Abstract
A variable number tandem repeat polymorphism in the coding region of the circadian clock PERIOD3 (PER3) gene has been shown to affect sleep. Because circadian rhythms and sleep are known to modulate sympathovagal balance, we investigated whether homozygosity for this PER3 polymorphism is associated with changes in autonomic nervous system (ANS) activity during sleep and wakefulness at baseline and after sleep deprivation. Twenty-two healthy participants were selected according to their PER3 genotype. ANS activity, evaluated by heart rate (HR) and HR variability (HRV) indexes, was quantified during baseline sleep, a 40-h period of wakefulness, and recovery sleep. Sleep deprivation induced an increase in slow-wave sleep (SWS), a decrease in the global variability, and an unbalance of the ANS with a loss of parasympathetic predominance and an increase in sympathetic activity. Individuals homozygous for the longer allele (PER35/5) had more SWS, an elevated sympathetic predominance, and a reduction of parasympathetic activity compared with PER34/4, in particular during baseline sleep. The effects of genotype were strongest during non-rapid eye movement (NREM) sleep and absent or much smaller during REM sleep. The NREM-REM cycle-dependent modulation of the low frequency-to-(low frequency + high frequency) ratio was diminished in PER35/5 individuals. Circadian phase modulated HR and HRV, but no interaction with genotype was observed. In conclusion, the PER3 polymorphism affects the sympathovagal balance in cardiac control in NREM sleep similar to the effect of sleep deprivation.
Keywords: clock gene, autonomic nervous system, cardiovascular risk, slow wave activity
genetic factors have been identified as playing an important role in many physiological processes, especially those involved in cardiovascular control (5, 10, 34, 46, 57). Heart rate (HR) variability (HRV), defined as the variation in the interval between consecutive heart beats, is modulated by the autonomic nervous system and is an indicator of clinically relevant cardiovascular phenotypes. Reduced HRV is an independent predictor of cardiac disease and cardiac mortality (3, 14, 15, 23, 27, 33, 40, 51). The primary explanation for this predictive effect is that reduced HRV reflects a shift in the cardiac sympathovagal balance from parasympathetic to sympathetic control over the autonomic nervous system (35, 45). Vigilance state and endogenous circadian phase are major modulators of the autonomic nervous system. Sympathetic activity is higher during wakefulness than sleep, and, within sleep, the sympathetic contribution is greater in rapid eye movement (REM) sleep than in deep non-REM (NREM) sleep (7, 56). During continuous wakefulness under controlled behavioural conditions, HRV is not constant but varies with circadian phase such that global HRV is greatest in the early morning hours (25, 30, 32, 44, 52, 53). The impact of sleep, wakefulness, and circadian phase on autonomic control of HRV is also of interest because the frequency of onset of acute myocardial infarctions shows a marked circadian variation. Understanding the sources of individual differences in HRV and how these relate to interindividual differences in sleep and circadian physiology may increase the diagnostic use of HRV and may provide new avenues for preventive therapy.
Recently, we characterized the effects of a variable number tandem repeat (VNTR) polymorphism in the coding region of the circadian clock gene PERIOD3 (PER3) on sleep structure and the circadian modulation of waking performance during acute sleep deprivation (55). Those homozygous for the PER3 allele with five repeats (PER35/5) displayed more slow-wave sleep (SWS) and enhanced slow wave activity (SWA; i.e., EEG power density in the range of 0.75–4.5 Hz during NREM sleep). In addition, PER35/5 subjects, compared with those homozygous for the short, four-repeat allele (PER34/4), showed an increase in slow eye movements and theta activity during wakefulness, two markers of sleepiness and inattention (11), and a decline in waking performance, in particular during the early morning hours after sleep deprivation.
These physiological alterations in PER35/5 subjects are very similar to those observed in chronically sleep-deprived individuals (8) and are likely to involve the autonomic nervous system (16, 48, 60). Previous studies have shown that sleep deprivation induces a reduction of HRV and an increase in sympathetic activity, which are predictors of cardiovascular events (60). Under baseline conditions, SWS and SWA are associated with a reduced sympathovagal balance (2, 7, 59). Decrements in alertness and cognition are associated with a decrease in parasympathetic activity, suggesting that cardiac autonomic variation could be one of several potential mechanisms involved in the regulation of cognitive performance (6, 31).
The associations between sleep, sleep deprivation, and autonomic cardiac control and the effects of this polymorphism on sleep and circadian modulation of waking performance led us to hypothesize that this polymorphism may be a marker for individual differences in the autonomic nervous system. The aim of the present study was to assess whether the PER3 polymorphism predicts differences in autonomic cardiac control and its interaction with vigilance state and circadian phase. Autonomic cardiac control was estimated by HRV indexes during polysomnographically recorded baseline sleep as well as during sleep deprivation and subsequent recovery sleep.
METHODS
This study is based on the same experimental protocol and study participants described in a previous publication (55). Those methods germane to this study are reproduced below.
Participants.
Participants gave written informed consent to participate in this study, which was favorably reviewed by the University of Surrey Ethics Committee. Four hundred and four subjects (aged between 20 and 35 yr) were genotyped. Individuals provided buccal swab samples from which genomic DNA was extracted using the QuickExtract system (Epicentre Biotechnologies, Madison, WI). Genotyping was performed using PCR as previously described (1). Ten healthy PER35/5 subjects and fourteen healthy PER34/4 subjects completed the laboratory study. All participants were free from medication and nonsmokers. Participants were not suffering from any recent acute or chronic illness. All subjects selected for this study were healthy individuals with no history of cardiovascular disease, and all underwent a thorough physical examination and blood screening, which included medical questionnaires, hematology, serology, and biochemistry to exclude diseases of somatic origin, including possible cardiovascular diseases. This was also confirmed by the subjects’ general practioners. Of the 24 subjects, 2 subjects (one genotyped PER34/4 and one genotyped PER35/5) could not be included in the analysis (spectral analysis and/or ECG recording) because of poor quality of recordings. The analyses presented in this study are therefore based on 13 PER34/4 and 9 PER35/5 subjects. It should be emphasized that the study participants were recruited primarily on the basis of their genotype. No prescreening was carried out with respect to sleep preference or sleep-wake timing. However, subjects who needed to stay awake during the night due to professional or social reasons (e.g., shift workers) were excluded. Hence, all subjects had normal sleep-wake patterns, which implies regular wake and sleep episodes in relation with the day/night cycle. This enabled investigation into whether the PER3 polymorphism can lead to changes in sleep structure, autonomic nervous system control, and so forth.
Protocol.
Participants wore actiwatches (wrist-worn Actiwatch L, Cambridge Neurotechnology, Cambridge, UK) and completed sleep diaries for ∼3 wk before the laboratory study. After 2 wk, the sleep diary and actigraphy data were analyzed to characterize the habitual sleep-wake cycle in each participant. Participants were then instructed to adhere strictly to their average habitual sleep time for 1 wk before the laboratory study.
During the laboratory study, circadian and homeostatic aspects of sleep and cardiac regulation were quantified under baseline conditions, during ∼40 h of sleep deprivation under constant routine (CR) conditions, and during a subsequent recovery sleep.
Polysomnographic (PSG) recordings were obtained for the duration of the 4-day intensive physiological monitoring study. Eight EEGs (F1, C3, P3, and O1 vs. A2 and F2, C4, P4, and O2 vs. A1), electroocculogram (EOG), and electromyogram were recorded with a sampling frequency of 256 Hz using Siesta PSG units (Compumedics, Abbotsford, Victoria, Australia). The sleep analysis involved visual scoring on a 30-s epoch basis using standardized criteria (43) and spectral analysis of the sleep EEG (50).
The first night served as a habituation to the laboratory, and bed and wake times were scheduled according to each individual's habitual sleep time. The second night served as the baseline sleep episode, which was also scheduled according to the individual's habitual sleep schedule. After the baseline sleep episode, participants were kept awake under constant routine (CR) conditions for the duration of the habitual waking period, sleep period, and following wake period, i.e., ∼40 h. After the CR, volunteers went to bed in the laboratory at their habitual bedtime for a recovery sleep episode of 12 h. During the baseline and recovery sleep episodes, participants were in total darkness. Before the baseline night and after the recovery night, participants were exposed to typical indoor lighting. After the baseline sleep episode, participants were exposed to dim light (<5 lux at eye level) throughout the entire CR.
CR.
The CR protocol has previously been used to asses the circadian regulation of autonomic control of the heart (26, 53). It serves to verify endogenous circadian rhythms by either removing masking effects or distributing masking effects uniformly over the study period (18). The light-dark cycle is replaced by constant dim light (<5 lux at eye level), and the masking effects of the sleep-wake cycle as well as associated changes in posture and activity levels are removed by keeping the participants awake in a semirecumbent posture in bed during ∼40 h with a constant temperature at 18 ± 0.5°C. To asses the rhythm of plasma melatonin, which is a reliable circadian phase marker, an indwelling cannula was inserted into a forearm vein at the beginning of the 40-h period.
Wakefulness was verified by continuous EEG and EOG recordings. During the CR, subjects received hourly nutritional drinks as a substitute to main meals to meet their caloric demand, which was calculated for each subject using the Harris-Benedict formula with an activity factor of 1.3 (24). The substitute nutritional drink (Fortisip, Nutricia, Wilts, UK) consisted of 35% fat, 49% carbohydrate, and 16% protein. Subjects also drank up to 4 liters of additional water per 24-h period.
HR and HRV.
A two-derivation ECG was recorded throughout the 5-day laboratory study. R-R intervals, i.e., the length of time between the R peaks of consecutive QRS complexes, were calculated, and all traces were visually checked for artifacts by an investigator (A. U. Viola). The R wave peak detection and R-R signal were analyzed by Darwin software (Medilog Huntleigh Healthcare, Oxford Instruments). The R wave detection was confirmed and corrected visually by an investigator with relevant expertise (A. U. Viola). Occasional ectopic beats were identified and replaced with interpolated R-R interval data. Thoracic and abdominal movements were recorded using piezo sensor bands. All data acquisition and postacquisition analyses were carried out in accordance with established standards, including those put forth by the Task Force on HRV Interpretation (49).To align the HRV data with sleep stages and avoid excluding large sections of the recording contaminated by movement artifacts during wakefulness, we used a sampling period of 2.5 min for HRV estimation (12, 19), in accordance with the recommendations of the Task Force on HRV Interpretation. Power densities in the low-frequency (LF) band (0.04–0.15 Hz) and in the high-frequency (HF) band (0.15–0.50 Hz) were calculated for each 2.5-min segment by integrating the spectral power density in the respective frequency bands. Absolute and normalized power spectra and derived measures (i.e., LF and HF) were considered and used as indicators of sympathetic and parasympathetic activity, respectively. Moreover, the LF-to-(LF + HF) ratio [LF/(LF + HF) ratio] was used as an index of sympathovagal balance. The SD of normal R-R intervals (SDNN) was computed as a global indicator of the overall HRV for each 2.5-min segment. Even though the SDNN does not directly relate to parasympathetic or sympathetic activity, it directly illustrates R-R variation. The root mean square SD (RMSSD) and the percentage of the number of times consecutive normal sinus (NN) intervals exceeded 50 ms (pNN50) were also computed for each 2.5-min segment and are considered markers of parasympathetic activity. The indexes selected are those most commonly used in the analysis of HRV.
For baseline and recovery sleep episode analyses, values were considered from sleep onset (sleep stage 2). To include all participants in mean profiles of HRV indexes during wakefulness, and to exclude unstable periods, ∼4 h at the beginning and the 4 h at the end of the CR were excluded from analyses. The recovery sleep episode was restricted to the duration of the baseline sleep episode for comparisons between the two conditions. Time course analyses of HRV within and across NREM-REM cycles were based on four complete NREM-REM sleep cycles during baseline sleep and six during recovery sleep. To establish HRV profiles, individual differences in the occurrence and duration of sleep stages were normalized according to an established method (17). In short, for each individual, the unequal time spent in NREM and REM sleep episodes were subdivided into percentiles so that HRV data could be averaged over subjects.
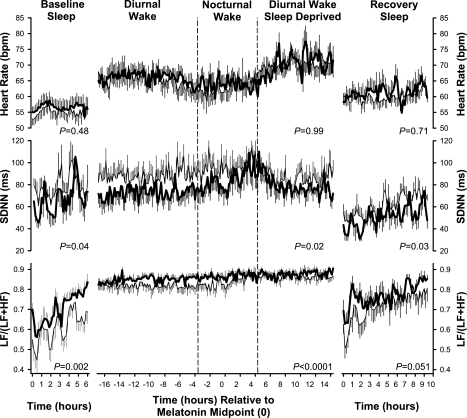
To illustrate the circadian variation in HR and HRV indexes (Fig. 1), all data were aligned with respect to the melatonin midpoint, which was defined as the midpoint between the upward and downward mean crossing (58). To assess the presence of circadian rhythmicity, and to quantify amplitude and phase, each individual time series, plotted relative to clock time, was considered to be rhythmic if the 95% confidence interval of the estimated amplitude of a sine wave with a circadian period of 24.2 h did not include 0. We chose 24.2 h because in humans the intrinsic circadian period is very tightly distributed around this value (13).
Fig. 1.
Effect of PERIOD3 gene (PER3) polymorphism during baseline sleep, diurnal wake, nocturnal wake, sleep-deprived diurnal wake, and recovery sleep on heart rate (HR) and two measures of HR variability {SD of normal R-R intervals (SDNN) and low frequency-to-low frequency + high frequency power ratio [LF/(LF + HF) ratio]}. The three wake periods are plotted relative to the timing of the plasma melatonin rhythm in 9 PER35/5 (thick solid line) and 13 PER34/4 (thin solid line) homozygotes. P values shown in the left, middle, and right indicate a significant effect of genotype, as assessed by ANOVA, during baseline sleep, wakefulness (i.e., diurnal wake, nocturnal wake and diurnal wake, sleep deprived) and recovery sleep, respectively. Data are plotted with a 10-min resolution and represent the average of four 2.5-min periods each. Error bars represent SEs.
Using PROC NLIN (SAS version 9.1, SAS Institute, Cary, NC), data were fitted to the following function: Value(sample ti) = mesor + amplitude × sin[(sample ti − phase)/24.2], in which the mesor, amplitude, and phase were free parameters, ti represents the clock time at which a sample was collected, and value represents the HRV measure being assessed.
Statistical analysis.
All statistical analyses were performed with SAS version 9.1. Comparisons of repeated measures between genotypes were made with mixed-model ANOVA for repeated measures (PROC MIXED). Genotype as well as time were factors in all analyses. Contrasts were assessed with the LSMEANS statement. All P values were based on Kenward-Roger's corrected degrees of freedom (29).
RESULTS
Reported results were based on data from 13 PER34/4 subjects (5 women and 9 men, age ± SE: 24.2 ± 0.9 yr, and body mass index ± SE: 21.7 ± 0.4 m/kg2) and 9 PER35/5 subjects (3 women and 6 men, age ± SE: 25.3 ± 1.3 yr, body mass index ± SE: 22.2 ± 0.6 m/kg2). Full details of sleep characteristics and alertness during sleep deprivation in the two genotypes have been reported elsewhere (55).
Endogenous circadian rhythm in HR and HRV variables.
As shown in Table 1, circadian variation was significant in 19 of 22 subjects for HR, in 16 of 22 subjects for SDNN, and in 12 of the 22 subjects for the LF/(LF + HF) ratio. The fitted maximum of the circadian rhythm in HR was located in the late afternoon. The maxima of the circadian rhythm in global variability, i.e., SDNN, was located at approximately 06:30 AM, and the maximum of the LF/(LF + HF) ratio was located between 03:00 and 05:00 AM. The two genotypes did not differ in amplitude or phase of these rhythms.
Table 1.
Number of subjects with a significant amplitude in the circadian rhythms of HR, SDNN, and LF/(LF+HF) ratio for mean fitted amplitudes and the timing of fitted maxima
|
Amplitude |
Maxima, Mean h:min ± SD | ||
|---|---|---|---|
| Means ± SD | Number of Subjects With Significant Amplitudes | ||
| HR, beats/min | |||
| All | 3.52±1.6 | 19/22 | 15:29±03:14 |
| PER35/5 | 3.25±1.91 | 8/9 | 14:41±03:13 |
| PER34/4 | 3.71±1.40 | 11/13 | 16:03±03:14 |
| SDNN, ms | |||
| All | 8.42±5.14 | 16/22 | 06:31±04:28 |
| PER35/5 | 7.91±6.45 | 6/9 | 06:35±03:31 |
| PER34/4 | 8.79±4.26 | 10/13 | 06:28±04:27 |
| LF/(LF + HF) ratio | |||
| All | 0.019±0.012 | 12/22 | 04:16±02:36 |
| PER35/5 | 0.014±0.008 | 5/9 | 03:12±03:30 |
| PER34/4 | 0.022±0.013 | 7/13 | 04:59±02:49 |
PER3, PERIOD3 gene; HR, heart rate; SDNN, SD of normal R-R intervals; LF/(LF + HF) ratio, low frequency-to-low frequency + high frequency ratio.
Time course of HRV indexes during baseline and recovery sleep and while awake during the CR.
Temporal profiles in HR, SDNN, and the LF/(LF + HF) ratio obtained for baseline sleep, the approximate 40-h period of wakefulness, and the recovery sleep episode are shown in Fig. 1. ANOVA during baseline sleep showed that PER35/5 individuals displayed significantly lower SDNN (P = 0.04) and a higher LF/(LF + HF) ratio (P = 0.002). The effects of the PER3 polymorphism on SDNN and the LF/(LF + HF) ratio persisted during wakefulness. The PER3 polymorphism also had a significant effect on SDNN (P = 0.03) and a strong trend toward significance on the LF/(LF + HF) ratio (P = 0.051) during recovery sleep. In contrast to these measures of HRV, HR showed no difference between the two genotypes throughout the study.
Effects of sleep and sleep deprivation on HRV indexes.
The averages for HR, SDNN, RMSSD, pNN50, and LF/(LF + HF) ratio during baseline sleep, wakefulness during the CR (on day 1, night 1, and day 2), and recovery sleep are shown in Table 2. Sleep, compared with wakefulness, was associated with a reduction in HR, SDNN, and the LF/(LF + HF) ratio and an increase in RMSSD and pNN50. The contrast between baseline sleep and nocturnal wake showed a significant increase in HR and the LF/(LF + HF) ratio and a significant decrease in SDNN, RMSSD, and pNN50. Sleep deprivation had a significant effect on all HRV indexes in both genotypes. The contrast between baseline and recovery sleep showed a reduction in RMSSD and pNN50 and an increase in SDNN and LF/(LF + HF) ratio during recovery sleep. This sleep deprivation effect was also seen during wakefulness, where the contrast between diurnal wake at baseline and diurnal wake while sleep deprived showed a significant decrease in pNN50 and a trend toward an decrease in RMSSD and an increase in the LF/(LF + HF) ratio. HR was affected significantly only by vigilance state, i.e., sleep versus wakefulness, and not by sleep deprivation.
Table 2.
Effect of vigilance state and sleep deprivation on HR, SDNN, RMSSD, pNN50, and LF/(LF + HF) ratio in all subjects and separately for the two genotypes
| Baseline Sleep | Diurnal Wake | Nocturnal Wake | Diurnal Wake, Sleep Deprived | Recovery Sleep |
Contrast, P value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Sleep vs. Recovery Sleep | Baseline Sleep vs. Diurnal Wake | Baseline Sleep vs. Nocturnal Wake | Diurnal Wake vs. Diurnal Wake, Sleep Deprived | Diurnal Wake vs. Nocturnal Wake | ||||||
| HR, beats/min | ||||||||||
| All | 55.75±1.52 | 65.94±1.75 | 63.24±1.88 | 67.17±3.19 | 60.06±1.65 | 0.12 | <0.0001 | 0.002 | 0.55 | 0.17 |
| PER35/5 | 56.67±2.44 | 65.90±2.42 | 63.54±2.74 | 68.95±2.97 | 60.61±2.76 | 0.37 | 0.004 | 0.08 | 0.37 | 0.44 |
| PER34/4 | 55.11±2.00 | 65.96±2.58 | 63.02±2.65 | 66.08±4.89 | 59.68±2.13 | 0.21 | <0.0001 | 0.01 | 0.97 | 0.26 |
| SDNN, ms | ||||||||||
| All | 72.74±5.07 | 79.27±5.34 | 87.73±5.81 | 81.30±4.20 | 51.49±3.74 | 0.0001 | 0.04 | 0.0006 | 0.81 | 0.009 |
| PER35/5 | 65.09±8.07 | 73.76±7.70 | 82.98±7.69 | 81.34±5.21 | 45.80±4.19 | 0.02 | 0.08 | 0.008 | 0.42 | 0.07 |
| PER34/4 | 78.03±6.35 | 83.08±7.36 | 91.01±8.39 | 81.28±6.15 | 55.42±4.84 | 0.002 | 0.23 | 0.02 | 0.75 | 0.06 |
| RMSSD, ms | ||||||||||
| All | 71.21±6.15 | 49.46±3.78 | 50.87±3.88 | 42.14±2.69 | 44.29±4.94 | <0.0001 | <0.0001 | <0.0001 | 0.09 | 0.67 |
| PER35/5 | 61.50±9.26 | 44.83±5.68 | 47.53±5.56 | 41.98±4.70 | 38.09±4.84 | 0.01 | 0.002 | 0.05 | 0.63 | 0.61 |
| PER34/4 | 77.95±7.96 | 52.66±5.04 | 53.18±5.41 | 42.24±3.39 | 48.59±7.53 | 0.0001 | <0.0001 | <0.0001 | 0.08 | 0.91 |
| pNN50 | ||||||||||
| All | 42.34±3.55 | 25.71±3.55 | 25.80±2.87 | 20.01±2.28 | 22.78±3.74 | <0.0001 | <0.0001 | <0.0001 | 0.03 | 0.97 |
| PER35/5 | 33.70±5.73 | 20.84±4.07 | 22.47±4.13 | 19.10±3.70 | 17.38±4.50 | 0.005 | 0.0003 | 0.01 | 0.48 | 0.63 |
| PER34/4 | 48.32±3.86 | 29.07±3.99 | 28.11±3.92 | 20.58±3.01 | 26.52±5.40 | <0.0001 | <0.0001 | <0.0001 | 0.03 | 0.73 |
| LF/(LF + HF) ratio | ||||||||||
| All | 0.68±0.02 | 0.84±0.01 | 0.85±0.01 | 0.87±0.01 | 0.74±0.03 | 0.03 | <0.0001 | <0.0001 | 0.08 | 0.24 |
| PER35/5 | 0.73±0.02 | 0.86±0.01 | 0.87±0.01 | 0.88±0.01 | 0.78±0.03 | 0.23 | <0.0001 | <0.0001 | 0.53 | 0.61 |
| PER34/4 | 0.65±0.03 | 0.82±0.02 | 0.85±0.02 | 0.87±0.01 | 0.71±0.04 | 0.04 | <0.0001 | <0.0001 | 0.08 | 0.3 |
Values are means ± SE. RMSSD, root mean square SD; pNN50, percentage number of times of consecutive normal sinus intervals exceeded 50 ms. Recovery sleep and nocturnal wake episodes were restricted to the same duration as baseline sleep.
Genotype- and sleep stage-dependent variations in HRV indexes during baseline and recovery sleep.
Analysis of the effect of genotype and sleep stage during baseline (Table 3) revealed that both genotype and sleep stage had significant effects on HRV indexes. pNN50 was significantly lower and the LF/(LF + HF) ratio was significantly higher in the PER35/5 group compared with the PER34/4 group, but only so in NREM sleep.
Table 3.
Genotype and sleep stage effect on HR, SDNN, RMSSD, pNN50, and LF/(LF + HF) ratio during baseline sleep episodes
|
Baseline Sleep |
Stage Contrast, P Value (NREM vs. REM) | ||
|---|---|---|---|
| NREM Sleep | REM Sleep | ||
| HR, beats/min | |||
| All | 55.10±1.55 | 58.62±1.63 | 0.12 |
| PER35/5 | 56.19±2.49 | 59.39±2.54 | 0.38 |
| PER34/4 | 54.35±2.02 | 58.08±2.19 | 0.38 |
| P value (PER34/4 vs. PER35/5) | 0.58 | 0.69 | |
| SDNN, ms | |||
| All | 68.22±5.26 | 94.36±7.61 | 0.01 |
| PER35/5 | 59.06±7.30 | 91.91±12.36 | 0.03 |
| PER34/4 | 74.57±7.01 | 96.06±10.02 | 0.08 |
| P value (PER34/4 vs. PER35/5) | 0.25 | 0.75 | |
| RMSSD, ms | |||
| All | 71.26±6.54 | 75.79±8.44 | 0.67 |
| PER35/5 | 58.07±8.37 | 73.28±12.79 | 0.37 |
| PER34/4 | 80.39±8.80 | 77.52±11.62 | 0.84 |
| P value (PER34/4 vs. PER35/5) | 0.15 | 0.78 | |
| pNN50 | |||
| All | 43.51±3.65 | 35.88±3.64 | 0.15 |
| PER35/5 | 33.59±5.77 | 32.61±5.77 | 0.90 |
| PER34/4 | 50.38±3.81 | 38.13±4.55 | 0.06 |
| P value (PER34/4 vs. PER35/5) | 0.02 | 0.44 | |
| LF/(LF + HF) ratio | |||
| All | 0.61±0.03 | 0.76±0.02 | <0.0001 |
| PER35/5 | 0.67±0.03 | 0.77±0.04 | 0.05 |
| PER34/4 | 0.57±0.04 | 0.76±0.02 | <0.0001 |
| P value (PER34/4 vs. PER35/5) | 0.04 | 0.93 | |
Values are means ± SE. REM, rapid eye movement; NREM, non-REM.
Sleep stage had a significant effect on SDNN (P = 0.01) and the LF/(LF + HF) ratio (P < 0.0001) when analyzed for the two genotypes combined. Within each genotype, sleep stage also had an effect, such that SDNN and the LF/(LF + HF) ratio were higher during REM than NREM sleep for both genotypes. In the PER34/4 subjects, pNN50 was lower during REM sleep than NREM sleep, but this did not reach statistical significance (P = 0.06).
These sleep stage associations were also observed during the recovery sleep episode.
HR (means ± SE; NREM: 59.76 ± 1.71 and REM: 64.60 ± 1.71, P = 0.05), SDNN (NREM: 52.10 ± 3.25 and REM: 72.40 ± 5.29, P = 0.002), and the LF/(LF + HF) ratio (NREM: 0.72 ± 0.02 and REM: 0.84 ± 0.03, P = 0.004) were higher during REM than NREM. However, no significant differences were observed between the two genotypes.
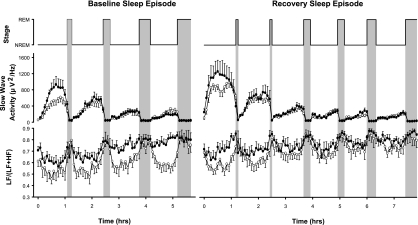
Normalizing the individual NREM-REM cycles allowed their influence on HRV profiles to be characterized. Figure 2 shows mean ± SE time courses of SWA and the LF/(LF + HF) ratio during the baseline and recovery sleep episodes. SWA dynamics displayed the well-known ultradian rhythmicity, with high SWA during NREM sleep and low SWA during REM sleep and the decline over consecutive NREM episodes. The NREM-REM cycle was associated with a low LF/(LF + HF) ratio during NREM sleep and high sympathetic predominance during REM. In the first NREM episode of baseline sleep, PER35/5 individuals had more SWA and a higher LF/(LF + HF) ratio than PER34/4 individuals. Although the differences in SWA between the genotypes dissipated over subsequent NREM episodes, the difference in the LF/(LF + HF) ratio became more pronounced. Whereas in PER34/4 individuals the LF/(LF + HF) ratio continued to display the typical decline during NREM episodes, this decline was absent in PER35/5 individuals.
Fig. 2.
Effect of the PER3 polymorphism on normalized slow wave activity (SWA) and normalized sympathovagal balance [LF/(LF + HF) ratio] during the first four non-rapid eye movement (NREM) and REM (NREM-REM) sleep cycles of baseline sleep and the first six NREM-REM sleep cycles for recovery sleep in 9 PER35/5 (solid symbols) and 13 PER34/4 (open symbols) individuals. Error bars represent SEs.
After sleep deprivation, SWA was enhanced in both genotypes, particularly at the beginning of the recovery sleep episode. Surprisingly, this increase in SWA was not accompanied by a reduction in the LF/(LF + HF) ratio in recovery sleep relative to baseline sleep in either genotype. However, the LF/(LF + HF) ratio was lower in PER34/4 than PER35/5 individuals in the first NREM episode. In subsequent NREM episodes of the recovery sleep, modulation of the LF/(LF + HF) ratio over NREM-REM cycles became smaller in both genotypes and resembled the pattern of PER35/5 individuals during baseline.
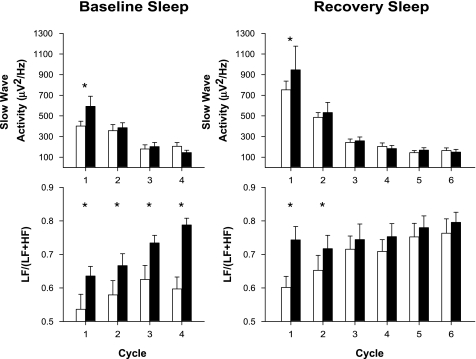
Averaging these data for NREM and REM sleep separately for each NREM-REM cycle (Fig. 3) demonstrated that SWA was significantly higher in PER35/5 individuals during the first cycle for baseline. The LF/(LF + HF) ratio was also significantly higher in this group, an effect that persisted throughout the baseline night and for the first NREM-REM sleep cycle during the recovery night.
Fig. 3.
Effect of the PER3 polymorphism on SWA and sympathovagal balance [LF/(LF + HF) ratio]. Data were averaged for NREM sleep, separately for each NREM-REM sleep cycle in baseline and recovery sleep episodes in 9 PER35/5 (solid bars) and 13 PER34/4 (open bars) individuals. Error bars represent SEs. *Significant difference between genotypes (P < 0.03).
DISCUSSION
These data show that acute sleep deprivation leads to a greater sympathetic influence on the autonomic control of the heart. This effect is more pronounced during sleep than wakefulness. Individuals homozygous for the longer PER3 allele have less global variability in HR, as indicated by a lower SDNN and less parasympathetic activity. This is also seen from the pNN50 as well as a greater sympathetic predominance on autonomic nervous system control compared with those homozygous for the shorter PER3 allele, in particular during NREM sleep. Because these parameters have previously been associated with adverse cardiovascular events (28, 36), these data indicate that sleep loss may increase cardiovascular vulnerability, for which the PER3 polymorphism may be a genetic marker.
The circadian variation of HR and HRV indexes during wakefulness, as observed during the CR, is very similar to that described by others. The maximum of the circadian rhythm of HR was located in the late afternoon, in accordance with previous studies (9, 26, 53, 56). The maximum global variability in the early morning hours (approximately 6:30 AM) has also been reported in other studies (53, 56). These data confirm the profound impact of the circadian timing system on autonomic control of the heart. We did not observe a major effect of the PER3 polymorphism on either the amplitude or timing of these circadian rhythms. Thus, and in accordance with previous assessments of markers of circadian phase (55), the PER3 polymorphism does not appear to affect circadian timing of HR variables during wakefulness, even though circadian phase modulated HR and HRV.
The effect of sleep and sleep stage on HR and HRV indexes, which has previously been shown to be considerable (2, 41, 59), was also observed in the present study. Autonomic balance varies in synchrony with the NREM-REM cycle. Detailed analyses of the influence of NREM and REM sleep confirmed that the sympathovagal balance shifts toward parasympathetic dominance during NREM sleep and toward sympathetic dominance during REM sleep. In accordance with previous studies (7, 37, 47), the time course of sympathovagal balance within and across NREM-REM cycles follows the time course of SWA. This balance is shifted most toward parasympathetic dominance in the second half of the first NREM episode, when SWA is at its maximum. This parasympathetic dominance becomes smaller over consecutive NREM episodes, when SWA declines. These and similar observations by others are in accordance with the notion that high levels of SWA are associated with parasympathetic dominance.
Comparison of the two genotypes, however, revealed that the time course of SWA and sympathovagal balance can dissociate. In the first NREM episode of baseline sleep, SWA was higher in PER35/5 individuals compared with PER34/4 individuals, but the syampathovagal balance indicated less parasympathetic dominance in PER35/5 than PER34/4 individuals. This dissociation between SWA and sympathovagal balance became even more pronounced in the subsequent NREM episode, during which SWA no longer differed between the genotypes. Whereas in PER34/4 individuals sympathovagal balance continued to shift toward parasympathetic dominance after the transition from REM sleep to NREM sleep, this shift was not observed in PER35/5 individuals.
The analysis of recovery sleep provided further evidence of the dissociation between SWA and sympathovagal balance. While SWA was enhanced, the parasympathetic contribution was not, and the NREM-REM cycle-dependent modulation of the sympathovagal balance was diminished during recovery sleep. In fact, our data indicate that during recovery sleep after sleep deprivation, parasympathetic indexes decrease and sympathetic predominance increases. This indicates that from an autonomic control of the heart perspective, recovery sleep appears not to have the characteristics of restorative sleep, i.e., low sympathetic predominance.
The sleep deprivation-induced sympathovagal phenotype is similar to that observed in PER35/5 individuals during baseline. Whether the mechanisms underlying the PER35/5 phenotype and the sleep deprivation phenotype are related remains unclear. It is of interest, though, that sleep propensity, as assessed by sleep latency and theta activity during wakefulness, is higher in PER35/5 individuals, and this may indicate that these individuals are in a state of sleep deprivation even under baseline conditions (55). In summary, these data show that the PER3 polymorphism affects the sympathovagal balance during baseline sleep and recovery sleep in a direction similar to the effects of sleep loss.
Whereas previous studies have indicated that when SWA increases, parasympathetic activity increases, as indexed by a decrease in the LF/(LF + HF) ratio (7), the present study shows that these two variables can dissociate. For example, after sleep deprivation, SWA is enhanced, but the LF/(LF + HF) ratio was also enhanced. Furthermore, this dissociation was also observed when comparing the two polymorphisms. PER35/5 individuals displayed greater SWA during the baseline night and a greater LF/(LF + HF) ratio compared with PER34/4 individuals. Hence, it can be assumed that the time course of SWA and the autonomic nervous system exhibit an ultradian rhythm with similar phases, although not all aspects of these EEG and autonomic variables are under the same central control.
Numerous studies have shown a circadian pattern of the risk for cardiovascular disorders, with a morning increase in all major cardiovascular events, predominantly between 06:00 and 12:00 hours (20, 38, 54). Furthermore, it has been postulated that the nocturnal peak incidence of myocardial infarction might be triggered by changes in autonomic nervous control of the cardiovascular system around the clock. In this study, the PER3 polymorphism affected the sympathovagal balance during normal day and night activities with a similar pattern to that induced by sleep deprivation, which, in turn, is habitually associated with an increased cardiovascular risk. Given the well-known associations among HRV indexes, mortality, and cardiovascular events, together with the epidemiological evidence for a link between short sleep duration and cardiovascular events (21), the data suggest an important role for this PER3 polymorphism in the overall control of autonomic balance during sleep and wake cycles.
The PER3 VNTR polymorphism has been linked with many distinctive phenotypes, including diurnal preference and delayed sleep phase syndrome (1, 42), an increased risk of breast cancer (61), bipolar disorder (39), drug dependence (62), sleep structure (55), and cognitive performance (22). Of course, caution should be exercised when affirming a direct role for PER3 in the control of autonomic balance, and we cannot rule out the possibility that the VNTR polymorphism is a genetic marker that is linked to another locus responsible for these phenotypes. However, the PER3 VNTR polymorphism is not an single-nucleotide polymorphism marker but a variation in four or five repeats of a 54-nucleotide sequence, which creates an 18-amino acid difference in the protein. The VNTR repeats contain clusters of predicted casein kinase-1ε phosphorylation sites, which are known to affect circadian period (4). Hence, we hypothesize that a variation in these repeats could affect phosphorylation of PER3, in addition to differences that will occur at the tertiary structural level. Therefore, we would expect these significant structural differences to impact on PER3 function and be related, either directly or indirectly, to the phenotype that we observed. Taken together, it is interesting to hypothesize that the PER3 polymorphism can represent an important clinical factor linked to an augmented cardiovascular risk and may provide novel approaches for research and preventive therapy.
GRANTS
This work was supported by Biotechnology and Biological Sciences Research Council Grants BSS/B/08523 and BB/F022883/1.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26: 413–415, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Baharav A, Kotagal S, Gibbons V, Rubin BK, Pratt G, Karin J, Akselrod S. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology 45: 1183–1187, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 88: 927–934, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Blau J PERspective on PER phosphorylation. Genes Dev 22: 1737–1740, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boomsma DI, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta Genet Med Gemellol (Roma) 39: 181–191, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Boon P, Moors I, De HV, Vonck K. Vagus nerve stimulation and cognition. Seizure 15: 259–263, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clin Neurophysiol 112: 992–996, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep 16: 100–113, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol Heart Circ Physiol 273: H1761–H1768, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Busjahn A, Voss A, Knoblauch H, Knoblauch M, Jeschke E, Wessel N, Bohlender J, McCarron J, Faulhaber HD, Schuster H, Dietz R, Luft FC. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms and heart rate variability in twins. Am J Cardiol 81: 755–760, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Regul Integr Comp Physiol 277: R640–R649, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Cogliati C, Colombo S, Ruscone TG, Gruosso D, Porta A, Montano N, Malliani A, Furlan R. Acute beta-blockade increases muscle sympathetic activity and modifies its frequency distribution. Circulation 110: 2786–2791, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284: 2177–2181, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Atherosclerosis Risk In Communities. Circulation 102: 1239–1244, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol 145: 899–908, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Delamont RS, Julu PO, Jamal GA. Sleep deprivation and its effect on an index of cardiac parasympathetic activity in early nonREM sleep in normal and epileptic subjects. Sleep 21: 493–498, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res 626: 190–199, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab 282: E297–E303, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Dumont M, Jurysta F, Lanquart JP, Noseda A, van de Borne P, Linkowski P. Scale-free dynamics of the synchronization between sleep EEG power bands and the high frequency component of heart rate variability in normal men and patients with sleep apnea-hypopnea syndrome. Clin Neurophysiol 118: 2752–2764, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Elliot WJ Cyclic and circadian variations in cardiovascular events. Am J Hypertens 14: 291S–295S, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47: 833–839, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Groeger JA, Viola AU, June CY, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 31: 1159–1167, 2008. [PMC free article] [PubMed] [Google Scholar]
- 23.Guzzetti S, La Rovere MT, Pinna GD, Maestri R, Borroni E, Porta A, Mortara A, Malliani A. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J 26: 357–362, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4: 370–373, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol 27: 197–200, 2000. [PubMed] [Google Scholar]
- 26.Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA 101: 18223–18227, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huikuri HV, Makikallio TH, Raatikainen MJ, Perkiomaki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death: appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation 108: 110–115, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kamen PW, Tonkin AM. Application of the Poincare plot to heart rate variability: a new measure of functional status in heart failure. Aust NZ J Med 25: 18–26, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983–997, 1997. [PubMed] [Google Scholar]
- 30.Kerkhof GA, Van Dongen HP, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens 11: 373–377, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women's Health and Aging Study I. J Am Geriatr Soc 54: 1751–1757, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol Regul Integr Comp Physiol 267: R819–R829, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Kudaiberdieva G, Gorenek B, Timuralp B. Heart rate variability as a predictor of sudden cardiac death. Anadolu Kardiyol Derg 7, Suppl 1: 68–70, 2007. [PubMed] [Google Scholar]
- 34.Kupper NH, Willemsen G, van den BM, de BD, Posthuma D, Boomsma DI, de Geus EJ. Heritability of ambulatory heart rate variability. Circulation 110: 2792–2796, 2004. [DOI] [PubMed] [Google Scholar]
- 35.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Malliani A, Lombardi F, Pagani M, Cerutti S. Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 5: 274–286, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G Autonomic modulation of the cardiovascular system during sleep. N Engl J Med 328: 347–349, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Maron BJ, Kogan J, Proschan MA, Hecht GM, Roberts WC. Circadian variability in the occurrence of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 23: 1405–1409, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE Jr, Schork NJ, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 141: 234–241, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98: 1510–1516, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Otzenberger H, Gronfier C, Simon C, Charloux A, Ehrhart J, Piquard F, Brandenberger G. Dynamic heart rate variability: a tool for exploring sympathovagal balance continuously during sleep in men. Am J Physiol Heart Circ Physiol 275: H946–H950, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Pereira DS, Tufik S, Louzada FM, Benedito-Silva AA, Lopez AR, Lemos NA, Korczak AL, D'Almeida V, Pedrazzoli M. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep 28: 29–32, 2005. [PubMed] [Google Scholar]
- 43.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: National Institutes of Health, 1968.
- 44.Scheer FA, van Doornen LJ, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms 14: 202–212, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation 85: I77–I91, 1992. [PubMed] [Google Scholar]
- 46.Singh JP, Larson MG, O'Donnell CJ, Tsuji H, Corey D, Levy D. Genome scan linkage results for heart rate variability (the Framingham Heart Study). Am J Cardiol 90: 1290–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Takase B, Akima T, Satomura K, Ohsuzu F, Mastui T, Ishihara M, Kurita A. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomed Pharmacother 58, Suppl 1: S35–S39, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 50.Thakor NV, Tong S. Advances in quantitative electroencephalogram analysis methods. Annu Rev Biomed Eng 6: 453–495, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji H, Venditti FJ Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 90: 878–883, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Van Dongen HP, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int 18: 85–98, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res 16: 148–155, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Venditti FJ, John RM, Hull M, Tofler GH, Shahian DM, Martin DT. Circadian variation in defibrillation energy requirements. Circulation 94: 1607–1612, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, von Schantz M, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol 17: 613–618, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Viola AU, Simon C, Ehrhart J, Geny B, Piquard F, Muzet A, Brandenberger G. Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J Biol Rhythms 17: 539–547, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Voss A, Busjahn A, Wessel N, Schurath R, Faulhaber HD, Luft FC, Dietz R. Familial and genetic influences on heart rate variability. J Electrocardiol Suppl 29: 154–160, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, Dijk DJ, Czeisler CA. Do plasma melatonin concentrations decline with age? Am J Med 107: 432–436, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Zemaityte D, Varoneckas G, Sokolov E. Heart rhythm control during sleep. Psychophysiology 21: 279–289, 1984. [DOI] [PubMed] [Google Scholar]
- 60.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, Demeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol 98: 2024–2032, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T. Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol Biomarkers Prev 14: 268–270, 2005. [PubMed] [Google Scholar]
- 62.Zou Y, Liao G, Liu Y, Wang Y, Yang Z, Lin Y, Shen Y, Li S, Xiao J, Guo H, Wan C, Wang Z. Association of the 54-nucleotide repeat polymorphism of hPer3 with heroin dependence in Han Chinese population. Genes Brain Behav 7: 26–30, 2008. [DOI] [PubMed] [Google Scholar]