Abstract
Although endothelin-1 (ET-1)-induced organ hypoperfusion after trauma-hemorrhage is improved by estrogen administration, it remains unclear whether estrogen receptor (ER) subtypes play any role in the attenuation of ET-1-induced vasoconstriction in any specific organ bed. To investigate this, isolated perfusion experiments in the heart, liver, small intestine, kidney, and lung were carried out in sham, at the time of maximum bleedout (MBO; i.e., 5-cm midline incision, with removal of 60% of circulating blood volume over 45 min to maintain a mean blood pressure of 40 mmHg), and 2 h after trauma-hemorrhage and resuscitation (T-H/R). Organ-specific ET-1-induced vasoconstriction was evaluated, and the effects of 17β-estradiol (E2) and ER-specific agonists propylpyrazole triol (PPT; ERα agonist) and diarylpropionitrile (DPN; ERβ agonist) were determined. ET-1 induced the greatest vasoconstriction in sham animals, with the strongest response in the kidneys, followed by the small intestine and liver. ET-1-induced responses were weakest in the heart and lungs. ET-1-induced vasoconstriction was evident at the time of MBO but was significantly decreased at 2 h after T-H/R. ERβ plays an important role in cardiac performance, as evidenced by improved heart performance (+dP/dt) in the presence of DPN. DPN also induced a greater effect than PPT in the reduction of ET-1-induced vasoconstriction in the kidneys and lungs. In contrast, PPT attenuated ET-1-induced vasoconstriction in the liver, whereas both DPN and PPT were equally effective in the small intestine. The increased +dP/dt values induced by E2, DPN, or PPT were evident at the time of MBO but were significantly decreased at 2 h after T-H/R. These data indicate that the effects of ET-1 on vasoconstriction and the role of ER subtypes in estrogen-induced vasorelaxation are organ specific and temporally specific after trauma-hemorrhage.
Keywords: maximum bleedout, 17β-estradiol, diarylpropionitrile, propylpyrazole triol
although significant advances in intensive care medicine have been made, sepsis leading to multiple organ failure continues to remain the major cause of death in the injured host (5, 6, 7, 11, 19). The functions of various organs after hemorrhagic shock are depressed, and such changes persist for a prolonged period despite fluid resuscitation (30–34). It is also known that there are sex hormone-mediated gender differences in cardiovascular and immune system functions (16, 26). In this regard, 17β-estradiol (E2) plays an important role in blood vessel dilation and improvement in organ perfusion and function after trauma-hemorrhage and resuscitation (T-H/R) or vascular injury (2, 10, 15, 17, 18, 20, 21, 24, 25, 27, 29). The mechanism of action of E2 appears to be multifactorial and related to improved circulation via nitric oxide (NO)-induced vasodilation and decreased synthesis of vasoconstricting molecules, such as endothelin (ET) and angiotensin II (2, 4, 20, 22).
ET-1 is a potent vasoconstricting peptide produced by endothelial cells and exerts its vasoconstricting effects locally on underlying vascular smooth muscle cells (23). A previous study (2) by our laboratory has shown that E2 attenuates ET-1-induced vasoconstriction after T-H/R. E2 influences ET-1-mediated vasoconstriction via downstream mechanisms that are regulated by estrogen receptors (ERs): ERα and ERβ (2, 17, 28). Nonetheless, the determination of downstream mechanisms that mediate E2-induced vasodilation is complicated by the fact that E2 is known to have both endothelium-dependent and -independent vasodilatory effects (9, 13, 14). A previous study (17) has shown tissue-specific expression of ERα and ERβ. The salutary effects of estrogen administration on organ function after T-H/R are both receptor subtype and tissue specific (36–38). However, our previous studies were carried out using in vivo systems, which allowed interactions between organ systems. To examine the organ-specific effects of E2, we used an isolated organ perfusion technique to determine the vasoactive effects of ET-1 in various organ beds and the role of ER subtypes in E2-induced vasorelaxation after T-H/R. In this manner, interactions between organ systems were prevented.
MATERIALS AND METHODS
Animal model of trauma-hemorrhage.
Male (275–325 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were fasted for 16 h before the experiment but were allowed water ad libitum. All experiments were performed in adherence with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama (Birmingham, AL). Rats were anesthetized with 1.5% isoflurane with air inhalation and then subjected to soft tissue trauma via a 5-cm ventral midline laparotomy, which was closed in layers before the onset of hemorrhage. Both femoral arteries and one femoral vein were cannulated with polyethylene (PE)-50 tubing for bleeding, monitoring of mean arterial pressure [blood pressure (BP)], and fluid resuscitation. BP was monitored using a BP analyzer (Digi-Med, Louisville, KY). Animals were restrained in the supine position, and the areas of incision were bathed with 1% lidocaine (Elkins-Sinn, Cherry Hill, NJ) to minimize postoperative pain. After the procedure, anesthesia was removed, and animals were maintained in a custom restrainer box, where they were allowed to awaken. When the BP reached ∼120 mmHg, animals were bled rapidly to a pressure of 40 mmHg (i.e., severe hypotension) within 10 min. The BP of 40 mmHg was maintained by removing additional blood until the animal was no longer able to maintain BP at 40 mmHg, i.e., maximum bleedout (MBO) was reached. BP was then maintained by an infusion of Ringer lactate (RL) intravenously in small volumes until 40% of the shed blood volume was returned in the form of RL (∼90 min from the onset of hemorrhage). Animals were then resuscitated with four times the shed blood volume with RL at a constant rate using a Harvard Apparatus pump (Holliston, MA; four times the MBO volume) over a period of 60 min. This entire procedure is referred to as T-H/R. After resuscitation, rats were lightly anesthetized with 1.5% isofluorane, the catheters were removed, the vessels were ligated, and skin incisions were closed with sutures. Animals were maintained in a conscious state without heparin injection throughout the T-H/R procedure. Sham-operated animals underwent the same surgical procedure but were neither bled nor resuscitated. The time required for MBO was ∼45 min; the volume of MBO was ∼60% of the calculated circulating blood volume. Measurements were carried out at 4.5 h after surgical operation in shams (mimicking the time of 2 h postresuscitation in the T-H/R group), after reaching MBO volume in the MBO group (i.e., 45 min after the onset of hemorrhage), and at 2 h postresuscitation in the T-H/R group.
Preparation of isolated hearts and measurements of coronary perfusion pressure and heart performance.
Animals were anesthetized with 1.5% isoflurane with air inhalation, and anesthesia was maintained by an injection of pentobarbital sodium (30 mg/kg body wt) via the femoral vein. Animals were also injected with 300 μl heparin (Abraxis) to prevent the formation of blood clots. The heart was isolated and perfused through the carotid artery with a consistent perfusion flow of 5 ml/min with aerated (95% O2-5% CO2 oxygenated) Krebs-Ringer-HCO3 buffer (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2.2H2O, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 0.026 mM Ca-EDTA, 11.1 mM d-glucose, and 0.22 g/l Na-pyruvate; pH 7.4) at 37°C. Perfusion pressure was determined by a BP analyzer (Digi-Med) placed in line before the carotid artery inlet cannula. The flow rate of 5 ml/min resulted in a perfusion pressure of ∼30 mmHg.
A balloon catheter, made using a condom tip (Trojan Brand, Princeton, NJ), was inserted into the left ventricle. The balloon was filled with 150 μl saline and connected to the heart performance analyzer (Digi-Med) to measure heart performance (+dP/dt). The initial +dP/dt value in sham animals was ∼500 mmHg/s.
Upon stabilization of perfusion basal tension, ET-1 or vehicle dose-response curves were performed. The dose-response curve to ET-1 was evaluated using increasing doses of ET-1 (American Peptide, Sunnyvale, CA), resulting in 0, 0.1, 1.0, 10, and 100 ng/ml final concentrations in the Krebs-Ringer-HCO3 buffer reservoir. The length of time between the administration of each dose was 10 min. Data are presented as percentage changes in perfusion pressure and were used as a measurement of ET-1-induced vessel constriction.
Dose-response curves of coronary perfusion pressure and +dP/dt to E2, diarylpropionitrile (DPN), and propylpyrazole triol (PPT) were also determined. The dose-response curves were evaluated using increasing doses of E2, DPN, and PPT, resulting in 10−9, 10−8, 10−7, and 10−6 M final concentrations in the Krebs-Ringer-HCO3 buffer reservoir. The length of time between the administration of each dose was 10 min. Data are presented as percentage changes in perfusion pressure or +dP/dt and were used as a measurement of the effects of E2, DPN, and PPT on perfusion pressure or +dP/dt.
Preparation of isolated livers and perfusion pressure measurements.
As previously described, the portal vein was cannulated with PE-90 tubing for perfusion and monitoring of perfusion pressure. The vena cava was cannulated with 0.062-in. silicone tubing to drain the perfusate. The liver was perfused at a constant flow rate of 5 ml/min with 37°C aerated Krebs-Ringer-HCO3 buffer. This flow rate induced a perfusion pressure of ∼40 mmHg in sham animals. Perfusion pressure was determined by a BP analyzer placed in line before the portal vein inlet cannula. After the perfusion pressure was stable, ET-1-induced vasoconstriction dose-response curves and effects of E2, DPN, or PPT on ET-1-induced vasoconstriction were determined as follows: one final concentration dose of 10−5 M E2, 10−6 M DPN, and 10−6 M PPT or an equal volume of vehicle was added to the Krebs-Ringer-HCO3 buffer reservoir. Once the perfusion pressure was stable (15 min), ET-1-induced vasoconstriction dose-response curves were performed using increasing concentrations (10−9–10−5 M) of ET-1 in the reservoir. The next dose in the series was added once maximal response of the previous dose had been established and the baseline tension was stable (10 min). The dose-response curves were expressed as percent increases from the maximal ET-1-induced perfusion pressure. According to preliminary experiments, the responses of ET-1 on perfusion pressure were too weak to be determined in the liver 2 h after T-H/R; therefore, the vasoresponse curves of the above agents were only determined in sham animals and at MBO but not in animals subjected to T-H/R.
Preparation of isolated lungs and kidneys and perfusion pressure measurements.
Measurements of perfusion in the lung and kidney were similar to those of the liver; however, the lungs were perfused from the pulmonary artery and kidneys were perfused via the renal artery. The ET-1-induced vasoconstriction dose-response curve and effects of E2, DPN, or PPT on ET-1-induced vasoconstriction were also determined in the isolated lung and kidney, and the methods were the same as those described above.
Preparation of isolated small intestines and perfusion pressure measurements.
After anesthesia, 300 μl heparin was injected via the femoral vein to prevent blood from clotting during small intestine perfusion. The branches of blood vessels to and from the cecum, ascending colon, duodenum, and transverse colon were then ligated. The ascending colon, cecum, large intestine, and duodenum were carefully removed with minimal bleeding. Approximately 15 cm of small intestine remained, allowing blood flow to be supplied via the superior mesenteric artery. The superior mesenteric artery and portal vein were cannulated with PE-50 and PE-90 catheters, respectively. The isolated intestine was perfused through the superior mesenteric artery catheter (PE-50) at a constant flow rate of 5 ml/min with 37°C aerated Krebs-Ringer-HCO3 buffer. Perfusion pressure was determined by a BP analyzer placed in line before the superior mesenteric artery inlet cannula. The ET-1-induced vasoconstriction dose-response curves and effects of E2, DPN, or PPT on ET-1-induced vasoconstriction were determined.
Statistical analysis.
All data are presented as means ± SE. Statistical differences among groups were determined by one-way ANOVA followed by Tukey's test; differences were considered significant if P < 0.05.
RESULTS
ET-1-induced changes in perfusion pressure in a variety of organs.
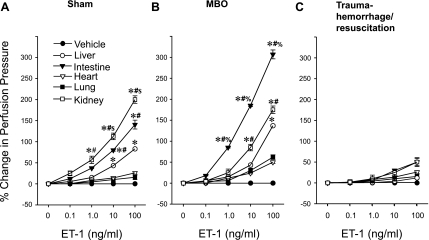
As shown in Fig. 1A, the increase in perfusion pressure induced by ET-1 was dose dependent (1.0–100 ng/ml) in the liver, intestine, and kidney. ET-1 had no effect in the lungs or heart of sham animals. At doses between 10 and 100 ng/ml, ET-1 induced the highest perfusion pressure in the kidneys followed by the intestine and then the liver (Fig. 1, A and B). In the MBO group, the highest responses to ET-1 were in the intestine and then the kidney and then the liver; however, no significant responses were observed in the lung and heart (Fig. 1B). ET-1 did not induce significant responses in all tested organs 2 h after T-H/R (Fig. 1C).
Fig. 1.
Endothelin (ET)-1-induced percent changes from baseline in organ perfusion pressure in sham (A), maximum bleedout (MBO; B), and trauma-hemorrhage and resuscitation (T-H/R; C) animals. Data are presented as means ± SE; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle in all organs; #P < 0.05 vs. the corresponding concentration of ET-1 in hepatic tissue; $P < 0.05 vs. the corresponding concentration of ET-1 in intestinal tissue. %P < 0.05 vs. the corresponding concentration of ET-1 in kidney.
Effects of E2, DPN, and PPT on coronary perfusion pressure and +dP/dt.
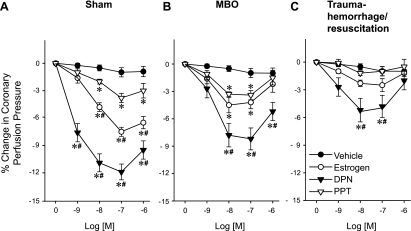
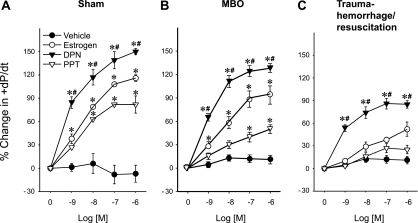
Coronary perfusion pressures were significantly decreased by E2, DPN, and PPT in sham animals (Fig. 2A) and at the time of MBO. DPN reduced coronary perfusion pressure greater than PPT (Fig. 2B). In contrast, at 2 h after T-H/R, coronary perfusion pressures were only decreased by DPN at 10−8–10−7 M (Fig. 2C). +dP/dt values were increased significantly by E2, DPN, and PPT in sham animals and at the time of MBO; however, DPN induced a significantly greater +dP/dt response compared with PPT (P < 0.05; Fig. 3, A and B). In the T-H/R group (Fig. 3C), +dP/dt values increased significantly only in the presence of DPN.
Fig. 2.
Estrogen (17β-estradiol)-, diarylpropionitrile (DPN)-, and propylpyrazole triol (PPT)-induced percent changes from baseline in coronary perfusion pressure in sham (A), MBO (B), and T-H/R (C) animals. Data are presented as means ± SE of percent changes; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle; #P < 0.05 vs. the corresponding concentration of PPT.
Fig. 3.
Estrogen-, DPN-, and PPT-induced percent changes from baseline in heart performance (+dP/dt) in sham (A), MBO (B), and T-H/R (C) animals. Data are presented as means ± SE of percent changes; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle; #P < 0.05 vs. the corresponding concentration of PPT.
Effects of E2, DPN, and PPT on organ-specific ET-1-induced perfusion pressure.
As shown in Fig. 4, the ET-1-induced increase in hepatic perfusion pressure (10–100 ng/ml) was significantly attenuated by E2, DPN, and PPT in sham animals and in animals at the time of MBO. PPT was significantly more effective than DPN at an ET-1 dose of 100 ng/ml in sham animals and at the time of MBO (Fig. 4, A and B).
Fig. 4.
Effects of estrogen, DPN, and PPT on ET-1-induced percent changes from baseline in hepatic perfusion pressure in sham (A) and MBO (B) animals. Data are presented as means ± SE; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle; #P < 0.05 vs. the corresponding concentration of DPN.
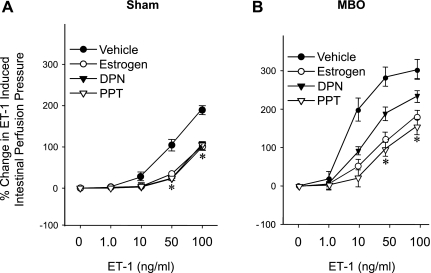
Figure 5 shows that ET-1-induced intestinal perfusion pressures were significantly attenuated by E2, DPN, and PPT when ET-1 was present at concentrations of 50–100 ng/ml. Both DPN and PPT were equally effective.
Fig. 5.
Effects of estrogen, DPN, and PPT on ET-1-induced percent changes from baseline in intestinal perfusion pressure in sham (A) and MBO (B) animals. Data are presented as means ± SE; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle.
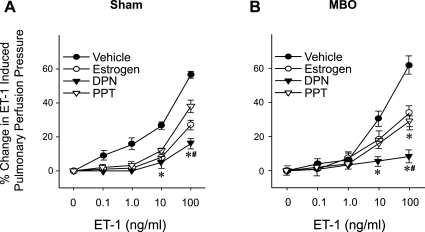
E2, DPN, and PPT significantly attenuated ET-1-induced pulmonary perfusion pressure when ET-1 was present at concentrations between 0.1 and 10 ng/ml in sham animals and at 10 mg/ml ET-1 in the MBO group (Fig. 6). DPN attenuation of the ET-1-induced increase in pulmonary perfusion pressure was significantly greater than that of PPT in sham animals and at the time of MBO.
Fig. 6.
Effects of estrogen, DPN, and PPT on ET-1-induced percent changes from baseline in pulmonary perfusion pressure in sham (A) and MBO (B) animals. Data are presented as means ± SE; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle; #P < 0.05 vs. the corresponding concentration of PPT.
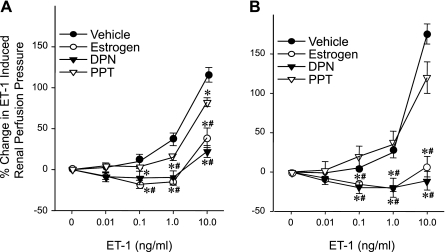
As shown in Fig. 7, DPN and E2 significantly attenuated the ET-1-induced increase in renal perfusion pressure at 0.1–10 ng/ml ET-1 in the sham and MBO groups. This effect was not observed with PPT in either the sham or MBO groups.
Fig. 7.
Effects of estrogen, DPN, and PPT on ET-1-induced percent changes from baseline in renal perfusion pressure in sham (A) and MBO (B) animals. Data are presented as means ± SE; n = 6–7 animals. Data were compared by one-way ANOVA and Tukey's test. *P < 0.05 vs. the corresponding concentration of vehicle; #P < 0.05 vs. the corresponding concentration of PPT.
DISCUSSION
A growing body of evidence has indicated that E2 administration after an ischemic insult improves cardiac output, organ perfusion, and function (8, 35). Possible mechanisms of action for E2 under such conditions might include the attenuation of the release of the vasoconstrictive peptide ET-1 (2, 36). A recent study (2) has shown that E2 not only reduces ET-1 production but also inhibits its vasoconstrictive action on the vasculature. The present study extends these findings by demonstrating that the vasoactive effects of ET-1 are organ dependent and that the salutary effects of estrogen are both receptor subtype and organ specific.
ET-1 increased perfusion pressure in a dose-dependent manner in the liver, intestine, and kidney. In contrast, these effects were not observed in the lung or heart. A previous study (3) has shown that blood perfusion in peripheral organs, such as the kidney, intestine, liver, lung, muscle, and skin, was significantly decreased after T-H/R. In contrast, blood perfusion to central organs, such as the heart and brain, was maintained (3). In this study, the peripheral organs, such as the kidneys, intestines, and liver, were more responsive to the vasoconstrictive action of ET-1 compared with central organs, such as the heart. This may explain, in part, why blood perfusion in peripheral organs was reduced after T-H/R. Although the precise reason why ET-1 had no effect in the lungs or heart is not known, it is possible that there are fewer ET-1 receptors in those organs and that may be reason for the lack of responsiveness.
The present data also show that the effects of ET-1-induced vasoconstriction were significantly decreased at 2 h after T-H/R but not at the time of MBO. Thus, the importance of ET-1 in regulating blood flow after trauma-hemorrhage appears to be greater early after injury but wanes later. This might be related to the importance of maintaining blood flow to the central organs under low-flow conditions, such as those observed at MBO.
In the present study, coronary perfusion pressure was significantly decreased by E2, DPN (ERβ agonist), and PPT (ERα agonist) in sham animals and at the time of MBO. However, at 2 h after T-H/R, coronary perfusion pressures were only decreased by DPN between concentrations of 10−8 and 10−7 M. Furthermore, the coronary perfusion pressures reduced by DPN were significantly greater compared with PPT in all groups. These findings indicate that ERβ is the predominate ER that regulates the vasodilatory actions of E2. Cardiac performance (+dP/dt) was also increased significantly by E2, DPN, and PPT in sham animals and at the time of MBO. DPN induced a significantly greater +dP/dt response than E2 or PPT in all groups, further supporting a predominant role for ERβ in the beneficial effect of E2 under such conditions.
The administration of E2 protects against organ damage after ischemia-reperfusion or T-H/R. This protective effect of E2 after T-H/R is, in part, related to a reduction in ET-1-induced vasoconstriction (2). In the present study, the effect of E2 on vasorelaxation was both receptor and organ dependent. ERβ played an important role in cardiac, kidney, and lung function, whereas ERα mediated vasodilation in the liver. Moreover, both receptor subtypes were involved in vasorelaxation in the small intestine. While our previous findings have demonstrated that the effects of ER subtypes on vasodilation vary between organ systems (35, 36), these experiments were carried out using in vivo systems, which allowed interactions between organ systems. The findings here used an isolated organ system, removing potential confounding factors that might be evident in a pure in vivo model. The precise mechanism by which E2 attenuates ET-1-mediated vasoconstriction remains unknown. Nonetheless, E2 stimulates the release of endothelium-derived relaxing compounds, such as NO and PGI2, which induce vasodilation (12, 20), and previous findings from our laboratory have shown that E2-mediated attenuation of ET-1-induced vasoconstriction is prevented by NO synthase or cyclooxygenase inhibitors (35).
Multiple organ failure or dysfunction, secondary to a systemic inflammatory response, remains the major cause of mortality and morbidity after trauma (17, 18). The present study focused on the liver, small intestine, and lung, critical organs in the development of organ dysfunction in trauma patients (1). Since selective ER agonists have varied responses in different organs, ER and organ-specific treatments might be possible after low-flow conditions. Studies have shown that estrogen not only improved organ perfusion but also improved heart performance (35, 36). It could therefore be argued that if E2 is given to an intact animal and it only vasodilates all organs, then that should result in decreased perfusion pressure. However, since E2 also improves heart performance, that should counteract the decreased perfusion pressure and thus maintain the animal's BP. Improved dP/dt induced by E2, DPN, or PPT was significantly decreased at 2 h after T-H/R compared with MBO, suggesting that the optimal time for treatment is during low-flow conditions and before resuscitation. The administration of E2 or DPN at the time of MBO was more effective than at 2 h after T-H/R, and E2 treatment correlates with improved survival in a rat model of T-H/R (preliminary observations).
The present study was conducted using organs from intact animals. It could therefore be argued that these results may be applicable only to 5α-dihydrotestosterone-replete animals. Although we have not tested the effects of E2 and ER agonists in organs from female rats, our previous studies (4, 36–38) have shown that cardiovascular functions are depressed in females in cycles other than the proestrus cycle. Thus, based on our previous studies, it would appear that similar salutary effects of E2 and ER agonists will also be observed in females with low E2 levels. This, however, remains to be determined. Furthermore, it remains to be determined whether similar salutary effects of E2 and ER agonists are also observed with aging.
In conclusion, this study shows that the effects of ET-1 on vasoconstriction are organ specific and that the role of ER subtypes in E2-induced vasorelaxation are also dependent on the tissue. Moreover, the maximal effects of E2 are evident under low-flow conditions, such as at the time of MBO. Thus, ER-specific treatments might be warranted after trauma-hemorrhage to maintain physiological function in different organ beds.
GRANTS
Funding from National Institute of General Medical Sciences Grant R37-GM-39519 supported this investigation.
Acknowledgments
The authors thank Drs. Alexis McBrayer and Martin Schwacha for help in editing the manuscript and Bobbi Smith for assistance with manuscript preparation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams CA Jr, Hauser CJ, Adams JM, Fekete Z, Xu DZ, Sambol JT, Deitch EA. Trauma-hemorrhage-induced neutrophil priming is prevented by mesenteric lymph duct ligation. Shock 18: 513–517, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. 17β-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am J Physiol Heart Circ Physiol 292: H245–H250, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ba ZF, Wang P, Koo DJ, Cioffi WG, Bland KI, Chaudry IH. Alterations in tissue oxygen consumption and extraction after trauma and hemorrhagic shock. Crit Care Med 28: 2837–2842, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Ba ZF, Yokoyama Y, Toth B, Rue LW, III, Bland KI, Chaudry IH. Gender differences in small intestinal endothelial function: inhibitory role of androgens. Am J Physiol Gastrointest Liver Physiol 286: G452–G457, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 10: 79–89, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Bolz SS, Pohl U. Indomethacin enhances endothelial NO release–evidence for a role of PGI2 in the autocrine control of calcium-dependent autacoid production. Cardiovasc Res 36: 437–444, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Deitch EA Multiple organ failure: pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckhoff DE, Bilbao G, Frenette L, Thompson JA, Contreras JL. 17-Beta-estradiol protects the liver against warm ischemia/reperfusion injury and is associated with increased serum nitric oxide and decreased tumor necrosis factor-alpha. Surgery 132: 302–309, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Gisclard V, Miller VM, Vanhoutte PM. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther 244: 19–22, 1988. [PubMed] [Google Scholar]
- 10.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320: 134–139, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Hechtman HB, Shepro D. Lung metabolism and systemic organ function. Circ Shock 9: 457–467, 1982. [PubMed] [Google Scholar]
- 12.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med 187: 917–928, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. J Cardiovasc Pharmacol 38: 657–665, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Jiang C, Sarrel PM, Poole-Wilson PA, Collins P. Acute effect of 17β-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am J Physiol Heart Circ Physiol 263: H271–H275, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock 23: 1–10, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Knoferl MW, Jarrar D, Angele MK, Ayala A, Schwacha MG, Bland KI, Chaudry IH. 17β-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol 281: C1131–C1138, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863–870, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ Res 83: 224–229, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol 75: 400–407, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Rao BR Isolation and characterization of an estrogen binding protein which may integrate the plethora of estrogenic actions in non-reproductive organs. J Steroid Biochem Mol Biol 65: 3–41, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Rogers J, Sheriff DD. Role of estrogen in nitric oxide- and prostaglandin-dependent modulation of vascular conductance during treadmill locomotion in rats. J Appl Physiol 97: 756–763, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46: 325–415, 1994. [PubMed] [Google Scholar]
- 24.Selzman CH, Turner AS, Johnson SM, Cain BS, Harken AH, Whitehill TA. Chronic estrogen replacement inhibits aortic intimal hyperplasia independent of serum lipids. J Card Surg 12: 228–234, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Selzman CH, Whitehill TA, Shames BD, Pulido EJ, Cain BS, Harken AH. The biology of estrogen-mediated repair of cardiovascular injury. Ann Thorac Surg 65: 868–874, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, Harbrecht BG, Peitzman AB, Billiar TR, Maier RV, Remick DG, Minei JP. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma 64: 572–578, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Terrell AM, Crisostomo PR, Markel TA, Wang M, Abarbanell AM, Herrmann JL, Meldrum DR. Postischemic infusion of 17-beta-estradiol protects myocardial function and viability. J Surg Res 146: 218–224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA 82: 7889–7893, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17Beta-estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 40: 205–212, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Ba ZF, Cioffi WG, Bland KI, Chaudry IH. Hepatocellular dysfunction after severe hypotension in the absence of blood loss is associated with the increased IL-6 and PGE2. J Surg Res 80: 136–142, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Singh G, Rana MW, Ba ZF, Chaudry IH. Preheparinization improves organ function after hemorrhage and resuscitation. Am J Physiol Regul Integr Comp Physiol 259: R645–R650, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Zhou M, Rana MW, Singh G, Ba ZF, Ayala A, Chaudry IH. ATP-MgCl2 restores renal microcirculation following trauma and severe hemorrhage. Can J Physiol Pharmacol 70: 349–357, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res 79: 39–46, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Wang P, Chaudry IH. Pentoxifylline increases gut ketogenesis following trauma and hemorrhagic shock. Crit Care Med 26: 101–107, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama Y, Schwacha MG, Bland KI, Chaudry IH. Effect of estradiol administration on splanchnic perfusion after trauma-hemorrhage and sepsis. Curr Opin Crit Care 9: 137–142, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol 40: 185–194, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol 79: 963–970, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function following trauma-hemorrhage. Shock. In press. [DOI] [PMC free article] [PubMed]