Abstract
When recovering from heart failure (HF), the myocardium displays a marked plasticity and can regain normal gene expression and function; however, recovery of substrate oxidation capacity has not been explored. We tested whether cardiac functional recovery is matched by normalization of energy substrate utilization during post-HF recovery. HF was induced in dogs by pacing the left ventricle (LV) at 210–240 beats/min for 4 wk. Tachycardia was discontinued, and the heart was allowed to recover. An additional group was studied in HF, and healthy dogs served as controls (n = 8/group). Cardiac free fatty acids (FFAs) and glucose oxidation were measured with [3H]oleate and [14C]glucose. At 10 days of recovery, hemodynamic parameters returned to control values; however, the contractile response to dobutamine remained depressed, LV end-diastolic volume was 28% higher than control, and the heart mass-to-body mass ratio was increased (9.8 ± 0.4 vs. 7.5 ± 0.2 g/kg, P < 0.05). HF increased glucose oxidation (76.8 ± 19.7 nmol·min−1·g−1) and decreased FFA oxidation (20.7 ± 6.4 nmol·min−1·g−1), compared with normal dogs (24.5 ± 6.3 and 51.7 ± 9.6 nmol·min−1·g−1, respectively), and reversed to normal values at 10 days of recovery (25.4 ± 6.0 and 46.6 ± 6.7 nmol·min−1·g−1, respectively). However, similar to HF, the recovered dogs failed to increase glucose and fatty acid uptake in response to pacing stress. The activity of myocardial citrate synthase and aconitase was significantly decreased during recovery compared with that in control dogs (58 and 27% lower, respectively, P < 0.05), indicating a persistent reduction in mitochondrial oxidative capacity. In conclusion, cardiac energy substrate utilization is normalized in the early stage of post-HF recovery at baseline, but not under stress conditions.
Keywords: dilated cardiomyopathy, fatty acids, glucose, tachypacing
the capacity of the failing heart to recover and restore, at least in part, a normal structure and function has been well demonstrated, both in animal models (12, 13, 26, 28) and in patients (3, 25). In pacing-induced heart failure (HF), an established model of dilated cardiomyopathy, hemodynamic and neurohormonal alterations, as well as myocyte function, return to control level within the first 2 wk of recovery after discontinuation of cardiac stimulation (28). Moreover, a number of studies have been performed in end-stage HF patients sustained with mechanical left ventricular (LV) assist devices while awaiting cardiac transplantation. The availability of ventricular tissue samples collected during the procedures of assist device implantation and removal has stimulated a strong interest in the molecular and cellular alterations occurring before and after mechanical unloading. Mechanical assistance favors reverse remodeling at structural, contractile, electrophysiological, and molecular levels in the failing myocardium (5, 11, 32). None of these studies, however, explored potential changes in cardiac energy substrate utilization during postfailure recovery.
The failing heart displays profound alterations in myocardial substrate selection, specifically a decrease in the uptake and oxidation of plasma free fatty acids (FFA) and an increase in glucose uptake and oxidation, which may significantly contribute to the progressive deterioration of cardiac function (2, 19, 29, 30). A major role of these alterations in the pathophysiology of HF is suggested also by a number of experimental and clinical investigations showing marked beneficial effects of pharmacological metabolic modulators (8, 14, 16). In addition, our laboratory previously found that the increase in glucose uptake in HF is associated with higher NADPH levels produced by glucose-6-phosphate dehydrogenase (G6PD), a key enzyme of the oxidative pentose phosphate pathway, which can enhance myocardial superoxide generation by NAD(P)H oxidase, thus suggesting a link between accelerated glucose metabolism and oxidative stress in the failing heart. In vivo studies on myocardial metabolism involve methods and invasive protocols that are not feasible in critical patients with LV assist devices; therefore, animal models of HF remain a necessary alternative to study the reversibility of metabolic changes.
In the present study, we tested whether cardiac functional recovery is matched by a normalization of myocardial energy substrate metabolism in dogs allowed to recover from pacing-induced HF. Our laboratory has previously shown that severe HF induced by sustained tachypacing shifts cardiac muscle to a higher utilization of glucose as a metabolic substrate, while it reduces FFA consumption (21), thus displaying a metabolic pattern strikingly similar to the one described in patients with dilated cardiomyopathy (6, 18). In addition, pacing-induced HF offers the key advantage of being reversible (12, 28) and, therefore, allows assessment of metabolic changes during postfailure recovery. To compare cardiac substrate metabolism of normal, failing, and recovering dogs, we combined measurements in conscious animals obtained by infusing isotope-labeled FFA and glucose, with quantifications of enzyme activities in freeze-clamped myocardial biopsies sampled from the beating heart at the end of the experimental protocols. Since a hallmark of HF in patients is the loss in metabolic flexibility in response to increased work demand (1, 18), substrate metabolism was assessed both at basal and increased heart rate.
MATERIALS AND METHODS
Surgical instrumentation and hemodynamic measurements.
Twenty-four adult, male, mongrel dogs (25–27 kg) were sedated with acepromazine maleate (1 mg/kg im), anesthetized with pentobarbital sodium (25 mg/kg iv), ventilated with room air, and chronically instrumented, as previously described (23, 26, 32). A thoracotomy was performed in the left fifth intercostal space. A catheter was placed in the descending thoracic aorta. A solid-state pressure gauge (P6.5; Konigsberg Instruments) was inserted into the LV through the apex. A Doppler flow transducer (Craig Hartley) was placed around the left circumflex coronary artery, and a human, screw-type, unipolar myocardial pacing lead was fixed in the LV wall. Wires and catheters were run subcutaneously to the intrascapular region, the chest was closed in layers, and the pneumothorax was reduced. Antibiotics were given after surgery, and the dogs were allowed to fully recover. After 7–10 days of recovery from surgery, dogs were trained to lie quietly on the laboratory table. The protocol was approved by the Institutional Animal Care and Use Committee of the New York Medical College and conform to the guiding principles for the care and use of laboratory animals published by the National Institutes of Health.
Experimental protocol.
HF was induced in 16 dogs by pacing the LV at 210 beats/min for 3 wk, then the pacing rate was increased to 240 beats/min (23). Dogs were considered to be in end-stage HF when LV end-diastolic pressure (LVEDP) was ≥25 mmHg, and hemodynamic hallmark was associated with clinical signs of severe decompensation (23). Eight dogs were utilized at this stage for the final experiment and then killed (HF group), whereas, in eight of the pacing dogs, the pacemaker was turned off when the LVEDP reached 25 mmHg. These dogs were allowed to recover, and the final in vivo experiment was performed 10 days later (Rec group). Since it was necessary to harvest large cardiac biopsies at the end of the in vivo experiment, we used a separate group of eight chronically instrumented, normal dogs for control (Con).
Experiments were conducted in intact, conscious dogs placed on the laboratory table following an overnight fast. In Rec, hemodynamic variables were recorded, and echocardiographic measurements were performed at four time points: baseline, 3 wk (which corresponds to compensated failure), end-stage failure, and 10 days of recovery. The experiments were performed at spontaneous heart rate, with the pacemaker turned off. At each time point, the contractile response to adrenergic stimulation was tested by infusing dobutamine at 5, 10, and 15 μg·kg−1·min−1 iv, with 5 min of infusion at each dose. The last dobutamine test was performed the day before the terminal experiment, to avoid catecholamine-induced alterations in myocardial enzyme activities.
For the terminal experiment in Con, HF, and Rec dogs, the coronary sinus was cannulated under fluoroscopic guidance, and the stability of its position was frequently checked. The coronary sinus catheter was introduced via a superficial leg vein; therefore, this procedure did not require any sedation. Then the radioisotopic tracers [9,10-3H]oleate (0.7 μCi/min) and [U-14C]glucose (20 μCi as a bolus, followed by 0.3 μCi/min) were infused through a peripheral vein to track the metabolic fate of FFA and glucose utilized by cardiac muscle as source of energy (21). During the infusion, Con and Rec were paced at 130 beats/min to match the spontaneous heart rate of HF dogs. After 40 min of tracer infusion, paired blood samples were withdrawn from the aorta and coronary sinus. Then the pacemaker was reactivated for 10 min at the rate of 210 beats/min to induce pacing stress, and a new set of paired blood samples was collected. In one Con, one HF, and two Rec dogs, measurement with isotopes was not possible due to technical problems with coronary sinus catheterization. At the end of this procedure, the dogs were anesthetized with 30 mg/kg iv of pentobarbital sodium, intubated, and ventilated, and the heart was exposed through an incision and retraction of the fifth intercostal space, as previously described (16, 21). A large transmural biopsy (∼10 g) was taken from the LV anterior free wall while the heart was still beating, immediately freeze-clamped with tongs precooled in liquid nitrogen, and stored at −70°C for subsequent measurements of enzyme activities. The remainder of the heart was then removed and weighted to determine the heart weight-to-body weight ratio, and additional samples were frozen in isopentane for subsequent histological analysis.
Hemodynamics, echocardiographic recordings, and calculated parameters.
The aortic catheter was attached to a P23ID strain-gauge transducer to measure aortic pressure. LV pressure was measured using the solid-state pressure gauge. The first derivative of LV pressure, LV dP/dt, was obtained using an operational amplifier (National Semiconductor LM 324). Coronary blood flow was measured with a pulsed Doppler flow meter (model 100, Triton Technology). All signals were recorded on an eight-channel direct-writing oscillograph (Gould RS 3800). The analog signals were also stored in computer memory through an analog-digital interface (National Instruments), at a sampling rate of 250 Hz. The triple product, an index of cardiac metabolic demand, was calculated as heart rate × LV systolic pressure × maximum dP/dt. Two-dimensional and M-mode echocardiography was also performed (Sequoia C256; Acuson, Mountain View, CA). Images were obtained from a right parasternal approach at the midpapillary muscle level, according to the criteria of the American Society of Echocardiography. Our laboratory has used these methods in previous studies (16, 21, 23).
Total and labeled metabolites.
Oxygen content and total cardiac substrate concentrations were measured in arterial and coronary sinus blood samples, as previously described (21). In brief, blood gases and oxygen content were measured by using a blood-gas analyzer and a hemoglobin analyzer, respectively. FFA concentration was determined spectrophotometrically in plasma. Glucose and lactate concentrations were measured in blood deproteinized with ice-cold 1 M perchloric acid (1:2 vol/vol) using spectrophotometric enzymatic assays. The concentrations of labeled metabolites were determined in arterial and coronary sinus blood samples. In particular, [3H]oleate activity was measured in plasma, whereas [14C]glucose activity was determined in blood deproteinized with ice-cold 1 M perchloric acid (1:2 vol/vol). 3H2O and 14CO2 activities were also measured in plasma and whole blood, respectively.
Myocardial O2 consumption (MV̇o2) was calculated by multiplying the arterial-coronary sinus difference in oxygen content by mean coronary blood flow. Mean coronary blood flow and the specific activities of [3H]oleate and [14C]glucose were multiplied, respectively, by the arterial-coronary sinus difference in 3H2O and in 14CO2 to calculate the rates of FFA and glucose oxidation. Arterial and coronary sinus concentration of lactate were used to calculate net chemical lactate uptake. MV̇o2 and rates of substrate consumption were normalized by cardiac weight.
Enzyme activities and metabolites in cardiac tissue.
The activity of the mitochondrial enzyme medium chain acyl-CoA dehydrogenase (MCAD) was measured as a marker of the capacity for fatty acid β-oxidation, and citrate synthase and aconitase were used as indexes of the oxidative capacity of the citric acid cycle. MCAD and citrate synthase activity were measured spectrophotometrically in extracts from powdered LV tissue, as previously described (16, 21). Aconitase activity was assayed spectrophotometrically, as described by Nulton-Persson and Szweda (20). Briefly, 25 μl of a 5% heart tissue homogenate was added to 375 μl of incubation mixture that contained 5 mM citrate, 0.5 mM MgCl2, 0.5 mM NADP+, and 1 U/ml isocitrate dehydrogenase, pH 7.4 (400 μl total volume at 37°C). Changes in absorbance at 340 nm were measured for 5 min. Triglyceride and glycogen concentration in tissue was determined using enzymatic spectrophotometric assays, as previously described (16, 21).
Protein expression.
Protein expression of MCAD and citrate synthase was determined by Western blot. In brief, 75 μg of protein of each sample were loaded on polyacrylamide gel for electrophoresis. After transfer, membranes were incubated with polyclonal anti-MCAD (1:200, Alexis Biochemicals) and monoclonal anti-citrate synthase (1:500, Chemicon), diluted in 1% milk. For loading control, membranes were stripped and reprobed with anti-calsequestrin (1:3,000, Affinity Bioreagents) (15).
Superoxide production in LV tissue.
Our laboratory has previously shown that the increased production of superoxide in LV tissue homogenates can be normalized by blocking G6PD, a key enzyme of the oxidative pentose phosphate pathway (9, 10). This suggests a potential link between altered glucose utilization and oxidative stress in the failing heart. To determine whether this metabolic alteration favoring oxidative stress is reversed during postpacing recovery, we pulverized LV tissue in liquid nitrogen and prepared homogenates in MOPS (50 mmol/l)-sucrose (250 mmol/l) buffer at pH 7.4. Only freshly prepared homogenates were used for biochemical assays. To detect superoxide, the homogenates were brought to a final volume of 50 μl and incubated for 30 min at 37°C without adding any drug, and matched samples were pretreated for 30 min with 6-aminonicotinamide (5 mmol/l), an inhibitor of G6PD. Incubated samples (20 μl) were placed in plastic scintillation minivials containing 5 μM lucigenin for the detection of superoxide, measured as arbitrary units (AU) per milliliter, in a final volume of 1 ml of air-equilibrated Krebs solution buffered with 10 mmol/l HEPES-NaOH (pH 7.4). Our laboratory has described these methods previously (9, 10).
Histomorphometric measurements.
Tissue sections were obtained from LV free wall samples rapidly frozen in isopentane precooled in liquid nitrogen and stored at −70°C until used. Six-micrometer-thick sections were stained with 1% Griffonia Simplicifolia Lectin 1 for 1 h at room temperature to evidence capillaries and to delineate cardiomyocytes. Digital pictures were recorded under fluorescent light, and computer-assisted measurements of capillary density and myocyte cross-sectional area were performed using Mocha image analysis software (Jandel Scientific, San Rafael, CA) (24).
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed by employing commercially available software (SigmaStat 3.0). Hemodynamic and metabolic changes at different time points in the same group (Rec) and differences between groups (Con, HF, and Rec) were compared by one- and two-way ANOVA followed by Tukey post hoc test. For all of the statistical analyses, significance was accepted at P < 0.05.
RESULTS
Hemodynamics and β-adrenergic response.
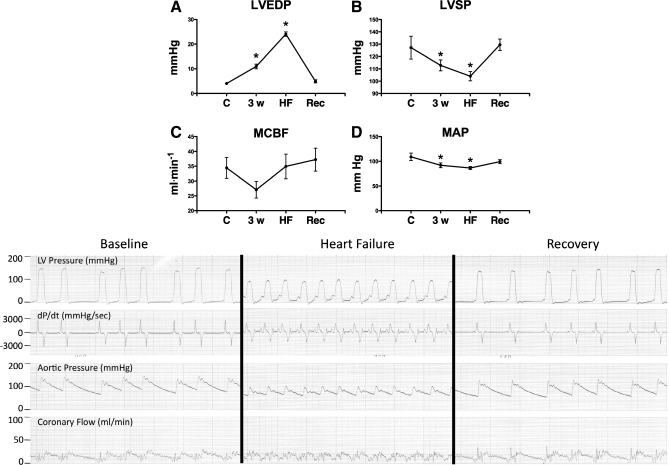
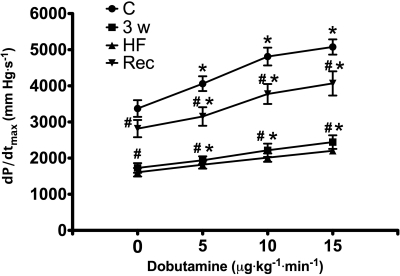
Spontaneous heart rate increased significantly from 99.3 ± 6.5 beats/min (control) to 127.0 ± 4.6 beats/min in HF and then dropped to 100.0 ± 12.1 beats/min after recovery [not significant (NS) vs. control]. Figure 1 shows the other main hemodynamic changes in the Rec group. LVEDP reached 24 ± 2.6 mmHg at 30 ± 0.6 days of pacing; therefore, that time point is indicated as week 4. LVEDP and systolic pressure returned to control levels at 10 days of recovery, while blood flow in the left circumflex coronary artery did not display significant differences relative to control at any time point. The contractile response to β-adrenergic stimulus is shown in Fig. 2. As expected, maximum dP/dt was significantly depressed at baseline and during dobutamine infusion at 3 and 4 wk of pacing, with no significant differences between these two time points. At 10 days of postpacing, both baseline and drug-induced changes had significantly recovered compared with HF; however, these values were still ∼20% lower than control.
Fig. 1.
Hemodynamics at control (C), after 3 wk of pacing (3w), heart failure (HF), and recovery (Rec). A: left ventricular (LV) end-diastolic pressure (LVEDP); B: LV systolic pressure (LVSP); C: mean coronary blood flow (MCBF); D: mean arterial pressure (MAP). Values are means ± SE; n = 8. *P < 0.05 vs. C. Representative tracings are shown below the graphs. dP/dt, first derivative of LV pressure.
Fig. 2.
Changes in maximum dP/dt (dP/dtmax) in response to dobutamine at C, 3w, HF, and Rec. Values are means ± SE; n = 8. *P < 0.05 vs. C; #P < 0.05 vs. C at the respective time points.
Echocardiographic measurements.
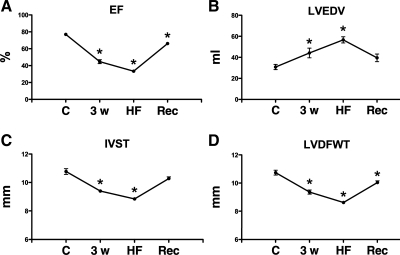
At 10 days postpacing, ejection fraction was still slightly, but significantly, lower than control (Fig. 3). Morphometic parameters assessed echocardiographically were still altered during recovery compared with control. Specifically, LV end-diastolic volume and diameter were significantly higher, while septum and LV free wall thickness were significantly smaller.
Fig. 3.
Echocardiographic measurements at C, 3w, HF, and Rec. A: ejection fraction (EF); B: LV end-diastolic volume (LVEDV); C: LV end-diastolic diameter (LVEDD); D: LV diastolic wall thickness (LVDWT). Values are means ± SE; n = 8. *P < 0.05 vs. C.
Cardiac weight and histomorphometric analysis.
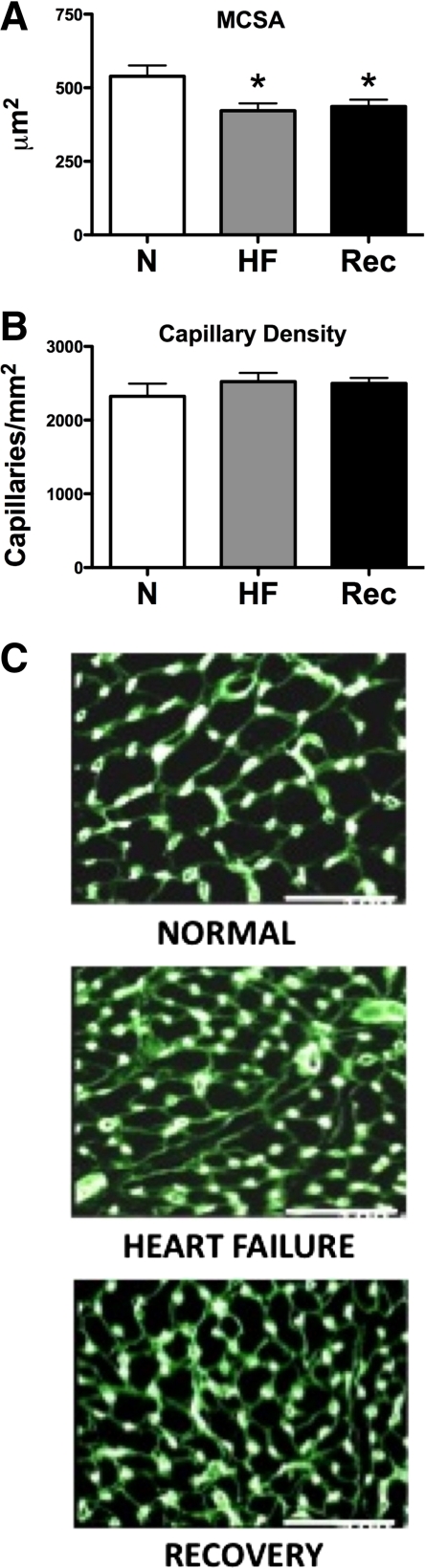
Body weight was 25.5 ± 0.8 kg in Con, 24.6 ± 1.3 kg in HF (NS vs. Con), and 22.0 ± 0.5 kg in Rec (P < 0.05 vs. Con). Heart weight was 190.7 ± 3.7 g in Con, 225.8 ± 10.3 g in HF (P < 0.05 vs. Con), and 214.8 ± 8.5 g in Rec (NS vs. Con). The heart weight-to-body weight ratio was 7.5 ± 0.2 g/kg in Con, 9.3 ± 0.3 g/kg in HF, and 9.8 ± 0.4 g/kg in Rec (both P < 0.05 vs. control). The histological analysis showed no significant differences in capillary density, but reduced cardiomyocyte cross-sectional area in both HF and Rec compared with control (Fig. 4).
Fig. 4.
Histological morphometrics in normal (N; n = 6), HF (n = 8), and Rec (n = 8) dogs. MCSA, cardiomyocyte cross-sectional surface area (A). B: capillary density. Values are means ± SE. *P < 0.05 vs. C. C: representative histological preparations.
Myocardial energetics and metabolic alterations.
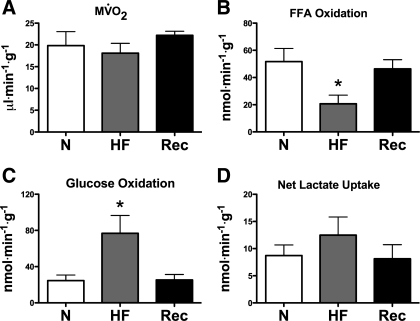
As shown in Fig. 5, there were no significant baseline differences in MV̇o2 among Con, HF, and Rec. At matched MV̇o2, FFA oxidation was ∼60% lower in HF and returned to values not significantly different from Con in the Rec group. Glucose oxidation followed a reciprocal pattern, increasing more than twofold in HF, and returning to Con values in Rec.
Fig. 5.
Cardiac oxygen and energy substrate consumption in N (n = 7), HF (n = 7), and Rec (n = 6) dogs. A: myocardial O2 consumption (MV̇o2); B: free fatty acid (FFA) oxidation; C: glucose oxidation; D: net lactate uptake. Values are means ± SE. *P < 0.05 vs. C.
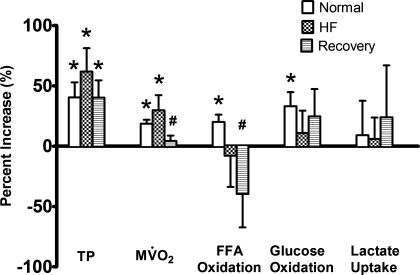
Baseline triple product (values × 103) in Con was 16,450.5 ± 1,691.6 mmHg2·beat·s−2, fell in HF down to 7,045.4 ± 2,068.4 mmHg2·beat·s−2 (P < 0.05 vs. Con), and recovered in Rec to 10,401.1 ± 994.279 mmHg2·beat·s−2 (NS vs. Con). Pacing stress significantly increased the triple product in all groups, with no significant differences among them (Fig. 6). The change in triple product was well reflected by an increase in MV̇o2 in all groups, but to a significantly lesser extent in Rec compared with Con and HF. A further indication that the metabolic demand was changing differently in the various groups was given by the percent increase in coronary blood flow: 16.4 ± 3.3% in Con, 20.7 ± 4.1% in HF (NS vs. Con), and 5.3 ± 1.8% in Rec (P < 0.05 vs. Con). Finally, substrate oxidation increased significantly in response to the pacing stress only in Con, with a significantly lower percent change in FFA oxidation in Rec compared with Con. Despite these marked differences in pacing stress response, we did not find any significant change in the concentration of triglyceride and glycogen (Table 1) in tissue samples collected when the heart was still beating and paced.
Fig. 6.
Percent change of triple product (TP), MV̇o2, FFA oxidation, glucose oxidation, and lactate uptake in N (n = 7), HF (n = 7), and Rec (n = 6) dogs, in response to pacing stress at 210 beats/min. Values are means ± SE. *P < 0.05 vs. baseline, i.e., heart rate fixed at 135 beats/min. #P < 0.05 vs. N.
Table 1.
Myocardial tissue concentration of triglycerides and glycogen
| Triglycerides | Glycogen | |
|---|---|---|
| Normal | 16.19±3.19 | 39.50±5.05 |
| Heart failure | 24.17±5.12 | 45.61±5.59 |
| Recovery | 19.72±7.44 | 44.04±3.50 |
Values are means ± SE in μmol/gram wet wt; n = 8 for all groups.
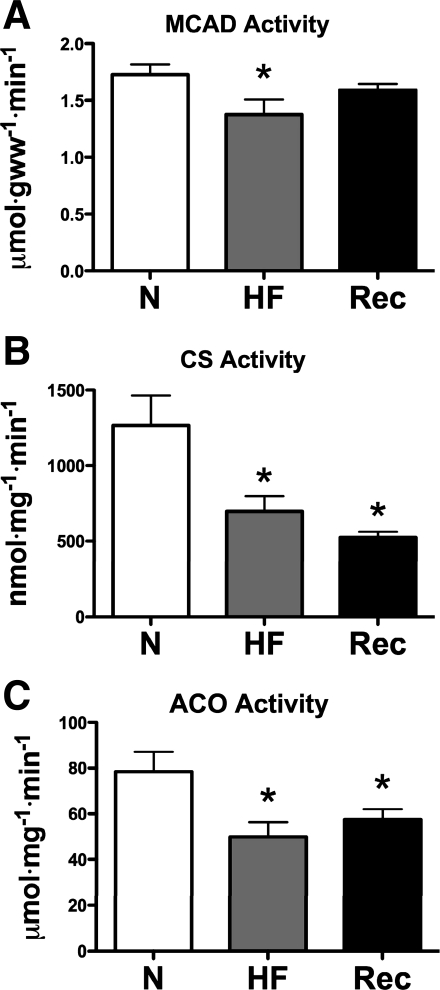
Figure 7 shows alterations in the activity of three key metabolic enzymes in LV myocardium. Consistent with reduced FFA oxidation, MCAD activity was significantly lower in HF, but not in Rec, compared with Con. However, citrate synthase and aconitase activities were lower in HF and did not return to normal levels in Rec. Changes in protein expression of these enzymes were consistent with changes in activity only for MCAD, but not for citrate synthase (Fig. 8).
Fig. 7.
Enzyme activities, medium-chain acyl CoA dehydrogenase (MCAD; A), citrate synthase (CS; B), and aconitase (ACO; C), in N (n = 8), HF (n = 8), and Rec (n = 7) dogs. Values are means ± SE. gww, Gram wet weight. *P < 0.05.
Fig. 8.
Protein expression of MCAD and CS. Values are means ± SE; n = 8 for all groups. *P < 0.05.
Superoxide production in LV homogenates was increased in HF [177 ± 24 AU/mg in Con and 611 ± 147 AU/mg in HF (n = 5 per group); P < 0.05]. The excess superoxide production was completely abolished by pretreatment with 6-aminonicotinamide, an inhibitor of G6PD, indicating involvement of the oxidative pentose phosphate pathway (10). Superoxide production returned to normal values in Rec (163 ± 31 AU/mg).
DISCUSSION
The present study shows that basal cardiac energy substrate oxidation is largely normalized at an early stage of postfailure recovery, when reverse morphological remodeling is not complete and contractile function is still impaired. Specifically, we found that: 1) baseline hemodynamics returned to control level, while ejection fraction and β-adrenergic response were still partially depressed compared with control; 2) LV was still dilated, its walls, as well as cardiomyocyte cross-sectional area, were thinner than control, and heart weight was increased; and 3) baseline FFA and glucose oxidation were normalized, but showed no increase in response to pacing stress, while activities of aconitase and citrate synthase remained lower than control.
The increasing use of cardiac support devices and resynchronization therapy to sustain cardiac function in HF patients has stimulated a strong interest in the processes of reverse remodeling (7, 17, 25). In particular, a remarkable cellular and molecular plasticity of failing myocardium in response to mechanical unloading has been shown in tissue samples harvested from patients supported by LV assist devices and awaiting cardiac transplant (3, 5, 11, 32). Similarly, previous reports described morphological, neurohormonal, and functional reverse changes that characterize the recovery of tachycardia-induced cardiomyopathy in dogs and rabbits (12, 13, 28). However, no prior clinical and experimental studies provided information regarding reverse changes in cardiac substrate oxidation during postfailure recovery. FFA and glucose utilization changes profoundly in severe HF. The failing heart oxidizes less FFA, which constitutes the main source of energy for the healthy cardiac muscle, and utilizes more glucose, as we and others have described in animal models and patients (29). These changes may play an important role in the evolution of HF toward decompensation, as suggested by the beneficial effects of metabolic therapies (8, 14, 16). Moreover, our laboratory has recently proposed a mechanistic link between enhanced glucose shunt through the oxidative pentose phosphate pathway and myocardial superoxide production in both pacing-induced and ischemic HF: part of the excess NADPH generated by this pathway can feed NADPH-oxidase, a major superoxide-producing enzyme (9, 10).
Characterization of the cardiac energy substrate metabolism requires paired arterial and coronary blood sampling to directly measure isotope-labeled substrate oxidation in vivo and myocardial biopsies for analysis of enzyme activities. These methods are not feasible in patients in end-stage failure supported by ventricular assist devices; therefore, a large animal model of postfailure recovery becomes a necessary alternative. Pacing-induced HF is reversible, and, in addition, chronic dogs allow direct metabolic measurements in the conscious state. Based on previous, detailed descriptions of the time course of postpacing recovery (28), we selected an early time point when many of the functional parameters are already normalized. At that stage, however, cardiac chambers are still abnormally enlarged, and a hypertrophic process continues for the following weeks (28). Since the structural remodeling was persistent, we expected a sustained alteration in substrate selection. In fact, glucose utilization is abnormally high in the pathologically hypertrophic heart (27, 31), and superoxide generated by NADPH oxidase is an important pro-hypertrophic stimulus (4). We found instead that alterations in cardiac substrate oxidation were completely normalized when measured at spontaneous heart rate. Importantly, glucose oxidation returned to control values, and we also found that the pentose phosphate pathway-related excess superoxide production, which was elevated in HF as in our laboratory's previous studies (9, 10), returned to normal values 10 days postpacing. FFA oxidation returned to control levels, consistent with a normalization of the activity and protein expression of MCAD, a representative enzyme of mitochondrial β-oxidation. On the other hand, citrate synthase activity remained lower also during recovery, suggesting that the reversal of changes in cardiac substrate metabolism is complex and heterogeneous. Previous studies observed downregulation of the mRNA for genes encoding key metabolic enzymes, such as the glucose transporters GLUT-1 and -4 and carnitine palmitoyl-transferase in patients with both nonischemic and ischemic HF supported with LV assist devices (22). These findings are similar to what our laboratory previously observed in pacing-induced HF (16), and such alteration was present even after several months of ventricular assistance.
A further indication of an incomplete recovery of myocardial energy substrate metabolism was the finding that, different from healthy, normal hearts, the myocardium of recovering dogs was metabolically insensitive to pacing stress, as evidenced by the lack of change in MV̇o2 and substrate oxidation, despite a similar percent increase in the triple product in all groups. Compared with control, recovering hearts appeared able to maintain a normal performance in response to pacing stress at the expense of much less fuel consumption. Therefore, while the metabolic rigidity found in the dogs in HF confirmed our laboratory's previous report in patients with dilated cardiomyopathy (18), pacing stress revealed a persistent abnormality of the recovering heart. Based on the experimental methodology adopted in the present study, it is not possible to define the mechanisms responsible for ability of the recovering heart to respond to acute pacing stress with no apparent change in myocardial energy expenditure, as assessed by the MV̇o2. It is important to note, however, that our measurements were limited to the oxidation of exogenous substrate. This is an inherent limitation of our experimental in vivo model. We cannot exclude that, despite reduced exogenous FFA and glucose utilization, the stressed hearts were utilizing more endogenous substrate. Another possibility is that the recovering heart was more energetically efficient (i.e., greater power generation by the LV for a given MV̇o2). The activity of citrate synthase and aconitase was still as low as they were in HF, indicative of a decreased mitochondrial oxidative capacity in the citric acid cycle. On the other hand, the same alteration was present also in failing hearts, which, on the contrary, responded to pacing stress with an increase in MV̇o2.
There are several other limitations of the present study that need to be addressed. First, tachypacing causes a dilated cardiomyopathy that obviously evolves and recovers at much faster rate than the human disease. Therefore, although numerous reports have documented the high reliability of this model, some of the observed changes may not fully reflect those observable in patients. Second, the histological analysis was primarily aimed at assessing possible changes in capillary density, whereas the real myocyte size cannot be measured without knowing both cross-sectional area and length. Third, based on the abnormal substrate oxidation during pacing stress in recovering hearts, it is very likely that even more pronounced differences would be found in response to dobutamine. We did not infuse radioisotopes during the dobutamine test. Fourth, the relevance of in vitro activity measurements of enzyme activities is limited in that they are not a direct measure of mitochondrial oxidative capacity. Finally, we found an inconsistency between citrate synthase activity and protein expression, which is difficult to explain. Of note, more consistent with reduced activity, our laboratory has previously shown a marked reduction in citrate synthase gene expression in failing hearts (16).
In summary, the present study shows that alterations in energy substrate selection in the unstimulated heart are reversed in postfailure recovery, even before reversal of morphological remodeling is not complete and contractile function is still impaired. On the other hand, during this early phase of post-HF recovery, there is a persistent decrease in the activity of key mitochondrial enzymes and an abnormal oxidative response to acute pacing stress.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant P01-HL-74237 (F. A. Recchia, H. N. Sabbah, and W. C. Stanley). F. A. Recchia is an Established Investigator of the American Heart Association.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersson B, Blomström-Lundqvist C, Hedner T, Waagstein F. Exercise hemodynamics and myocardial metabolism during long-term beta-adrenergic blockade in severe heart failure. J Am Coll Cardiol 18: 1059–1066, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 116: 434–448, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 355: 1873–1884, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Piacentino V 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res 91: 517–524, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40: 271–277, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Drakos SG, Terrovitis JV, Anastasiou-Nana MI, Nanas JN. Reverse remodeling during long-term mechanical unloading of the left ventricle. J Mol Cell Cardiol 43: 231–242, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, and Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48: 992–998, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, Recchia FA, Gupte SA. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail 13: 497–506, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol 41: 340–349, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR, Burkhoff D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 102: 2713–2719, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Howard RJ, Stopps TP, Moe GW, Gotlieb A, Armstrong PW. Recovery from heart failure: structural and functional analysis in a canine model. Can J Physiol Pharmacol 66: 1505–1512, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Kawai H, Mohan A, Hagen J, Dong E, Armstrong J, Stevens SY, Liang CS. Alterations in cardiac adrenergic terminal function and beta-adrenoceptor density in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 278: H1708–H1716, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 112: 3280–3288, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Lei B, Lionetti V, Young ME, Chandler M, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in sever heart failure. J Mol Cell Cardiol 36: 567–576, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, d'Agostino C, Hintze TH, Stanley WC, Recchia FA. Carnitine palmitoyl transferase-I inhibition prevents vent ricular remodeling and delays decompensation in pacing induced heart failure. Cardiovasc Res 66: 454–461, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Mudd JO, Kass DA. Reversing chronic remodeling in heart failure. Expert Rev Cardiovasc Ther 5: 585–598, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L'Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 293: H3270–H3278, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer S The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem 276: 23357–23361, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 106: 606–612, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Razeghi P, Young Me Ying J, Depre C, Uray IP, Kolesar J, Shipley GL, Moravee CS, Davies PJ, Frazier OH, Taegtmeyer H. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology 97: 203–209, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ Res 83: 969–979, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah HN, Sharov VG, Gupta RC, Mishra S, Rastogi S, Undrovinas AI, Chaudhry PA, Todor A, Mishima T, Tanhehco EJ, Suzuki G. Reversal of chronic molecular and cellular abnormalities due to heart failure by passive mechanical ventricular containment. Circ Res 93: 1095–1101, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Soliman OI, Geleijnse ML, Theuns DA, Nemes A, Vletter WB, van Dalen BM, Motawea AK, Jordaens LJ, ten Cate FJ. Reverse of left ventricular volumetric and structural remodeling in heart failure patients treated with cardiac resynchronization therapy. Am J Cardiol 101: 651–657, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Soppa GK, Lee J, Stagg MA, Felkin LE, Barton PJ, Siedlecka U, Youssef S, Yacoub MH, Terracciano CM. Role and possible mechanisms of clenbuterol in enhancing reverse remodelling during mechanical unloading in murine heart failure. Cardiovasc Res 77: 695–706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 115: 2033–2041, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Spinale FG, Holzgrefe HH, Mukherjee R, Arthur SR, Child MJ, Powell JR, Koster WH. LV and myocyte structure and function after early recovery from tachycardia-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 268: H836–H847, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer H, Ballal K. No low-fat diet for the failing heart? Circulation 114: 2092–2093, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem 276: 44390–44395, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Zafeiridis A, Jeevanandam V, Houser SR, Margulies KB. Regression of cellular hypertrophy after left ventricular assist device support. Circulation 98: 656–662, 1998. [DOI] [PubMed] [Google Scholar]