goal of these studies is to evaluate the utility of pharmacological sEH inhibition for clinical use. Our findings demonstrate for the first time that 1) a selective sEH inhibitor is cardioprotective when administered either before or at the end of regional myocardial ischemia; 2) mice with sEH gene deletion sustain smaller damage after in vivo myocardial I/R compared with wild-type (WT) control mice; and 3) exogenously administered sEH substrate 14,15-EET reduces I after myocardial I/R, whether administered before or at the end of the ischemic period. Finally, we provide the first immunohistochemical evidence of sEH localization in cardiac myocytes of left ventricular (LV) tissue.
MATERIALS AND METHODS
Animals.
The Institutional Animal Care and Use Committee at the Portland Veterans Affairs Medical Center approved this study, and all animals received treatment in compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, National Research Council; National Academy Press, 1996). Study animals were allowed access to phytoestrogen-free food (no. 2014, Harlan Teklad, Madison, WI) and water ad libitum until induction of anesthesia.
A colony of sEHKO mice with targeted deletion of the EPHX2 gene is maintained in house. These mice have been backcrossed to C57BL/6 for at least seven generations, and, therefore, homozygous sEHKO mice were compared with WT C57BL/6 mice obtained from Jackson Laboratories. Phenotype of the sEHKO mice and genotyping procedure have been previously described (21).
Regional myocardial I/R.
Male mice between the ages of 16 and 24 wk were anesthetized with isoflurane (induction 4–5 vol%; maintenance 1–2 vol%), intubated with a 20-G plastic intravenous catheter, and mechanically ventilated using a rodent ventilator (Inspira, volume-controlled, Harvard Apparatus). ECG and rectal temperature were continuously monitored using a PC-based recording device (PowerLab 8/30, ADInstruments, Colorado Springs, CO). The animals were positioned in a right lateral decubital position on a heating pad, and rectal temperature was maintained at 37°C throughout the experiment. A PE-10 catheter was inserted into the left jugular vein for intravenous drug infusion. Using a dissecting microscope, a left-sided thoracotomy was performed in the fourth intercostal space. Heparin (1 U/gram body wt intraperitoneal) was given to prevent clotting during temporary occlusion of the left coronary artery (LCA) in all groups, except for the preischemia 14,15-EET group (which experienced excessive bleeding in pilot studies following heparin administration). A ligature (8-0 Monosof MV-135-4, 3/8 5-mm tapered needle, Syneture, Norwalk, CO) was placed around the LCA ∼2 mm distal from the left atrial margin. The suture was fed through a short section of PE-10 tubing to form a snare to allow temporary occlusion. The LCA was occluded for 40 min; occlusion was confirmed by persistent ECG changes during occlusion and visual paling of the LV. After 40 min, the snare was released, and reperfusion was confirmed with visual hyperemia of the LV and return of the ECG changes. After 2 h of reperfusion, the LCA was reoccluded, and fluorescent polymer microspheres (0.4% solution, diameter 1–10 μm; Duke Scientific, Palo Alto, CA) were infused via needle puncture of the LV apex. The microspheres showed a maximum excitation at 360-nm-long UV light and delineated the nonperfused, ischemic area at risk (AAR) as a negative image. Heart was then excised and rinsed in normal saline, and the right and left atria were removed under the dissecting microscope. The remaining left and right ventricles were weighed and then sliced into seven sections (diameter = 1 mm) for imaging and staining.
Measurement of AAR and I.
Heart sections were immediately photographed with a digital camera (Canon Powershot A620) under UV light for determination of AAR. I was determined by staining the heart sections in 1% 2,3,5-triphenyltetrazolium chloride (TTC) in phosphate buffer at pH 7.4 for 10 min, followed by 10% neutral buffered formalin bath overnight. Pictures were taken the next day under full spectrum light. TTC stained noninfarcted myocardium red, while infarcted myocardium remained white. Both AAR and I areas were calculated in a blinded fashion using standard imaging software (Photoshop Elements 4). A calibration ruler was present in each picture to convert pixels into volume. TTC slices were volume corrected for dehydration following formalin immersion.
Drugs.
The 14,15-EET was a kind gift from Dr. J. R. Falck and also obtained from BIOMOL. It was dissolved in a vehicle composed of 100% ethanol and saline (1:3), resulting in a final ethanol solution of 25%. The selective sEH inhibitor, 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester (AUDA-BE), was kindly provided by Dr. Bruce Hammock. AUDA-BE was dissolved in sesame oil, as previously described (20). The EET antagonist, 14,15-EEZE, was obtained from Cayman Chemical and dissolved in 25% ethanol. EEZE acts as direct EET antagonist on a putative EET receptor (3).
14,15-EET (2.5 μg/g body wt iv) or AUDA-BE (10 μg/g ip) was given either before LCA occlusion or before reperfusion and compared with vehicle control. EEZE (2.5 μg/g body wt iv) was given 15 min before reperfusion (see Fig. 1 for experimental protocol and time line). The 14,15-EET concentration chosen was previously reported to be cardioprotective in rats (5), and the AUDA-BE concentration used was based on our laboratory's previously published pharmacokinetic studies (20). To compare the magnitude of cardioprotection found within the epoxide pathway with that of other cardioprotective agents, we used SNC-162, a nonpeptide δ-opioid receptor agonist. δ-Opioid receptor agonists produce cardioprotection against myocardial I/R (14). SNC-162 (0.1 μg/g iv over 10 min) was dissolved in 10% DMSO.
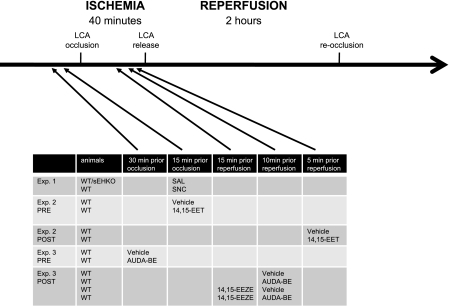
Fig. 1.
Experimental protocol and time course. Male mice were subjected to 40 min of occlusion of the left coronary artery (LCA) and 2 h of reperfusion. The LCA was reoccluded at the end of each experiment to assess the ischemic area at risk (AAR), as describe in materials and methods. The figure shows the type of animals and the time of drug injection for each experiment. WT, wild-type C57BL\6J; sEHKO, soluble epoxide hydrolase (sEH) knockout mice; WTSNC, WT pretreated with the δ-opioid agonist SNC-162 per infusion for 10 min before occlusion; PRE, given before ischemia; POST, given during ischemia before reperfusion; AUDA-BE, sEH inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester; 14,15-EET, 14,15-epoxyeicosatrienoic acids; 14,15-EEZE, 14,15- epoxyeicosa-5(Z)-enoic acid; SAL, saline.
Immunoblotting.
Proteins from LV tissue were dissolved in SDS sample buffer (2% SDS, 10% glycerol, 80 mM Tris, pH 6.8, 0.15 M β-mercaptoethanol, 0.02% bromphenol blue) and separated on 4–20% linear gradient SDS-polyacrylamide gels (Bio-Rad) in a minigel apparatus (Mini-PROTEAN 3, Bio-Rad) and transferred to polyvinylidene difluoride membranes (Amersham). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline-Tween 20 (10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20) for 60 min at room temperature and incubated overnight at 4°C with primary rabbit anti-sEH antibody (1:2,000 in 5% dry milk), as previously described (20). After washing, the antigens were detected with fluorescent secondary antibodies (Cy5-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-mouse, 1:2,500 in phosphate-buffered saline Tween (PBST) with 5% BSA) provided with and according to the protocols of the ECL-Plus Western Blotting Detection Kit (Amersham). Immunoreactivity was detected simultaneously using a Typhoon Trio variable mode imager (Amersham).
Immunohistochemistry.
Mouse LV myocardial samples were fixed with fresh 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. The sections were mounted on superfrost glass slides. Sections were kept at 60°C overnight and then deparaffinized with xylene followed by washing in 100, 96, 80, and 70% ethanol. Heat unmasking of epitopes was done by boiling the samples three times in citrate buffer (pH 6) at 600 W in a microwave oven. After cooling down to room temperature and 1 h of blocking in 5% dry milk, slides were incubated overnight at room temperature with primary antibodies (rabbit anti-sEH antibody, 1:50), as previously described (7). After washing in PBS, sections were incubated with secondary FITC-conjugated goat anti-rabbit IgG (1:100 in 5% dry milk, Invitrogen) and Alexa Fluo 597 donkey anti-sheep IgG (1:200 in 5% dry milk, Invitrogen) for 4 h. After washing, sections were placed in an autofluorescence-reduction solution (10 mM CuSO4, 50 mM ammonium acetate) for 30 min, and then mounted with Slow Fade Gold Antifade Reagent with 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen). The sections were viewed on Zeiss Axiovert 200 fluorescent microscope and MetaMorph imaging system (Universal Imaging). Negative controls included sections in which the primary antibodies were omitted (incubated with secondary antibodies only), or sections in which both primary and secondary antibodies were omitted. Further negative controls included use of sEH primary antibody after preincubation with its specific blocking peptide and use of tissue from a sEHKO mouse heart. No sEH signal was detectable in any of the negative controls.
Statistics.
Data were analyzed using a commercially available statistics program (Prism 5.0). Differences between groups were assessed with Student's t-test and one-way ANOVA with post hoc Newman-Keuls test as appropriate. All data were expressed as means ± SE, unless otherwise noted. A value of P < 0.05 was considered to indicate statistical significance.
RESULTS
sEH gene deletion reduces I after myocardial I/R.
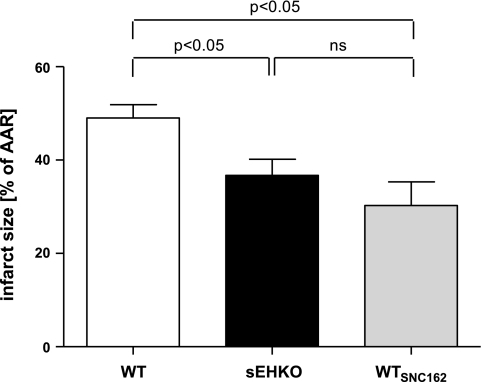
sEHKO mice sustained significantly reduced I/AAR compared with WT C57BL\6J mice. The observed reduction of I/AAR size in sEHKO mice was comparable to the effect of preischemic administration of SNC-162 (WTSNC) (Fig. 2). The ischemic insult was similar in both groups, as evidenced by similar AARs normalized to biventricular heart volume (Table 1). Temperature was kept at 37°C during the entire duration of the experiment and showed no difference between WT, sEHKO, and WTSNC mice. Heart rates recorded before LCA occlusion and 30 min of reperfusion were significantly lower in sEHKO (535 ± 16 beats/min) and WTSNC (494 ± 18 beats/min) mice compared with WT controls (619 ± 12 beats/min; P < 0.05).
Fig. 2.
sEH depletion reduces infarct size (I) after regional myocardial ischemia-reperfusion (I/R). Shown is the I as percentage of AAR for WT, sEHKO, and WTSNC. I/AAR is significantly reduced in sEHKO mice. The reduction is comparable to opioid-induced cardioprotection. Values are means ± SE; n = 5–10 animals per group. P < 0.05. ns, Not significant.
Table 1.
Weight and heart volumes
| Body Weight, g | Biventricular Weight, mg | Area At Risk, mm3 | Infarct Volume, mm3 | AAR/Biventricular Volume, % | |
|---|---|---|---|---|---|
| WT | 28±1 | 114±5 | 39±5 | 18±2 | 29±3 |
| sEHKO | 25±1 | 115±3 | 35±4 | 13±2* | 27±3 |
| SNC | 26±1 | 115±6 | 31±3 | 9±2* | 27±2 |
| Pre | |||||
| Vehicle | 28±1 | 131±3 | 38±4 | 21±3 | 28±3 |
| 14,15-EET | 27±0 | 130±4 | 36±2 | 10±2* | 27±2 |
| Post | |||||
| Vehicle | 28±0 | 128±2 | 43±4 | 20±2 | 32±3 |
| 14,15-EET | 27±1 | 127±5 | 33±5 | 12±2* | 27±4 |
| Pre | |||||
| Vehicle | 27±1 | 117±3 | 43±2 | 20±2 | 33±2 |
| AUDA-BE | 27±1 | 123±3 | 39±3 | 12±2* | 28±2 |
| Post | |||||
| Vehicle | 27±0 | 121±3 | 40±3 | 19±1 | 30±2 |
| AUDA-BE | 26±1 | 120±8 | 46±4 | 14±3 | 35±1 |
| EEZE | 27±1 | 122±4 | 42±4 | 18±2 | 33±4 |
| EEZE+AUDA-BE | 27±1 | 122±9 | 42±5 | 16±3 | 32±4 |
Values are means ± SE. Shown are the body weight, wet biventricular weight, area at risk (AAR), infarct volume, and AAR as a percentage of biventricular volume for all 5 experiments. WT, wild type; sEHKO, soluble epoxide hydrolase knockout; SNC, SNC-162; Pre, before left coronary artery occlusion; Post, before reperfusion; 14,15-EET, 14,15-epoxyeicosatrienoic acid; AUDA-BE, 12-(3-adamantan-1-yl-ureido)-dodecanoic acid butyl ester; EEZE, 14,15-epoxyeicosa-5(Z)-enoic acid.
P < 0.05 compared with vehicle.
14,15-EET reduces myocardial I independent of timing of treatment.
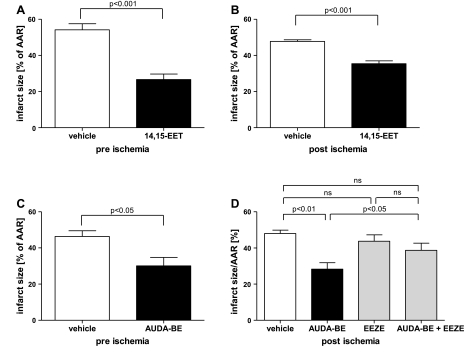
I was significantly reduced by 14,15-EET compared with vehicle, whether 14,15-EET was administered before ischemia or before reperfusion, as shown in Fig. 3, A and B. However, 14,15-EET injection before reperfusion was less effective in reducing I/AAR compared with preischemic delivery. The ischemic insult was similar in all four groups tested, as shown by similar AAR normalized to biventricular heart volume (Table 1). There was no significant difference in heart rate and rectal temperature between treatment and vehicle groups.
Fig. 3.
14,15-EET and sEH inhibition reduce I after myocardial I/R. Shown is the I as percentage of AAR for the following treatment conditions tested: 14,15-EET injection (intravenous) before ischemia (A); 14,15-EET injection (intravenous) before reperfusion (B); AUDA-BE injection (intraperitoneal) before ischemia (C); and AUDA-BE injection (intraperitoneal) before reperfusion with or without EEZE, an EET antagonist, compared with the corresponding vehicle (D). Values are means ± SE; n = 4–8 animals per group.
Inhibition of sEH reduces myocardial I independent of timing of treatment, and cardioprotection is blocked by the EET antagonist EEZE.
Administration of AUDA-BE significantly reduced I after myocardial I/R compared with vehicle control, whether it was given before ischemia or before reperfusion (Fig. 3, C and D). I did not significantly differ between preischemic and prereperfusion administration of AUDA-BE. The EET antagonist EEZE abolished the cardioprotective effect of AUDA-BE given before reperfusion (Fig. 3D). The ischemic insult was similar in all four groups tested, as shown by similar AAR normalized to biventricular heart volume (Table 1). Rectal temperature showed no significant difference between vehicle and treatment groups. Heart rate was significantly higher in the AUDA-BE preischemic group compared with the vehicle control at baseline (555 ± 24 vs. 496 ± 17 beats/min, P < 0.05).
Expression and regional distribution of sEH in LV tissue.
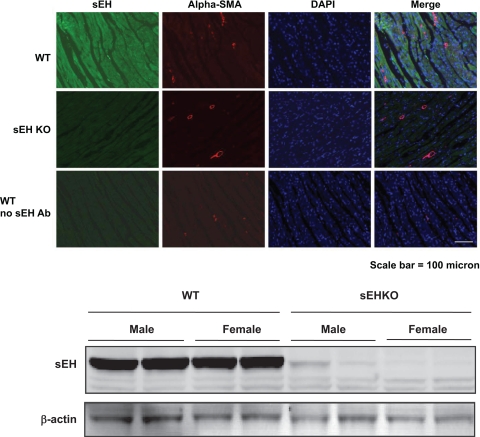
Immunhistochemistry demonstrated that sEH is abundantly expressed in WT LV cardiomyocytes, but absent in sEHKO hearts (Fig. 4A). Coimmunostaining with α-smooth muscle actin antibody, which stains myofibroblasts, showed no enhancement for sEH in this cell type. DAPI was used to visualize the nucleus, which did not enhance for sEH. The presence of sEH in LV tissue was also confirmed by immunoblotting, which showed robust expression of sEH protein in WT hearts and the absence of sEH protein in sEHKO hearts (Fig. 4B).
Fig. 4.
sEH is localized in cardiomyocytes from left ventricular tissue. Top: sEH is readily expressed in cardiomyocytes of left ventricular tissue sample. Shown is the immunoreactivity for sEH (column 1), α-smooth muscle actin (Alpha-SMA) for smooth muscle cells and fibromyoplasts (column 2), 4,6-diamidino-2-phenylindole for nuclear staining (column 3), and the merged image (column 4) for left ventricular tissue from WT (row 1), sEHKO (row 2), and for WT without primary sEH antibody (Ab) (row 3). Bottom: immonublot for sEH protein expression in left ventricular sample from WT and sEHKO from male and female animals (n = 2).
DISCUSSION
The main findings of our study are as follows: 1) sEH gene deletion is protective against regional myocardial I/R injury in vivo; 2) pharmacological inhibition of sEH is cardioprotective, whether the inhibitor is administered before or at the end of ischemia, and this cardioprotective effect is mediated by endogenous EET; 3) exogenously administered 14,15-EET reduces I in a collateral-deficient model of regional myocardial I/R, when administered before or at the end of LCA occlusion; and 4) sEH is abundantly expressed in LV cardiac myocytes from WT. These findings suggest that sEH and its main substrate 14,15-EET are important modulators of myocardial I/R injury, and that strategies aimed at increasing 14,15-EET, including sEH inhibition, may serve as a therapeutic strategy in myocardial I/R injury.
Augmentation of the levels of P-450 epoxygenase products EETs, either through inhibition or gene deletion of sEH or through administration of exogenous EETs, promotes tolerance to ischemic stress. For instance, AUDA-BE (10 μg/g ip, the dose used in the present study) reduces sEH activity in brain tissue and protects against experimental stroke (20). Similarly, sEHKO mice sustain reduced cerebral infarcts after experimental stroke (21). Recently, Gross et al. (4) showed a marked increase in 14,15-EET in coronary venous blood sample following intracoronary treatment with AUDA, the active component of AUDA-BE. Isolated hearts from sEHKO mice demonstrate improved functional recovery after 20 min of global ischemia (16). Likewise, augmentation of endogenously derived EETs by CYP2J2 epoxygenase overexpression improves functional recovery in isolated heart preparations after global I/R (15). Finally, preischemic administration of exogenous 14,15-EET reduces I in vivo in rat and dog models of regional myocardial I/R (5, 13).
Thus the current data confirm and extend our and others' previous reports showing a beneficial effect of sEH inactivation and EETs augmentation. Specifically, we expanded on studies using isolated heart preparations from sEHKO mice by demonstrating that these mice are protected from in vivo myocardial I/R using a clinically relevant LCA occlusion model. Furthermore, in the previous studies demonstrating infarct reduction by EETs administration, EETs were given preocclusion in rats (5), and both preocclusion and pre-reperfusion in dogs (13). Although the canine data were very strong and suggestive of an EETs-induced direct cardiomyocyte protection, EETs are coronary vasodilators, and dogs possess a variable and frequently robust coronary collateral circulation. Thus we felt it was important to confirm the “postischemic” protective effect of EETs in a collateral-deficient mouse model of regional I/R. The current data show that administration of EETs at the onset of reperfusion imparts tolerance to myocardial I/R in a collateral deficient species, suggesting that the salutary effect is indeed a direct cardiomyocyte phenomenon.
Soluble epoxide hydrolase is a bifunctional enzyme with a hydrolase activity located in the COOH-terminal and a phosphatase activity in the NH2-terminal (12). Most of the known biological functions of sEH are attributed to its hydrolase activity. The main substrate for sEH is 14,15-EET, resulting in rapid hydration to 14,15-DHET. That cardioprotection mediated by sEH inhibition is mainly due to augmenting endogenous 14,15-EET is supported by our observation that pre-reperfusion administration of EEZE abolished the cardioprotective effect.
In the current data, sEH inhibition just before reperfusion resulted in EET-mediated cardioprotection. This extends previously reported findings from other investigators. For example, the 14,15-EET antagonist 14,15-EEZE abolished the improved recovery of LV developed pressure in isolated hearts from sEHKO mice (16), and Gross et al. (4) showed that the cardioprotective effect of preischemic sEH inhibition was completely abolished by EEZE. However, both studies administered the sEH inhibitor and EET antagonist before the ischemia, rather than at the more clinically relevant time point of reperfusion onset.
Soluble epoxide hydrolase is known to be expressed in several tissues, including blood vessels (1). In the brain, it has been localized in neurons (9). sEH activity has been detected in heart tissue by immunoblot (16). However, it is not clear which cell type constitutes the source for sEH. Accordingly, we were able to confirm the abundant expression of sEH in LV tissue from WT mice and the absence in hearts from sEHKO mice. More importantly, our study is the first to localize sEH in cardiomyocytes from LV tissue by immunohistochemistry. This observation is consistent with the ability of sEH inhibition to induce cardioprotection independent of vasodilatation. sEH expression was also observed in coronary vessels.
The primary endpoint in the present study was reduction in I; blood pressure and cardiac function following regional myocardial ischemia were not assessed. Because EETs are vasodilators, it is possible that sEH inhibition or gene deletion may have resulted in a decrease in postischemic blood pressure. However, if hemodynamically significant, this would have resulted in reduced coronary perfusion pressure and increased I, which was not the case. Although sEH inhibition is expected to augment endogenous EETs, the effect will be most pronounced in tissues with high EET production, such as ischemic tissue. This (ischemic) tissue specificity distinguishes the current approach of pharmacological soluble epoxide hydrolase inhibition from other previously tested cardioprotective agents. Thus regional myocardial ischemia should limit the augmentation of EETs primarily to the ischemic area within the heart. Therefore, although EETs are vasodilatory, systemic vasodilation and hypotension are not expected.
We detected a difference in heart rate between sEHKO mice and controls at baseline, and it is possible that this would contribute to the reduction in I seen. However, the animals in the preischemic AUDA-BE group showed a reduction in I, despite increased heart rate, which makes a heart rate-dependent effect less likely. Indeed, Gross et al. (5) reported no differences in heart rate and blood pressure at 30 min of occlusion and 2 h of reperfusion in a rat model of regional myocardial I/R in between groups tested with a similar dose of 14,15-EET. In contrast to regional myocardial ischemia, where sEHKO mice are protected, in cardiac arrest-induced whole body ischemia, sEHKO mice were at a disadvantage, presumably due to excessive vasodilation and an inability to maintain blood pressure after cardiopulmonary resuscitation (6).
AUDA-BE is a potent and selective sEH inhibitor (8). The pharmacokinetic profile in C57BL\6J mice has recently been characterized, and the dose used in the present study (10 μg/g) results in plasma levels greater than the IC50 for the inhibitor within 1 h of administration (20). The fact that both sEH inhibition and exogenously administered 14,15-EET resulted in reduced I when given during ischemia before reperfusion suggests that this approach may be useful clinically, for example, before percutaneous coronary interventions or surgical revascularization in the setting of acute myocardial ischemia.
In summary, the findings in the present study represent the first description of successful cardioprotection by sEH inhibition before and during ischemia, and the first demonstration of in vivo cardioprotection by sEH gene deletion. The data support the concept of using sEH inhibition as a novel therapeutic treatment for myocardial cardioprotection.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1 NS44313 (N. J. Alkayed), Veterans Affairs Merit Review grant (Medical Research Service, DVA; D. M. Van Winkle), and Anesthesiology Research and Education Foundation (M. J. Merkel).
Acknowledgments
The authors thank Dr. Wenri Zhang for technical assistance. The authors thank the Portland Veterans Affair Medical Center Veterinary Medical Unit and the Transgenic Animal Core Facility of the Department of Anesthesiology and Peri-Operative Medicine, Research Division, for excellent animal care and service.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J Histochem Cytochem 54: 329–335, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, Hammock BD, Spector AA. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol 287: H2412–H2420, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol 294: H2838–H2844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol 42: 687–691, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation 76: 89–94, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92: 653–658, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem 47: 2110–2122, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci 27: 4642–4649, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Hurn PD, Alkayed NJ. Cytochrome P450 in neurological disease. Curr Drug Metab 5: 225–234, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311–333, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA 100: 1558–1563, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol 291: H537–H542, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther 89: 123–137, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res 95: 506–514, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Seubert JM, Sinal CJ, Graves J, Degraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem 268: 6402–6407, 1993. [PubMed] [Google Scholar]
- 20.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931–1940, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073–2078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]