Abstract
Cranial nerve visceral afferents enter the brain stem to synapse on neurons within the solitary tract nucleus (NTS). The broad heterogeneity of both visceral afferents and NTS neurons makes understanding afferent synaptic transmission particularly challenging. To study a specific subgroup of second-order neurons in medial NTS, we anterogradely labeled arterial baroreceptor afferents of the aortic depressor nerve (ADN) with lipophilic fluorescent tracer (i.e., ADN+) and measured synaptic responses to solitary tract (ST) activation recorded from dye-identified neurons in medial NTS in horizontal brain stem slices. Every ADN+ NTS neuron received constant-latency ST-evoked excitatory postsynaptic currents (EPSCs) (jitter <192 μs, SD of latency). Stimulus-recruitment profiles showed single thresholds and no suprathreshold recruitment, findings consistent with EPSCs arising from a single, branched afferent axon. Frequency-dependent depression of ADN+ EPSCs averaged ∼70% for five shocks at 50 Hz, but single-shock failure rates did not exceed 4%. Whether adjacent ADN− or those from unlabeled animals, other second-order NTS neurons (jitters <200 μs) had ST transmission properties indistinguishable from ADN+. Capsaicin (CAP; 100 nM) blocked ST transmission in some neurons. CAP-sensitive ST-EPSCs were smaller and failed over five times more frequently than CAP-resistant responses, whether ADN+ or from unlabeled animals. Variance-mean analysis of ST-EPSCs suggested uniformly high probabilities for quantal glutamate release across second-order neurons. While amplitude differences may reflect different numbers of contacts, higher frequency-dependent failure rates in CAP-sensitive ST-EPSCs may arise from subtype-specific differences in afferent axon properties. Thus afferent transmission within medial NTS differed by axon class (e.g., CAP sensitive) but was indistinguishable by source of axon (e.g., baroreceptor vs. nonbaroreceptor).
Keywords: myelinated, unmyelinated, C-fibers, capsaicin, quantal release, glutamate, convergence
visceral afferents, the ix and Xth cranial nerves, enter the brain at the solitary tract nucleus (NTS) (5). These afferents provide information for vital homeostatic reflexes that coordinate systemic control of cardiovascular, respiratory, and gastrointestinal function, as well as visceral aspects of integrated satiety, body temperature, neuroendocrine, and stress responses (19, 24, 41, 67). These diverse afferents belong to two broad classes, those with myelinated or unmyelinated axons, and each class distinctively expresses characteristic ion channels and receptors (32, 43, 48). Patterns of the distribution of afferent fibers outline a loose viscerotopy (46, 52). However, cellular heterogeneity, even within NTS subregions, is substantial and includes varied afferent sources (e.g., heart, airways, gastrointestinal, etc.), neurotransmitters, interconnections, cellular phenotypes, and projection targets (4, 11, 16, 62, 78). This heterogeneity challenges experimental approaches to better understand central transmission from specific afferents and the cellular basis of viscerosensory integration within the NTS.
Among cranial afferents, the aortic depressor nerve (ADN) has several unique properties that provide an anatomical basis for identification of a specific subset of NTS neurons connected to arterial baroreceptors. The ADN contains only axons from stretch-sensitive primary afferents (aortic baroreceptors) of the aortic arch (35, 68, 69). Thus the nerve trunk of the ADN contains only a single functional modality and is thus distinct from other peripheral nerve trunks that contain both mixtures of various afferent modality axons, as well as intermingled efferent axons. Tracer applied to ADN identifies both aortic baroreceptor cell bodies in the nodose ganglion, as well as their central terminations of the solitary tract (ST) within caudal NTS (21, 33, 53). These ADN-associated NTS second-order neurons lie within the medial subnucleus and are the first central neurons within the arterial baroreflex pathway that autonomically regulate heart rate on a beat-to-beat basis (5, 62).
With the use of tracer-identified NTS neurons (ADN+) in horizontal brain stem slices, our studies found that all ADN+ NTS neurons received ST-evoked excitatory postsynaptic currents (ST-EPSCs), consistent with direct, monosynaptic transmission from single ST afferent axons, i.e., second order. Capsaicin (CAP) tests (32) revealed higher synaptic failure rates of presumed C-type ST-EPSCs than CAP-resistant ST-EPSCs. Remarkably, these ST synaptic performance patterns held true for closely adjacent, ADN− second-order neurons, as well as neurons from unlabeled animals. Together, our findings suggest that afferent-activated pathways through medial NTS are defined by afferent-discrete inputs to second-order neurons, and differences in transmission performance were closely related to myelination phenotype but indistinguishable between ADN+ and non-ADN afferent modalities.
MATERIALS AND METHODS
NTS Slices
Hindbrains of male Sprague-Dawley rats (150–350 g, Charles River, Boston, MA) were prepared as previously described (33). All animal procedures were approved by the Institutional Animal Care and Use Committee, in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Labeling of Central Terminals of Aortic Baroreceptors
For the anatomical identification of NTS neurons receiving ADN central synaptic contacts, we used our procedure for dye labeling and quality control, as described previously (20, 33, 53). Rats were prepared in an initial surgery that placed tracer dye onto a cervical segment of the ADN trunk. Young rats (20–30 days old) were anesthetized with an anesthetic cocktail administered intramuscularly (1 ml/kg body wt, 56 mg/ml ketamine, 6 mg/ml xylazine, and 1 mg/ml acepromazine) (27). Using a dissection microscope, the ADN was located and separated from the surrounding tissue 1 cm peripheral to joining the superior laryngeal nerve and the nodose ganglia. We used the lipophilic fluorescent dye fast DiI (Molecular Probes, Eugene, OR). Dye containment was essential to minimize contamination of adjacent nerves and accomplished using a premolded shell that is then sealed in place with fresh dental molding compound (Coltene, Mahwah, NJ) (31, 33). In all cases, a minimum of 5 wk was allowed for transport of dye-containing lysosomes to NTS (42). This in vivo endocytic mechanism contrasts distinctly from the fate of such dyes in fixed tissue in which diffusion within the plasma membrane slowly disperses the dye (40). Retrograde transport of carbocyanines (25) fills the soma and proximal dendrites with fluorescent puncta, and such label can be recognized as intracellular by comparing focal planes within the neuron. Following removal of the NTS slice for electrophysiology, the remaining brain stem was surveyed at the dorsal motor nucleus of the vagus (DMNV) for retrogradely transported dye. NTS slices were rejected from animals in which DMNV showed filled cell bodies. Such retrograde dye indicates dye contamination that reached the peripheral vagal nerve trunk and subsequent retrograde transport along efferent axons. Retrograde vagal labeling meant that anterograde transport along vagal afferents would contaminate ST staining and include a mixture of vagal as well as ADN afferents, so that such slices were rejected from study. An additional group of animals that were equivalent in age (8–16 wk old at the time of death) but without dye surgery were used as naive rats for electrophysiological studies.
Neuron Slice Preparation, Identification, and Recordings
Brains were removed from deeply anesthetized (4% isoflurane) rats and placed in cold (0–2°C) artificial cerebral spinal fluid (aCSF) composed of the following (in mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 dextrose, 2 CaCl2, and bubbled with 95% O2-5% CO2 (pH 7.4). Slices (250 μm) were microtome cut (Leica VT-1000S Leica Microsystems, Bannockburn, IL) in a quasi-horizontal orientation using a sapphire knife (Delaware Diamond Knives, Wilmington, DE). Electrodes (1.8–3.5 MΩ) were filled with a solution composed of the following (in mM): 10 NaCl, 130 potassium-gluconate, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 1.0 Na2ATP, 0.1 NaGTP; pH 7.3, 295 mosM. aCSF used for recording was identical to that used for brain slicing procedures in aCSF. In experiments focused on glutamate release probability, these solutions were modified as described below.
For recording, slices were mounted in the perfusion chamber, and cell bodies medial to the ST and <250 μm from obex were visualized using infrared differential interference contrast (IR DIC) optics (Axioskop FS2+, Zeiss, Oberkochen, Germany). Next, fluorescent images of ADN contacts within NTS neurons at ×400 were captured (Axiocam, Zeiss) and compared with digitally superimposed real-time IR DIC images. By focusing through the cell and back, only neurons in which dye puncta clearly corresponded to the DIC image of the neuron soma surface or dendrite were considered anatomically identified as AND-contacted neurons (ADN+). In electrophysiological experiments, results were compared between ADN− neurons found in close proximity to ADN+ neurons within NTS. A group of second-order NTS neurons from the equivalent medial subregion was studied with identical methods from slices of naive rats. After locating such neurons, voltage-clamp recordings were made at 32–34°C using a Multiclamp 700B or Axoclamp 2A/B amplifier and pClamp 9 software (Molecular Devices, Union City, CA). No leak subtractions, liquid junction potentials, or series resistance compensations were performed. Input resistance was monitored throughout recordings. Cell recordings were halted if series or input resistance was not stable.
Remote Activation of ST Afferents
A concentric bipolar stimulating electrode (50 μm inner core diameter, 200 μm outer diameter, F. Haer, Bowdoinham, ME) was placed on the visible ST as it coursed rostrally at a distance of up to 5 mm but no less than 1 mm from the recorded neurons. Patterned bursts of five stimulus shocks (0.1-ms duration spaced at 20-ms intervals) were generated with a duty cycle of 3–6 s (Master-8, AMPI, Jerusalem, Israel). After determining the response threshold (see below), most studies were conducted at an intensity set at twice the event threshold. Responses to the burst of five such shocks were designated EPSC1, EPSC2, etc., to indicate the shock position within the sequence.
Latency Variability-Synaptic Jitter
The latency to event onset and its variation across trials are fundamental indicators of the synaptic transmission process. Analysis of latency was based on consideration of the responses to the first shock in the train of five (EPSC1). All events within a 5-ms window of EPSC1 were considered synchronized to the ST shock if they occurred regularly across an aggregate test series of ST shock sequences (generally bursts of five shocks) and had similar waveform. Individual events not meeting these ST-synced criteria (timing and waveform) were considered to be unrelated to ST activation and were excluded from analysis. Variability in latency (jitter) was calculated using EPSC1 as the standard deviation (SD) of latency and served as a critical index of synaptic order. In the present study, only monosynaptic events were analyzed as judged by EPSC1 jitter of <200 μs (31). Synaptic jitter calculations included at least 30 individual latency values for each neuron. These low-jitter ST-EPSCs typically have low rates of synaptic failure, high amplitudes, and substantial frequency-dependent depression (12, 31).
ST Stimulus Intensity-Recruitment Relations
Each ST axon should be activated to fire in all-or-none fashion and thus have a distinct stimulus threshold. In the horizontal slice, increments in ST shock intensity are initially ineffectual (subthreshold) and then with increased intensity exceed threshold to consistently evoke EPSCs (6, 8, 11, 12). Once the axon threshold is exceeded, conducted action potentials are intensity independent. The all-or-none character of ST intensity-recruitment relations indicates a reliance on activation of single axons impinging on individual neurons (1, 8). For testing, shock intensities were finely graded above and below threshold to establish the threshold value for the onset of reliably evoked responses. Shock intensities up to five times above threshold intensity were tested generally. The appearance of new ST-synced events or changes in shape of synaptic responses at increased intensity indicated recruitment of additional afferent fibers. These additional synaptic events also exhibited discrete stimulus thresholds and latency characteristics but had high jitter (>200 μs, i.e., polysynaptic) events that were not analyzed (8, 12).
Synaptic Pathway Failures
ST shocks sometimes fail to evoke a synaptic response. Serially connected, polyneuronal pathways, in addition to having high-jitter latencies, are particularly prone to synaptic failures (11, 31). Any ST shock that failed to produce an identifiable EPSC within the same 5-ms window used for analysis of the response latencies was counted as a synaptic failure. Failure rates were calculated as a percentage of total number of ST shocks delivered at each of the positions (i.e., EPSC1, EPSC2, etc.) within ST shock bursts. A minimum of 30 ST synaptic responses was examined to calculate failure rates.
CAP Synaptic Blockade
Following full synaptic characterization of the recorded neurons, the transient receptor potential vanilloid type 1 agonist CAP (100 nM) was tested in a subset of cells. This CAP test took place at the end of the experiment and established the ST axon for a given EPSC was CAP sensitive or CAP resistant. Previous studies of NTS synaptic responses and nodose ganglion neurons (32) have shown that CAP sensitivity of ST afferent axons corresponds to an unmyelinated afferent, and CAP resistance depends on myelinated afferent neurons. No cases of partial blockade of ST-EPSCs were found. Only a single test of CAP was used in a given slice, and, following the CAP test, recordings ceased.
Drugs
All drugs were applied by bath perfusion. The blocker 1,2,3,4-tetrahydro-6-nitro-2, 3-dioxo-benzo quinoxaline-7-sulfonamide was obtained from Sigma-RBI (Natick, MA). CAP and tetrodotoxin were obtained from Tocris Cookson (Ballwin, MO) and dissolved in 100 μl DMSO before dilution with external solution to a final concentration of 100 nM. DMSO alone at the highest concentration in external solution had no effect on NTS neurons or synaptic transmission.
Data Collection and Analysis
Variance-mean analysis of ST-EPSCs.
In a limited subset of neurons, variance-mean (V-M) analysis was used to assess the quantal release process for glutamate and compare these characteristic release relationships between ADN+ neurons and neurons from naive rats using methods as previously described (12). ST-EPSC amplitudes were measured as the net peak current (peak current minus the mean baseline current over 10 ms immediately preceding each stimulus shock). V was calculated as the square of SD of 30–80 successive ST-EPSC1 response amplitudes in each condition. V values were not corrected for baseline V, since the SD of the noise generally accounted for <1% of ST-EPSC amplitude in the control condition (12). For each neuron, we calculated M and V during each recording condition for each of ST-EPSC1. Although infrequent, failures were included for all V and M calculations. To modify the release probability, we constructed V-M relations for steady-state ST-EPSCs in 2, 0.5, and 0.25 mM extracellular Ca2+ with twofold Mg2+ replacement of the reduced Ca2+. Data were fit with a least squares regression method for each neuron using the equation: y = K1x + K2x2 (Origin version 7.5, OriginLab, Northampton, MA). For comparison of V-M relations, we estimated the maximal EPSC amplitude (EPSCmax) for each neuron, together with the theoretical constraint at minimal release (0 V at 0 M) to generate individual V-M relations for each neuron. This parabolic fit predicts average quantal sites (q) and number of functional release sites (N) for each neuron. Aggregate data normalized V and M by dividing by EPSCmax within neurons under control conditions before generating aggregate mean values. When possible, CAP was tested on these neurons following successful completion of the V-M measurements.
Statistical testing.
Statistical comparisons were made using paired or unpaired Student's t-test, repeated-measures ANOVA, one- or two-way ANOVA, and Bonferroni Dunn test for post hoc analysis, where appropriate (SigmaStat 3.5, SysStat Software, Point Richmond, CA). All summary data are presented as means ± SE. P values < 0.05 indicated significant differences.
RESULTS
Neuroanatomical Identification of Aortic Baroreceptive NTS Neurons
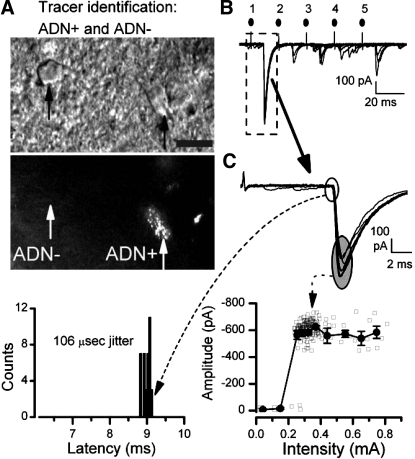
In rats with ADN tracer implantations, horizontal brain stem slices featured fields of fluorescent puncta medial to the ST and, under high magnification (Fig. 1A), these ADN markings formed characteristic clusters of centrally transported tracer dye. Clusters of dye puncta lay in close proximity to the soma membrane of individual neurons, and such dye-positive cells (ADN+) were considered to be anatomically identified second-order baroreceptive NTS neurons (Fig. 1A). Neurons with no colocalized fluorescence were designated ADN−. The relationship of ADN− neurons to ST, however, was unknown. Potentially, ADN− neurons might be other second-order neurons contacted by non-ADN ST afferents, or these cells could be higher order NTS neurons only indirectly or unrelated to ST. Without ADN labeling, such as in naive animals, second-order neurons in the medial subnucleus of NTS could be contacted by ADN or any other afferent reaching this region. These network connective distinctions cannot be resolved anatomically in this preparation and required the functional assessment of synaptic connections from ST.
Fig. 1.
The presence of fluorescent, anterogradely transported dye from the aortic depressor nerve (ADN) allowed anatomical identification of solitary tract nucleus (NTS) neurons directly receiving aortic baroreceptor terminals (ADN+) in vitro. A–C are from a single representative ADN+ neuron. A: under infrared differential interference contrast (IR DIC) microscopy, numerous cell bodies were apparent (top micrograph, black arrows) in thin brain stem slices used for recordings. Using fluorescence excitation, bright puncta of DiI label were revealed along the surface of some cell bodies and their proximal dendrites (bottom micrograph, right, white arrow, ADN+), and these were considered anatomically identified second-order baroreceptive NTS neurons. Note that adjacent dye-negative neurons (white arrow, left, ADN−) had similar appearance under IR DIC but lacked DiI puncta. Scale bar is 10 μm. B: shocks delivered to the solitary tract (ST) evoked large low-jitter excitatory postsynaptic currents (EPSCs) in this ADN+ neuron (original traces recorded from right ADN+ neuron depicted in A). Amplitudes depressed with shocks (1–5, solid circles above traces) were repeated at a 20-ms interval in a burst of 5 stimuli repeated each 3 s (7 trials displayed). C: expanded analysis of EPSC1 (dashed line box in B) examined the timing of the onset of the EPSC, latency (dashed line and arrow to bottom left), and the amplitude (dashed line and arrow to bottom plot). Examination of the timing of EPSC1 (open oval) showed that the latency of this ADN+ neuron varied minimally (bottom plot, left) with ∼9 ms and a jitter (SD of latency) of 106 μs (n = 38 trials), well below the 200-μs cutoff for monosynaptic pathways directly from ST. The amplitudes of EPSC1 (shaded oval) characteristically varied from shock to shock, but increasing shock intensity indicated a sharp threshold profile for evoking the EPSC and no change in amplitude at suprathreshold intensities (bottom plot, right). Shaded squares are individual values, and solid squares are means ± SE. Original synaptic response traces displayed in B and C were recorded using tests at twice the threshold intensity, but note that many trials are not displayed to increase clarity. Similar results were found in all ADN+ neurons. ADN− neurons were tested using identical protocols and analyses.
ADN+ NTS Neurons Receive Low-jitter ST-EPSCs
To test whether ADN+ or ADN− neurons were connected to ST afferent pathways, the synaptic responses to electrical shocks to ST were assayed, and synaptic timing and amplitude characteristics were examined (Fig. 1, B and C). After locating and attaining a stable patch recording from an ADN+ NTS neuron (Fig. 1A), activation of ST with electrical shocks evoked minimally variant ST-EPSC responses that depressed substantially with repeated shocks (Fig. 1B). Analysis of the latency indicated a low synaptic jitter and narrow histogram of latencies to successive stimuli (Fig. 1C). Increasing ST shock intensity failed to alter the amplitude of the observed responses, even using shocks at multiples of the threshold intensity (Fig. 1C). The stimulus intensity recruitment relationship displayed a single distinct ST threshold intensity, and increases in intensity above threshold did not alter the response amplitude or shape (Fig. 1C). This ST stimulus activation profile is consistent with responses activated by a single-afferent input, thought to arise from a single-afferent axon (11, 12). Note that no additional ST afferent contacts were recruited by large increases in ST stimulus intensity (Fig. 1C). We found no evidence that ADN+ neurons received multiple ST afferents converging on a single neuron. All ADN+, tracer-identified NTS neurons had generally similar individual ST response patterns and characteristics to this example.
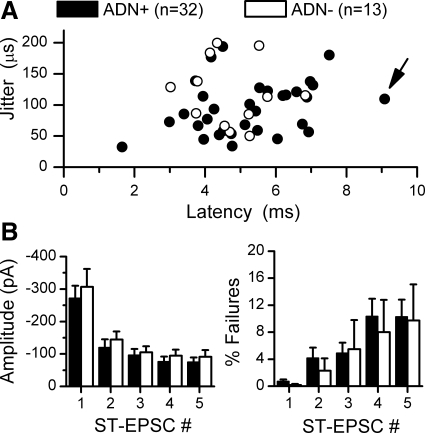
In their latency and amplitude characteristics, ADN dye-identified NTS neurons were generally quite similar to each other and to ADN− NTS neurons that met the criterion of having ST-EPSCs with <200-μs latency jitter (Fig. 2). Overall, in 45 NTS neurons from ADN-labeled animals, ADN+ and ADN− second-order neurons spanned a wide range of latencies but had limited jitter distributions. The highest jitter value for an ADN+ neuron (n = 32) was 192 μs, and latency-jitter paired values for individual neurons overlapped in their distributions between ADN+ and ADN− (n = 13) second-order ST contacts (Fig. 2). The range of absolute latency values (1.8–9.1 ms, Fig. 2A) in these neurons is surprisingly large, since they were physically intermixed with cell bodies within the medial portion of NTS. Neurons with different latencies were often directly adjacent and within 50 μm of one another. However, there was no systematic difference in mean latency or jitter (5.24 ± 0.27 vs. 4.72 ± 0.28 ms latency or 93.57 ± 7.66 vs. 113.01 ± 15.01 μs jitter, ADN+ and ADN−, respectively, P > 0.05). Likewise, the mean basal EPSC amplitudes and failure rates were equivalent for EPSC1 (Fig. 2B, P > 0.6). The 50-Hz bursts of five ST shocks depressed EPSC amplitudes by ∼70% at EPSC5, and ST-EPSC failure rates were similar across labeled and unlabeled neurons (P > 0.2 ADN+ vs. ADN−, Fig. 2B). No anatomically identified ADN+ second-order neurons exceeded 200-μs jitter, suggesting that this electrophysiological criterion is a reliable marker for anatomically monosynaptic ST contacts.
Fig. 2.
Dye-identified second-order NTS neurons (ADN+, n = 32) had comparable ST synaptic characteristics to adjacent ADN− NTS neurons, meeting the 200-μs cutoff for monosynaptic ST pathways (n = 13). A: using the procedures for study outlined in Fig. 1, latency-jitter paired values for ADN+ and ADN− second-order NTS neurons overlapped. The ADN+ neuron whose recordings were displayed in Fig. 1 is marked by an arrow. B: frequency-dependent depression during a burst of 5 shocks to ST (20-ms interval) were similar between ADN+ and ADN− neurons, and synaptic failure rates increased similarly as the stimulus burst progressed (P > 0.05).
CAP-sensitive ST-EPSCs Are Weak and Failure Prone
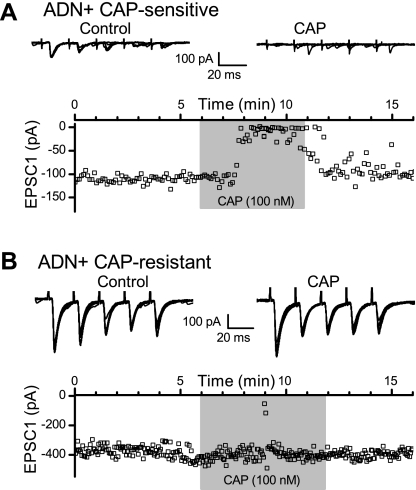
Applied to the peripheral ADN trunk, the transient receptor potential vanilloid type 1 agonist CAP selectively blocks axonal conduction of unmyelinated action potentials in the compound electroneurogram, while lightly myelinated ADN fibers continue to conduct in the Aδ range (34, 66). In slices, CAP blocks synaptic transmission along CAP-sensitive ST pathways to NTS thought to correspond to unmyelinated peripheral afferent axons (32, 43). In a subset of ADN+ NTS neurons, 100 nM CAP was used to test for blockade of ST-EPSCs (Fig. 3). CAP fully blocked evoked ST-EPSCs within 2–3 min in a subset of all neurons tested (Fig. 3A), and these neurons were classified as CAP sensitive. Note that spontaneous synaptic events remained in these neurons during CAP so that CAP actions are selective for afferent ST synaptic events (Fig. 3A). Blockade of CAP-sensitive ST-EPSCs reversed upon return to control aCSF (Fig. 3A, bottom). In the remainder of the neurons tested, the ST-EPSCs were unaffected (i.e., CAP resistant, Fig. 3B). Neurons with CAP-sensitive ST-EPSCs were considered to be second-order NTS neurons that received unmyelinated, primary visceral ST afferents, i.e., C-fiber axons. Conversely, CAP-resistant neurons were considered to receive myelinated ST afferents, i.e., A-fiber axons. In no case did CAP partially block ST-EPSCs in sensitive neurons, i.e., produce ST-EPSCs reduced in amplitude but persistent in 100 nM CAP. Such results suggest that individual second-order NTS neurons receive either unmyelinated or myelinated ST afferents, but not both.
Fig. 3.
Capsaicin (CAP) exposure (100 nM) identified CAP-sensitive and CAP-resistant NTS neurons with ADN+ puncta. CAP rapidly and reversibly suppressed ST-evoked EPSCs in CAP-sensitive neurons (A, shaded box) but had no effect on CAP-resistant ST transmission (B). Note that ADN+ CAP-sensitive neurons tended to have much smaller amplitude ST-EPSCs than CAP-resistant NTS neurons. Representative traces shown in the top portions of A and B include 10 control and 7 CAP trials for the CAP-sensitive neuron and include 11 control and 11 CAP trials for the CAP-resistant neuron. This presynaptic action of CAP reflects the presence of transient receptor potential vanilloid type 1 receptors of ST afferent terminals that arise from unmyelinated afferent axons (32). Note that, during CAP block of ST synced EPSCs, spontaneous synaptic events continue to occur.
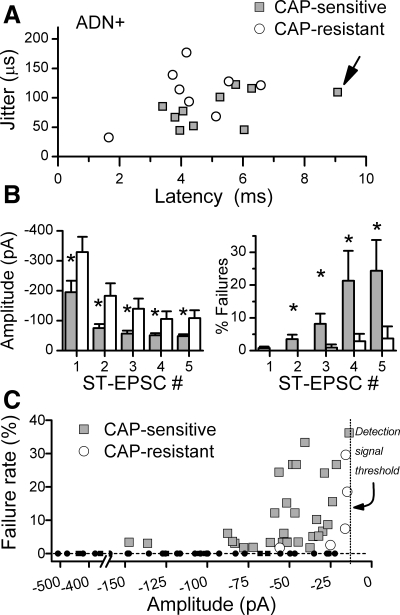
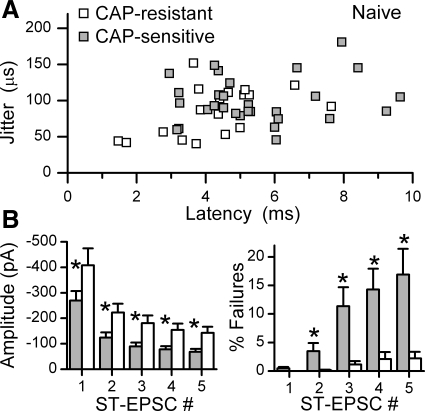
Within the group of ADN+ neurons, latencies of CAP-sensitive and CAP-resistant ST-EPSCs varied widely, but their distributions overlapped substantially (Fig. 4A). Although the longest latency ST-EPSC found in ADN+ neurons (Fig. 1, B and C) was CAP sensitive, average latencies and jitters (Fig. 4A) were similar (5.21 ± 0.54 vs. 4.37 ± 0.52 ms latency or 82.07 ± 9.33 vs. 109.15 ± 15.70 μs jitter, CAP sensitive n = 10 and CAP resistant n = 8, respectively, P > 0.05). However, the peak amplitudes of CAP-resistant ST-EPSCs were nearly 50% larger than in CAP-sensitive responses (P < 0.02, Fig. 4B, left). Furthermore, although the ST-EPSCs in all ADN+ neurons depressed to a similar degree (∼70%) within the burst of five ST shocks, CAP-resistant ST-EPSCs remained significantly larger than CAP-insensitive responses at all burst positions (EPSC1-EPSC5, P < 0.001, Fig. 4B, left).
Fig. 4.
A: across all ADN+ neurons tested with CAP (n = 18), latency-jitter values for ST-EPSCs had considerable overlap between CAP sensitive (shaded squares, n = 10) and CAP resistant (open circles, n = 8). The CAP-sensitive point marked by the arrow corresponds to the representative ADN+ neuron presented in detail in Fig. 1. B: the mean ST-EPSC amplitudes of CAP sensitive (left, shaded bars), however, were significantly smaller (*P < 0.02) than for CAP resistant (open bars) to the first as well as subsequent ST shocks (ST-EPSC #) during the burst of 5 stimuli. Failures of CAP-resistant ST-EPSCs (right, open bars) were quite low at all ST shock positions and remained <5% throughout. Failures for CAP-sensitive neurons were similar to CAP-resistant neurons at ST-EPSC1 (right, shaded bars), but increased substantially for later responses within the burst (*P < 0.001). C: a plot of the individual mean failure rates against the mean amplitude for successful, nonfailure events shows that failures occurred at event amplitudes well above the detection threshold of 5–15 pA. Failure rates were calculated from a minimum of 30 trials for EPSC1–EPSC5 for each neuron. Small solid points indicate zero failure points (dashed horizontal line), whereas larger points indicate mean event amplitudes at corresponding mean failure rates. The mean reliable level of detection of single events is represented by dotted vertical line, although this was adjusted in each neuron (range 5–20 pA). Note that no failures occurred in CAP-resistant EPSCs (open circles) until amplitudes were quite depressed (corresponding to EPSC3–EPSC5), whereas recurring failures were found in CAP-sensitive EPSCs (shaded squares) at amplitudes as high as 145 pA.
One of the most distinctive differences in synaptic performance between CAP-sensitive and CAP-resistant ST-EPSCs was in their failure rates. Careful inspection of the synaptic responses of some ADN+ neurons to bursts of ST shocks (Fig. 1B) showed that the first shock in the burst very rarely failed to release glutamate (i.e., EPSCs were present, Fig. 1B and Fig. 4B, right). EPSC1 failed at similar low basal rates (<1%) in all ADN+ neurons. However, in some neurons, many shocks failed to succeed in evoking an EPSC (Fig. 1B, shocks 2–5). Subsequent CAP tests revealed that CAP-sensitive, ADN+ ST-EPSCs had failure rates that increased >18-fold as the burst of five shocks progressed, while failure rates of CAP-resistant ST-EPSCs did not change within the burst (P < 0.02, Fig. 4B, right). Failures in CAP-sensitive neurons occurred at increasing rates as the burst progressed, and in all cases the increase in failures occurred concurrently with frequency depression of EPSC amplitude (Fig. 4C). Failure amplitudes were well above detection limits, however, and CAP-resistant events of similarly low amplitudes did not fail, whereas CAP-sensitive EPSCs of similar or greater amplitude failed at high rates (Fig. 4C). This frequency-dependent augmentation of synaptic failures suggests a use-dependent mechanism within the unmyelinated, presynaptic ST transmission process.
To examine whether ST transmission to ADN+ NTS neurons was unique to neurons contacted by baroreceptor afferents, similar tests were conducted in slices from naive animals, i.e., animals without dye implant surgery. In naive animals, second-order NTS neurons were identified electrophysiologically by their ST-EPSC jitter characteristics (<200 μs). Neurons were recorded within the same region as in the ADN-labeled neuron series. Latency-jitter distributions for CAP-sensitive and CAP-resistant ST-EPSCs for second-order NTS neurons (n = 47) from naive animals overlapped (Fig. 5A) and the pattern and range of values were quite similar to those in the ADN+ neurons (Fig. 4A). CAP-sensitive and CAP-resistant ST-EPSCs of unlabeled neurons overlapped in their latency-jitter distributions (Fig. 5A). With the benefit of larger sample sizes (Fig. 5A), the latencies of CAP-sensitive ST-EPSCs were significantly longer than for CAP-resistant responses (5.51 ± 0.35 ms, n = 28 vs. 4.22 ± 0.34 ms, n = 19, P = 0.014). ST-EPSC jitter values were comparable between the two naive groups (101.41 ± 6.00 vs. 84.16 ± 7.60 μs jitter, CAP sensitive and CAP resistant, respectively P > 0.05). Amplitudes of CAP-sensitive ST-EPSCs were substantially smaller than CAP-resistant responses (Fig. 5B, left, P = 0.01). Like the ADN+ neurons, ST-EPSCs failed more often in the CAP-sensitive group as the burst of five ST shocks progressed (Fig. 5B, right, P < 0.001). The general patterns were consistent with those for ADN-labeled neurons, suggesting that the differences identified by CAP were similar for all ST afferents terminating in medial NTS and not peculiar to ADN baroreceptor afferents. Thus high rates of afferent activation induce a use-dependent failure of ST afferent terminals to release glutamate only in the CAP-sensitive, unmyelinated ST inputs.
Fig. 5.
In a larger cohort of NTS neurons from naive animals, synaptically identified second-order neurons had similar synaptic response profiles to ADN+ neurons. A: CAP-sensitive (shaded squares, n = 19) and CAP-resistant (open squares, n = 28) ST-EPSCs generally overlapped in latency and jitter distributions. B: CAP-sensitive ST-EPSCs had significantly smaller amplitudes than CAP-resistant responses (P = 0.01) and failed more often as the burst of 5 shocks progressed (P < 0.001), suggesting a use-dependent increase in failures only in the CAP-sensitive transmission. *Post hoc testing significant differences between CAP-sensitive and -resistant values, respectively. The general patterns are consistent with those for ADN+ neurons (Fig. 4), suggesting that the differences identified by CAP are similar for all ST afferents and not peculiar to ADN baroreceptive NTS neurons.
V-M Analysis Indicates High Release Probability
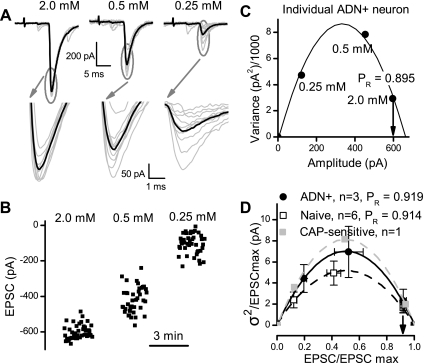
ST stimulation activates axons that arise from a potentially varied pool of cranial visceral afferents. It is possible that different afferents might have different glutamate release properties at their central terminations. To test this idea, we performed quantal analysis of ST-evoked EPSCs. At a given synapse, the probability of vesicle release (PR), N, and q can be estimated by determining the dependence of EPSC V on M amplitude under conditions that alter release (22, 38, 74). To compare the transmitter release characteristics at second-order NTS neurons, we conducted a V-M analysis of glutamate release for ST-EPSCs from ADN-labeled and naive animals. Within a single, representative ADN+ neuron, the mean amplitude of the ST-EPSC declined as the extracellular Ca2+ concentration was reduced, and the V increased in a characteristic fashion (Fig. 6, A and B). The V-M relationship of the ST-EPSC amplitudes at various Ca2+ conditions for this neuron (Fig. 6C) followed a parabolic model and the estimated probability of glutamate release, PR, was 0.89 in this ADN+ neuron at 2 mM Ca2+.
Fig. 6.
Variance-mean analysis of glutamate release for ST-EPSCs from ADN+ and naive NTS neurons. Within a single, representative ADN+ neuron, the variance of the ST-EPSC amplitudes increased as the extracellular Ca2+ concentration was reduced, while the mean amplitude declined in a characteristic fashion (A and B). The relationship between the variance and the mean of the ST-EPSC amplitude from A and B at various Ca2+ conditions was well described by a parabolic model (r2 values always exceeded 0.9). C: data were fit with a least squares method for each neuron using the equation: y = A + K1x + K2x2. In this case, r2 = 0.997 and A = −0.478, K1 = 16.95, and K2 = −23.69. This fit estimated the probability of glutamate release (PR) as 0.895 in this ADN+ neuron at 2 mM Ca2+. D: group averages of variance-mean relationships show that the average of three ADN+ neurons (solid line), r2 = 0.997, A = 0.081 ± 0.130, K1 = 20.40 ± 0.80, K2 = −20.43 ± 0.76, was similar to the relationship for the average of 6 naive neurons (dashed line), r2 = 0.999, A = 0.005 ± 0.111, K1 = 27.93 ± 0.80, K2 = −27.84 ± 0.57. The mean PR was similar for both groups of neurons at ∼0.91. In some long-lasting recordings (4 cases), neurons were exposed to CAP, and all three ADN+ neurons were CAP resistant and their curve overlapped with data from one naive neuron that was CAP sensitive (replotted as a shaded trace with shaded squares).
The V-M curves for three ADN+ neurons indicated similar basic properties for glutamate release onto these second-order neurons and resembled those constructed for second-order neurons from naive animals (Fig. 6D). Overall, the goodness of the model fit averaged 0.988 (n = 9, r2 ranging 0.912–1.00) and with the average of three ADN+ neurons as r2 = 0.997. The q averaged 30.9 ± 11.3 pA (range 17–53 pA) for ADN+ neurons and similarly 26.8 ± 2.3 pA (range 4–32 pA) for second-order neurons from naive animals (P > 0.23), while N, the number of contacts, averaged 17.4 ± 5.1 (range 12–27) sites for ADN+ neurons compared with the similar 22.4 ± 2.3 (range 9–22) sites for second-order neurons from naive animals (P > 0.6). Likewise, the PR for glutamate was remarkably similar across neurons in the 2.0 mM Ca2+ condition at 0.919 ± 0.023 (range 0.895–0.965) for ADN+ neurons and 0.914 ± 0.003 (range 0.883–0.946) for second-order neurons from naive animals (P > 0.9). In a few cases, neurons survived long enough to be tested with CAP at the end of the V-M experiment. The ADN+ neurons tested (n = 3) were found to be CAP resistant (Fig. 6). A single naive neuron was CAP sensitive, and its V-M profile fell within the error bars of the mean values for the CAP-resistant group. This very preliminary evidence suggests that glutamate release properties may be similar across CAP-sensitive and -resistant afferents. In terms of the function of the glutamate release machinery, transmission to ADN+ neurons was indistinguishable from ST transmission to other second-order NTS neurons.
DISCUSSION
In the present studies, we exploited unique neuroanatomical features of the ADN that permitted study of aortic baroreceptor-innervated, second-order NTS neurons. The ADN DiI label arrives only through aortic baroreceptor axons, and thus the dye in our experiments is transported solely anterogradely to the brain stem (33). In most afferent-containing peripheral nerve trunks, carbocyanine dyes label a mixture of both central afferent processes (anterograde transport), as well as central efferent processes and cell bodies of projection neurons (retrograde transport along efferent axons). In the case of the vagal nerve trunk, brain stem dye arrives anterogradely in the NTS and retrogradely in DMNV neurons (33). In contrast to early anatomical studies in which the ADN was cut for horseradish peroxidase staining (21, 44), lipophilic dye implants did not require disruption of the peripheral axon and thus avoided the potential for injury-induced sprouting and terminal redistribution (37, 58).
Guided by fluorescent ADN+ terminals, we recorded from medial NTS neurons bearing these baroreceptor contacts. Our work discerned five major findings from this large cohort of aortic baroreceptive NTS neurons. First, we found that ST stimulation always evoked synaptic responses, and these ST-EPSCs conformed to monosynaptic jitter criteria (chiefly, <200 μs jitter). Thus these anatomically identified neurons confirmed that the 200-μs criterion is a reasonable electrophysiological discriminator of monosynaptic ST contacts from synaptic contacts arising from indirect polysynaptic pathways (31). Second, comparisons of synaptic transmission characteristics of ADN+ neurons with ADN− neurons meeting monosynaptic criteria for ST-EPSCs (<200 μs jitter) revealed no differences for these neurons, both within medial NTS, whether unlabeled (ADN−) or from naive animals. Thus mean latency, jitter, and frequency-dependent depression were indistinguishable between separate cohorts of second-order NTS neurons, whether ADN+ and ADN−. In addition, this lack of difference indicated by direct, quantitative synaptic comparisons (ADN+ vs. ADN− and carbocyanine vs. naive) suggests that the presence of carbocyanine tracer in ST afferents (53) does not alter afferent properties (13, 15, 73, 80). Our results from unlabeled second-order neurons were recorded from the same medial NTS subnucleus of naive animals and presumably included neurons that received ADN afferent contacts, but clearly we do not know with certainty for those individual neurons in naive animals. Third, the single threshold in the ST recruitment characteristics of EPSCs to ADN+ neurons indicated dependence on a single-afferent fiber. We found no evidence that higher intensity stimuli recruited additional, directly convergent ST afferent inputs within individual neurons. Similar, single-threshold recruitment relations were found in unlabeled second-order neurons (ADN− and neurons from naive animals). Fourth, quantal release curves described similar, high-release probabilities for glutamate-mediating transmission across ADN+, ADN−, and naive neurons. The V-M relations were consistent with broadly uniform glutamate release mechanisms across ST afferents within medial NTS. Fifth, high frequencies of ST activation generated frequent failures in CAP-sensitive but not in CAP-resistant ST-EPSCs. Such results, in conjunction with previous work in nodose neurons (43, 51), suggest that presynaptic mechanisms are responsible for this use-dependent responses differentiated between myelinated and unmyelinated (C-fiber) afferent axons. Collectively, our findings suggest that afferent-activated pathways through medial NTS may be best defined by the presynaptic afferent contacts onto individual second-order neurons, but that glutamate transmission was remarkably uniform. Afferent myelination phenotype was associated with functional distinctions that are likely to span afferent modalities arising from different peripheral sources. Such results offer a tentative outline of basic principles underlying baroreceptor integration that may apply broadly across afferent information processing within medial NTS. If these presynaptic distinctions between myelinated and unmyelinated afferents found in medial NTS are generalized, then we might speculate and anticipate that such dichotomies will be found in other subregions of NTS, such as the commissural subnucleus, but currently evidence is lacking.
Second-order Baroreceptive NTS Neurons Receive Reliable, Low-jitter ST Inputs
The all-or-none stimulus intensity relations uniformly indicated that ADN+ neurons received single-afferent inputs. Our approach in using stimulus-recruitment profiles in NTS neurons is consistent with criteria widely used to distinguish single-fiber inputs in other central nervous system regions (2, 64, 65). In principle, increments in the stimulus intensity should have at least two possible recruitment effects: 1) addition of new, higher threshold axons, and/or 2) spread of stimulus currents that radiate more widely to activate axons more distant from the stimulation electrode tip. By our experimental design, we have attempted to decrease the likelihood of recruiting non-ST axons by placing our concentric bipolar stimulation electrode on the ST quite distant (1–5 mm) from the recorded cells. Stimulus spread to off-ST axons should be less likely to activate axons making contact with the recorded neurons. As our laboratory reported previously (see Fig. 4 in Ref. 9), moving the stimulation electrode off of the visible ST resulted in either no response or an elevation in threshold shock intensity for evoking the same response, a finding consistent with greater current intensity required to spread from the off-ST site to reach the same axon within the ST. When stimulation electrodes are placed quite close (∼150 μm) to NTS neurons in transverse slices (20, 77), shocks commonly recruit both EPSCs and monosynaptic inhibitory postsynaptic currents (IPSCs, i.e., local nonafferent axons) (18, 77).
Single shocks to ST rarely (<1%) failed to activate an ST-EPSC, and this is quite different from excitatory transmission characteristics in many forebrain neurons in which failure rates of ∼50% are common (2, 64, 65). The uniformly high reliability of ST-NTS transmission within medial NTS may relate, at least in part, to an unusually homogeneous and high probability of glutamate release. Our quantal analysis revealed that both ADN+ and other second-order NTS neurons averaged >0.91 PR at 2 mM Ca2+, similar to our laboratory's previous studies (12). NTS release probabilities are much higher and more uniform across neurons than other central synapses under similar conditions [calyx of Held, PR = 0.25–0.40 (54); mossy fiber-CA3 interneuron, PR = 0.34–0.51 (47), or mossy fiber-pyramidal, PR = 0.20–0.28 (79)]. All ST-NTS release curves had high regression coefficients for parabolic fits to V-M data, a finding consistent with uniform release site characteristics within the activated terminal fields (23). Estimates of active terminal numbers from the V-M analysis are generally consistent with ADN+ terminal staining surrounding single-cell bodies (53) (see Fig. 1). In addition to aortic baroreceptors, the medial subregion of NTS receives afferents from the heart and lungs, as well as subdiaphragmatic organs, (e.g., Refs. 29, 61), and such afferents generally display excitatory synaptic responses that similarly depress substantially to repeated activation. We do not know the staining efficiency of our ADN dye-labeling procedure. However, ADN− and second-order neurons from naive animals in our studies likely receive ST contacts from varied visceral afferents. Thus the general similarities in the present cross-sample (ADN+, ADN−, and naive) results support the conclusion that the basic glutamate transmission properties and general organization of the terminal architectures across these afferents are remarkably similar at second-order neurons within the medial NTS subnucleus.
Afferent Pathways to Second-order Neurons in Medial NTS: Evidence for Limited Monosynaptic Convergence
Thin horizontal slices of the brain stem offer optimal experimental access to NTS neurons for intracellular studies of afferent synaptic transmission, but clearly this procedure has the potential to damage or interrupt afferent pathways. Despite this possibility, every ADN+ neuron that we recorded responded with a low-jitter ST-EPSC to ST shocks. Such results suggest that the horizontal plane of sectioning preserves the course of ADN afferents along the ST to second-order neurons, at least over distances of up to 5 mm. If our slicing damages some ST afferent terminations, then we may be underestimating synaptic contacts to these neurons. Nonetheless, the similarity of latency and jitter distributions for ADN+ and other ADN− second-order neurons supports the view that baroreceptive afferent pathways are organized similarly to the ST pathways of the broader population of afferents in medial NTS. Since ours are functional measures, the results suggest that an afferent axon, such as one from an aortic baroreceptor, engages multiple synaptic contacts (17–22 estimated by V-M analysis) to produce EPSCs onto individual second-order neurons and rely on similar pre- and postsynaptic mechanisms. Thus our findings on ADN+ neurons are consistent with the concept that NTS is organized into “clusters, or groups, that receive their main sensory input from one type of vagal afferent,” as suggested for pulmonary afferents of medial NTS (45).
Our laboratory's previous work in horizontal slices found that single medial NTS neurons tend to receive one direct input (monosynaptic) from a single afferent plus additional indirect, ST-activated polysynaptic inputs (7–11, 31). Here, we found no evidence for monosynaptic convergence from multiple, different afferents onto these single neurons. This in vitro result closely resembles the overall findings of studies of afferent convergence in intact animals. In intact cats and rats, extracellular recordings outlined maps of the path of single-afferent axons to their targets in NTS, and overall such work suggests that afferents commonly divide into collateral axons in a pattern that is quite similar across several afferent subtypes (26, 30). Interestingly, these collaterals of individual afferent axons span considerable rostral-caudal and medial-lateral distances before making close appositions (presumed functional synaptic contacts) with different neurons (46). Anatomically, detailed evidence concerning the distribution of terminations arising from individual afferents within NTS is quite limited. In one study, intracellular labeling of both a single-afferent pulmonary fiber, together with a single NTS neuron, showed that one collateral formed multiple synaptic terminals on a single, ventrolateral NTS neuron (3). If this particular class of pulmonary afferents is broadly typical, then together these anatomical findings plus our electrophysiological studies suggest that cranial visceral afferents may rely on multiple contacts onto single neurons and that single afferents will collateralize to repeat this contact pattern onto multiple neurons. Neither the anatomical nor the electrophysiological approach, however, answers the important questions of whether multiple, different afferents contact individual NTS neurons or whether these branch maps indicate functional connections. From the perspective of our present findings, our afferent recruitment results in thin slices substantially agree with the anatomical and electrophysiological evidence available from intact animals.
Other approaches have been used to assess convergence from different peripheral afferent nerves onto single NTS neurons in intact animals. In general, this in vivo work has identified rather limited convergence and thus agrees well with our slice conclusions. Maximal shocks to whole peripheral nerve trunks in intact animals most commonly trigger spikes within a narrow time window in poststimulus histograms, and such surveys identified only single-afferent inputs to most single NTS neurons: 249/292 neurons (28), 38/45 neurons (17), 25/28 neurons (59), or 55/56 neurons (55). Convergence from arterial baroreceptors or cardiac mechanoreceptors, for example, was rare, <13% in NTS neurons activated by C-type pulmonary vagal afferents through right atrial injection of phenylbiguanide (60). Likewise, physiological activation of aortic or carotid sinus baroreceptors did not activate NTS neurons activated by cardiac vagal mechanoreceptors in dogs (72). Interestingly, when tested in cases of apparent convergence, responses commonly met monosynaptic criteria for only one nerve, with the remainder of inputs meeting polysynaptic criteria [e.g., carotid sinus and superior laryngeal nerves (55)]. Again, such in vivo results were consistent with our in vitro slice studies of medial NTS that have identified convergent, polysynaptic EPSCs and IPSCs driven by ST inputs, each with unique threshold intensities (8, 9, 11). However, even in thin horizontal slices, multiple (2–3) monosynaptic inputs excite particular phenotypic subsets of NTS neurons, many of which lie outside of the medial subnucleus [e.g., catecholaminergic (8)]. In horizontal NTS slices, multiple polysynaptic inputs are common in second-order medial NTS neurons and include ST-activated, polysynaptic IPSCs and EPSCs (6, 9, 11, 57), as are found in intact brain stem intracellular recordings (56, 75).
Another interesting corollary of afferent convergence is the evidence that unmyelinated and myelinated afferents do not mix at single medial NTS neurons. In our slices, CAP either completely blocked ST-EPSCs or was without effect in all ADN+ neurons, as well as other NTS neurons. This all-or-none CAP dichotomy in ADN+ neurons suggests that myelinated and unmyelinated ST afferents do not converge directly onto single NTS neurons, a finding consistent with a single axon mediating electrically activated ST-EPSCs (11, 32, 43). Again, such segregation of afferent phenotype by myelination has been observed in NTS neurons in vivo. In only 1 case out of 72, NTS neurons that were activated by unmyelinated cardiac vagal afferents also received myelinated inputs, although it is not clear whether all connections were monosynaptic (29).
CAP-sensitive ST Afferents Fail Synaptically at High Frequencies
Sensitivity of ST-EPSCs to CAP was all or none and subdivided NTS second-order neurons into two groups, whether ADN+ or ADN− (32). Interestingly, activation of CAP-sensitive ST-EPSCs with 50-Hz bursts of shocks revealed augmenting failure rates as each burst progressed. This form of synaptic failure progression was absent in CAP-resistant neurons, whether ADN+ or not. The mechanism for this failure difference clearly arises from the presynaptic ST afferent component of transmission. Glutamate release properties were quite uniform across afferents and in the small subset of CAP-tested neurons; the variation in individual V-M relations was not correlated with CAP sensitivity. Although further and much more extensive studies of glutamate release in CAP-sensitive and CAP-resistant neurons are necessary to resolve this issue properly, the degree of variation suggests that the basic glutamate release mechanism may be common between the two classes of afferent axons. Rather, it may be that afferent conduction and/or action potential properties of the CAP-sensitive afferents might be responsible for failure differences. Although the mechanism for these use-dependent failures is unclear, the complement of ion channels in nodose neurons differs systematically across the myelinated/unmyelinated classes (50, 70, 71, 76). Unmyelinated cranial visceral afferents have broad action potentials, lower rates of discharge, and frequency-dependent broadening within the nodose soma compared with myelinated afferents (49). CAP sensitivity of nodose and vagal ganglion neurons is found only in C-type, unmyelinated neurons, and neurons with conduction velocities in the myelinated range are uniformly CAP resistant (51). With respect to afferent frequency, the baroreflex effects of electrical activation of ADN C-fibers are nearly maximal at 5 Hz (36). Presently, little is known about the central presynaptic afferent and terminal or how closely peripheral nodose neuron properties represent central portions of these neurons.
NTS Afferent Processing and Integration
Our studies suggest that cranial visceral afferents and arterial baroreceptors, in particular, convey a consistently robust excitation to second-order NTS neurons within medial NTS. The mean synaptic currents (200–400 pA, on average) were quite large. Although the CAP-resistant, presumably myelinated inputs were substantially larger than the unmyelinated inputs, the synaptic responses generally generated initial spikes in the postsynaptic NTS neuron 80–90% of the time (6, 8). This synaptic conformation contrasts with the conventional view of central excitatory synaptic transmission in which integration most often occurs distally on relatively remote dendrites and single-fiber inputs may consist of single quanta (64). Thus NTS processing of afferent inputs follows a fundamentally different integration strategy in which basal communication is secured by large primary glutamate signals determined by the presynaptic, i.e., afferent properties. ST excitatory inputs appear to derive predominantly from individual afferents and thus would convey a single modality of information with a high safety factor. Instead of combining multiple small inputs converging from multiple redundant, unreliable sources, e.g., hippocampus (2), medial NTS appears to rely on limited large amplitude but highly reliable inputs. This model of medial NTS integration elevates the potential importance of modulation of the release process at presynaptic terminals, particularly by descending neural inputs, and this theme is supported by examples of powerful presynaptic regulation of cranial visceral afferent glutamate release by peptides: angiotensin, opioids, vasopressin, or cholecystokinin (7, 8, 12, 14, 39, 63).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-41119 and HL-56460.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron 57: 420–431, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci USA 91: 10380–10383, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders K, Ohndorf W, Dermietzel R, Richter DW. Synapses between slowly adapting lung stretch receptor afferents and inspiratory beta-neurons in the nucleus of the solitary tract of cats: a light and electron microscopic analysis. J Comp Neurol 335: 163–172, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Andresen MC, Doyle MW, Bailey TW, Jin YH. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz J Med Biol Res 37: 549–558, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus tractus solitarius. J Neurophysiol 74: 1518–1528, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Bailey TW, Hermes SM, Aicher SA, Andresen MC. Target-specific, dynamic pathway tuning by A-type potassium channels in solitary tract nucleus: cranial visceral afferent pathways to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol 582: 613–628, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey TW, Jin YH, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. J Neurosci 20: 1904–1911, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol 284: R1340–R1353, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Belugin S, Mifflin SW. Transient voltage-dependent potassium currents are reduced in NTS neurons isolated from renal wrap hypertensive rats. J Neurophysiol 94: 3849–3859, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Bonham AC Neurotransmitters in the CNS control of breathing. Respir Physiol 101: 219–230, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 264: H1674–H1685, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Brooks PA, Glaum SR. GABAB receptors modulate a tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat nucleus tractus solitarii in vitro. J Auton Nerv Syst 54: 16–26, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci 9: 11–25, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol 562: 535–551, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciriello J Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett 36: 37–42, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Clements JD Variance-mean analysis: a simple and reliable approach for investigating synaptic transmission and modulation. J Neurosci Methods 130: 115–125, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci 23: 105–113, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Cone RD Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Corbett EK, Batten TF, Kaye JC, Deuchars J, McWilliam PN. Labelling of rat vagal preganglionic neurones by carbocyanine dye DiI applied to the heart. Neuroreport 10: 1177–1181, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol 373: 63–86, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis JA Mouse and rat anesthesia and analgesia. Curr Protoc Neurosci Jan: Appendix 4B, 2008. [DOI] [PubMed]
- 28.Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol 360: 261–273, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donoghue S, Fox RE, Kidd C, Koley BN. The distribution in the cat brain stem of neurones activated by vagal non-myelinated fibres from the heart and lungs. Q J Exp Physiol Cogn Med Sci 66: 391–404, 1981. [DOI] [PubMed] [Google Scholar]
- 30.Donoghue S, Garcia M, Jordan D, Spyer KM. Identification and brain-stem projections of aortic baroreceptor afferent neurones in nodose ganglia of cats and rabbits. J Physiol 322: 337–353, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci 22: 8222–8229, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods 37: 37–48, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632–H640, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Fan W, Reynolds PJ, Andresen MC. Baroreflex frequency-response characteristics to aortic depressor and carotid sinus nerve stimulation in rats. Am J Physiol Heart Circ Physiol 271: H2218–H2227, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol Regul Integr Comp Physiol 277: R748–R756, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald M Capsaicin and sensory neurons–a review. Pain 15: 109–130, 1983. [DOI] [PubMed] [Google Scholar]
- 38.Foster KA, Regehr WG. Variance-mean analysis in the presence of a rapid antagonist indicates vesicle depletion underlies depression at the climbing fiber synapse. Neuron 43: 119–131, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 93: 2530–2540, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development 101: 697–713, 1987. [DOI] [PubMed] [Google Scholar]
- 41.Hollis JH, Lightman SL, Lowry CA. Integration of systemic and visceral sensory information by medullary catecholaminergic systems during peripheral inflammation. Ann N Y Acad Sci 1018: 71–75, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Ignatius MJ, Shooter EM, Pitas RE, Mahley RW. Lipoprotein uptake by neuronal growth cones in vitro. Science 236: 959–962, 1987. [DOI] [PubMed] [Google Scholar]
- 43.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J Neurosci 24: 4709–4717, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalia M, Welles R. Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res 188: 23–32, 1980. [DOI] [PubMed] [Google Scholar]
- 45.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Regulation of Breathing, edited by Dempsey JA and Pack AI. New York: Decker, 1995, p. 219–284.
- 47.Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol 554: 175–193, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson SN Morphological and biochemical cell types of sensory neurons. In: Sensory Neurons: Diversity, Development, and Plasticity, edited by Scott SA. New York: Oxford University Press, 1992, p. 27–59.
- 49.Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett 421: 62–66, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Li BY, Schild JH. Patch clamp electrophysiology in the nodose ganglia of the adult rat. J Neurosci Methods 115: 157–167, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods 164: 75–85, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loewy AD Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy AD and Spyer KM. New York: Oxford, 1990, p. 88–103.
- 53.Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res 581: 339–343, 1992. [DOI] [PubMed] [Google Scholar]
- 54.Meyer AC, Neher E, Schneggenburger R. Estimation of quantal size and number of functional active zones at the calyx of held synapse by nonstationary EPSC variance analysis. J Neurosci 21: 7889–7900, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mifflin SW Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Mifflin SW, Felder RB. An intracellular study of time-dependent cardiovascular afferent interactions in nucleus tractus solitarius. J Neurophysiol 59: 1798–1813, 1988. [DOI] [PubMed] [Google Scholar]
- 57.Miles R Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J Neurophysiol 55: 1076–1090, 1986. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura S, Myers RR. Myelinated afferents sprout into lamina II of L3–5 dorsal horn following chronic constriction nerve injury in rats. Brain Res 818: 285–290, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull 37: 397–404, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Paton JFR Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Paton JFR, Li YW, Deuchars J, Kasparov S. Properties of solitary tract neurons receiving inputs from the sub-diaphragmatic vagus nerve. Neuroscience 95: 141–153, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20: 1675–1688, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Poole SL, Deuchars J, Lewis DI, Deuchars SA. Subdivision specific responses of neurons in the nucleus of the tractus solitarius to activation of mu-opioid receptors in the rat. J Neurophysiol 98: 3060–3071, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Raastad M, Storm JF, Andersen P. Putative single quantum and single fibre excitatory postsynaptic currents show similar amplitude range and variability in rat hippocampal slices. Eur J Neurosci 4: 113–117, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, Hausser M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 450: 1245–1248, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds PJ, Fan W, Andresen MC. Capsaicin-resistant arterial baroreceptors. J Negat Results Biomed 5: 6, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rinaman L Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28: 50–60, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sapru HN, Gonzalez E, Krieger AJ. Aortic nerve stimulation in the rat: cardiovascular and respiratory responses. Brain Res Bull 6: 393–398, 1981. [DOI] [PubMed] [Google Scholar]
- 69.Sapru HN, Krieger AJ. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol 42: 344–348, 1977. [DOI] [PubMed] [Google Scholar]
- 70.Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type nodose sensory neurons: model interpretations of dynamic discharge characteristics. J Neurophysiol 71: 2338–2358, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol 78: 3198–3209, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Seagard JL, Dean C, Hopp FA. Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol 520: 243–253, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol 552: 547–559, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silver RA Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: theory, application and limitations. J Neurosci Methods 130: 127–141, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol 512: 149–162, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stansfeld CE, Wallis DI. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol 54: 245–260, 1985. [DOI] [PubMed] [Google Scholar]
- 77.Titz S, Keller BU. Rapidly deactivating AMPA receptors determine excitatory synaptic transmission to interneurons in the nucleus tractus solitarius from rat. J Neurophysiol 78: 82–91, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Kitzing E, Jonas P, Sakmann B. Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse. Adv Second Messenger Phosphoprotein Res 29: 235–260, 1994. [DOI] [PubMed] [Google Scholar]
- 80.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci 24: 5516–5524, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]