Abstract
Elevated blood glucose and free fatty acids induce oxidative stress associated with the incidence of cardiovascular disease. In contrast, laminar shear stress (LSS) plays a critical role in maintaining vascular health. The present study examined the mechanism for the antioxidant effect of LSS attenuating the oxidative stress induced by high glucose (HG) and arachidonic acid (AA) in human umbilical vein endothelial cells. HG and AA synergistically decreased cell viability and increased glutathione (GSH) oxidation and lipid peroxidation. The lipid peroxidation was markedly prevented by LSS as well as tetrahydrobiopterin (BH4) and GSH. LSS increased BH4 and GSH contents, and expression of GTP cyclohydrolase-1 and glutamylcysteine ligase (GCL) involved in their biosynthesis. Inhibition of GCL activity by DL-buthionine-(S,R)-sulfoximine and small-interfering RNA-mediated knockdown of GCL lessened the antioxidant effect of LSS. Therefore, it is suggested that LSS enhances antioxidant capacity of endothelial cells and thereby attenuates the oxidative stress caused by cardiovascular risk factors.
Keywords: laminar shear stress, high glucose, arachidonic acid, lipid peroxidation, glutamylcysteine ligase
shear stress, a hemodynamic force generated by blood flow, is known to regulate various vascular functions as well as gene expression in a magnitude- and flow pattern-dependent manner (22). The regions of arteries experiencing laminar shear stress (LSS) due to orderly blood flow are usually protected from atherosclerotic lesion formation, and thus LSS of relatively high level has been proposed to play anti-atherogenic roles. Differently from oscillatory shear stress, LSS usually does not stimulate inflammatory reactions but suppresses them probably by increasing nitric oxide (NO) production rather than reactive oxygen species (ROS) (8) or by induction of enzymes associated with antioxidant defense (25).
Chronic LSS enhances endothelial NO production by inducing endothelial nitric oxide synthase (eNOS) expression (5). A recent study demonstrated that LSS increases the content of tetrahydrobiopterin (BH4), an essential cofactor of eNOS, by activating GTP cyclohydrolase-1 (GTPCH-1), a rate-limiting enzyme for de novo synthesis of this cofactor, providing an additional mechanism for the increased NO production by LSS (34). However, BH4 is very prone to oxidation by ROS and can be depleted under oxidative stress conditions. Then, eNOS reaction can be “uncoupled” and produce ROS instead of NO, causing further increase of oxidative stress, rather than attenuation of oxidative stress.
Plasma free fatty acids are elevated in diabetic patients and play a role in the pathogenesis of diabetic vascular complications (35). High blood glucose and free fatty acids can cause the activation of protein kinase C and NAD(P)H oxidase, leading to ROS formation and inactivation of eNOS (15, 30). Indeed, high glucose (HG) and free fatty acids have been shown to produce an endothelial dysfunction by increasing ROS formation and decreasing antioxidant defenses (10). However, LSS effects on the oxidative stress induced by HG and free fatty acids are unknown. Therefore, assuming the potential prooxidant effect of HG plus free fatty acids and antioxidant effect of LSS, the present study investigated how LSS attenuates the oxidative stress induced by HG and arachidonic acid (AA) in human umbilical vein endothelial cells (HUVECs).
MATERIALS AND METHODS
Reagents.
Glucose, nitro-l-arginine methyl ester (l-NAME), glutathione (GSH), glutathione disulfide (GSSG), N-acetyl-l-cysteine (NAC), DL-buthionine-(S,R)-sulfoximine (BSO), 2-thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane,3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), 5,5′-dithiobis[2-nitrobenzoic acid], 1-methyl-2-vinyl-pyridium trifluoromethane sulfonate (M2VP), NADPH, GSH reductase, and dihydrorhodamine 123 (DHR 123) were purchased from Sigma-Aldrich-Fluka (St. Louis, MO). Apocynin was purchased from Calbiochem (San Diego, CA). AA and BH4 were purchased from Cayman Chemical (Ann Arbor, MI).
Cell culture and treatments.
HUVECs purchased from Clonetics Cambrex (Rockland, ME) were cultured on 0.2% gelatin-coated 100-mm tissue culture dishes (BD Biosciences, San Jose, CA) at 37°C and 5% CO2 in endothelial cell growth medium EBM-2 (Clonetics Cambrex) with a normal level of glucose (5.0 mM glucose, NG) supplemented with endothelial growth supplements and 10% FBS (GIBCO-BRL, Grand Island, NY).
In the first set of experiments, the effect of HG and AA inducing oxidative stress was examined. Cells were plated on a six-well plate at a density of 2 × 104 cells/cm and grown in a growth medium with a normal level of glucose (5.0 mM glucose, NG) for 48 h to reach ∼80% confluency. Cells were pretreated with test materials in a fresh medium for 1 h followed by treatments with additional 25 mM glucose (total 30 mM glucose, HG) and/or 20∼40 μM AA for 48 h.
In the second set of experiments, the antioxidant effect of LSS was examined. Cells on 100-mm culture dishes were exposed to LSS at 12 dyn/cm2 for 24 h. LSS was provided by rotating a Teflon cone (0.5°Cone angle) mounted on a culture dish, as described previously (2, 28). When specified, small-interfering RNA (siRNA) were transfected into HUVEC at 25 nM, according to the manufacturer's instruction. Cells at ∼50% confluency were treated with a mixture of siRNA and Lipofectamin RNAiMAX (Invitrogen, Carlsbad, CA) in Opti-MEM (Invitrogen) for 5 h, followed by incubation in a growth medium for 36 h. Human GCLm siRNA (no. 1299001) and control duplex oligoribonucleotide (no. 12935200) were purchased from Invitrogen. GCLm siRNA sequences were as follows: 5′-CCA GAU GUC UUG GAA UGC ACU GUA U-3′ (sense) and 5′-AUA CAG UGC AUU CCA AGA CAU CUG G-3′(antisense). Cell morphology was examined under an Eclipse TS100 inverted-phase microscope from Nikon (Melville, NY). Cell viability was assayed using MTT (6).
Analysis of lipid peroxidation.
The analysis of 2-thiobarbituric acid-reactive substances (TBARS) as a marker of lipid peroxidation was carried out as described previously (23). Briefly, cells were treated in a lysis buffer (20 mM Tris-Cl, 2.5 mM EDTA, and 1.0% SDS, pH 7.5). Cell lysates (100 μl) were mixed with 900 μl of 1.0% phosphoric acid and 1.0 ml of 0.9% TBA and then heated on a boiling water bath for 45 min. Standard solutions of 1,1,3,3-tetramethoxypropane, a precursor of malondialdehyde (MDA), were treated in the same way as cell lysates. After cooling, 1.5 ml of 1-butanol was added, and the mixture was centrifuged at 13,000 rpm for 15 min to separate into two layers. TBARS contents of the 1-butanol layer were spectrophotometrically determined at 532 nm. Additionally, TBA-MDA adducts were quantified by an HPLC method (13). The HPLC system (Waters, Milford, MA) consisted of a SAT/IN module, a 515 isocratic pump, a Rheodyne 7725i injector with a 20-μl sample loop, a 2487 ultraviolet (UV) detector, and a 2475 electrochemical detector. Separation was carried out on a 5 μm Agilent RP-18 (4.6 mm × 250 mm). The mobile phase consisted of 50% (vol/vol) 50 mM potassium phosphate (pH 6.3) and 50% (vol/vol) methanol. The flow rate was 1.0 ml/min. TBA-MDA adducts were detected with a UV detector at 532 nm. Protein content of cell lysates was determined by a DC protein assay kit (Bio-Rad, Hercules, CA).
Detection of ROS.
The production of ROS was determined by using oxidant-sensitive probe DHR 123 as described before (31). HUVECs were pretreated with or without 1.0 mM apocynin for 30 min and then loaded with 1.0 μM DHR 123 for 30 min before the treatment with HG and AA. After incubating the treated cells for 3 h, images of cells fluorescing due to the oxidation of DHR 123 to rhodamine 123 were obtained with the use of a Nikon Eclipse TE2000-U microscope. To extract the formed rhodamine 123, cells were ruptured in ice-cold 70% ethanol that contained 0.1 M HCl (31). The precipitated proteins were removed by centrifugation at 13,000 rpm for 15 min. The supernatants were neutralized with NaHCO3 and centrifuged again to remove precipitates. The fluorescence intensity of clear supernatant aliquots was measured at an emission wavelength of 580 nm (excitation at 485 nm) in a FLUO-star OPTIMA multidetection microplate reader (BMG Labtechnologies, Offenburg, Germany).
Analysis of GSH and GSSG.
Total GSH content (GSH + GSSG) was quantified by an enzymatic cycling assay method (1). GSSG content was determined by the same method after trapping GSH with M2VP (12). An HPLC method was also employed to detect GSH (32). Separation was carried out on a 5-μm Waters Xterra RP-18 column (4.6 mm × 150 mm) by eluting 50 mM potassium phosphate (pH 3.0) at 0.8 ml/min. GSH was detected with an electrochemical detector at an applied potential of +0.70 V. Data are presented as GSH equivalents in nanomoles GSH per milligram protein. One mole of GSSG is therefore expressed as 2 mol GSH equivalents. Protein content was determined using the acid-insoluble precipitates dissolved in 1.0 N NaOH.
Analysis of BH4.
Intracellular BH4 content was determined by an HPLC method as described previously (18). Briefly, cells were collected with a trypsin-EDTA treatment and suspended in 100 mM dithiothreitol containing 100 μM EDTA. The cell suspension was frozen immediately on dry ice and stored at −80°C. The cell suspension was thawed at 4°C and centrifuged at 13,000 rpm for 20 min to obtain the supernatant for analysis. Separation was carried out on a 5-μm Waters Xterra RP-18 (4.6 mm × 150 mm) using a mobile phase of 50 mM sodium acetate, 5.2 mM citrate, 60 μM EDTA, 160 μM dithiothreitol, and 5% methanol (pH 5.2). The flow rate was 0.7 ml/min. BH4 was detected with an electrochemical detector at an applied potential of +0.12 V.
Western blotting analysis.
Western blotting of the cell lysates was performed as previously described (18). Antibodies for GTPCH-1, glutamylcysteine ligase (GCL) catalytic subunit (GCLc), and its modulatory subunit (GCLm) were purchased from Santa Cruz Biotech (Santa Cruz, CA). Antibodies for eNOS and β-actin were from Cell Signaling (Danvers, MA) and Sigma-Aldrich, respectively.
RT-PCR analysis.
Total RNA was isolated from cells by using the RNeasy kit (Qiagen, Valencia, CA) as per the manufacturer's instruction. RT-PCR was performed using the GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) in a reaction mixture (20 μl) containing Maxime RT-PCR PreMix (iNtRON Biotechnology, Seongnam, ROK), 250 ng RNA, and 10 pmol of gene-specific primer sets. Amplification products were electrophoresed in an 1.5% agarose gel with a iVDye 100-bp DNA ladder (GenDEPOT, Barker, TX) as a size marker. The gel was ethidium bromide-stained, and band intensities were quantified using a Gel Doc system (Bio-Rad). The sequences of the primers were as follows: GTPCH-1 (GeneBank accession no., NM000161.2) 5′-TTG GTT ATC TTC CTA ACA AG-3′ (sense) and 5′-GTG CTG GTC ACA GTT TTG CT-3′ (antisense); eNOS (GeneBank accession no., NM000603.3) 5′-TGC TGG CAT ACA GGA CTC AG-3′ (sense) and 5′-TAG GTC TTG GGG TTG TCA GG-3′ (antisense); GCLc (GeneBank accession no., NM001498.2) 5′-CTG GGG AGT GAT TTC TGC AT-3′ (sense) and 5′-AGG AGG GGG CTT AAA TCT CA-3′ (antisense); GCLm (GeneBank accession no., NM002061.2) 5′-TTT GGT CAG GGA GTT TCC AG-3′ (sense) and 5′-TGG TTT TAC CTG TGC CCA CT-3′ (antisense); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (GeneBank accession no., NM002046) 5′-GCC AAA AGG GTC ATC ATC TC-3′ (sense) and 5′-GTA GAG GCA GGG ATG ATG TTC-3′ (antisense).
Statistical analysis.
Data are presented as means ± SE of three or more independent experiments. The statistical analyses were performed using the Sigma Stat 3.1 software program. Significant differences among the groups were determined by a one-way ANOVA. Duncan's multiple-range test was performed if differences were identified between the groups at P < 0.05.
RESULTS
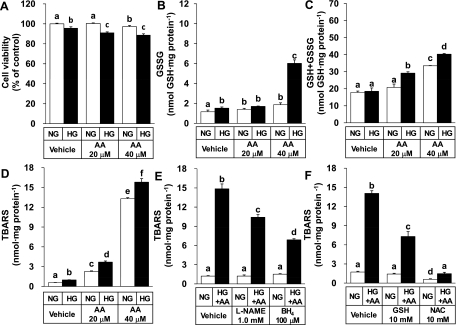
The study first examined if HG or AA can induce oxidative stress in cultured HUVECs. Cells were treated in a growth medium with a normal level of glucose (5.0 mM glucose, NG) or HG medium (30 mM glucose) containing different concentrations (0, 20 or 40 μM) of AA for 48 h. HG and AA decreased cell viability in a synergistic manner (Fig. 1A). GSSG content was also synergistically increased by HG and AA (Fig. 1B). Of interest, HG and AA increased the glutathione pool significantly (Fig. 1C). Therefore, it was indicated that HG plus AA increased both prooxidant generation and antioxidant synthesis.
Fig. 1.
High glucose (HG) and arachidonic acid (AA) induce oxidative stress in human umbilical vein endothelial cells (HUVECs). After 48 h cultivation in a normal glucose (NG) medium, cells were maintained in an NG (5.0 mM glucose) or HG (30 mM glucose) medium containing varied concentrations of AA for an additional 48 h. Cell viability was determined by 1,1,3,3-tetramethoxypropane,3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (A). Total glutathione (GSH) content [GSH + glutathione disulfide (GSSG)] was quantified by an enzymatic cycling assay method (C). GSSG content was determined by the same method after trapping GSH with 1-methyl-2-vinyl-pyridium trifluoromethane sulfonate (B). Lipid peroxidation was measured by a spectrophotometric thiobarbituric acid-reactive substances (TBARS) assay (D). In E and F, cells were pretreated for 1 h with an inhibitor [nitro-l-arginine methyl ester (l-NAME)] or a cofactor [tetrahydrobiopterin (BH4)] of nitric oxide (NO) synthase (E), and with GSH or N-acetyl-l-cysteine (NAC) (F). Cells were then maintained in an NG (5.0 mM glucose) or HG + AA medium (30 mM glucose + 40 μM AA) for 48 h and used for TBARS assay. All data represent means ± SE (n = 3∼6 experiments). Bars not sharing the same letter are significantly different from each other (P < 0.05).
TBARS content, a marker of lipid peroxidation, was dose-dependently increased by AA in both the NG and HG media (Fig. 1D). HG tended to enhance the TBARS formation. The lipid peroxidation was significantly attenuated by both an inhibitor (l-NAME) and cofactor (BH4) of nitric oxide synthase (Fig. 1E), implicating uncoupled eNOS activity might be involved. As expected, GSH and its precursor, NAC, prevented the lipid peroxidation induced by HG plus AA significantly (Fig. 1F).
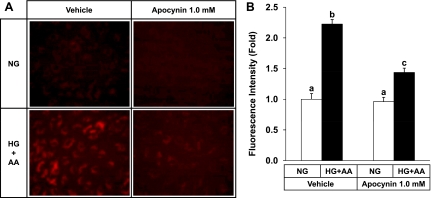
HG plus AA also markedly increased ROS formation detected by using DHR 123 (Fig. 2). As expected, ROS formation due to HG + AA was significantly attenuated by apocynin, an inhibitor of NAD(P)H oxidase (Fig. 2), confirming an involvement of NAD(P)H oxidase in oxidative stress under HG + AA conditions (15, 30).
Fig. 2.
HG plus AA increases reactive oxygen species (ROS) formation in HUVECs. Cells were loaded with 1.0 μM dihydrorhodamine 123 (DHR 123) for 30 min in the absence or presence of 1.0 mM apocynin, an inhibitor of NAD(P)H oxidase. Cells were then maintained in an NG (5.0 mM glucose) or HG + AA medium (30 mM glucose + 40 μM AA) for 3 h. Cells fluorescing due to oxidation of DHR 123 to rhodamine 123 were observed under a fluorescence microscope. Representative fluorescent images of cells, out of three experiments, are shown (A). B: formed rhodamine 123 was extracted from cells and quantified fluorophotometrically. Data represent means ± SE (n = 3). Bars not sharing the same letter are significantly different from each other (P < 0.05).
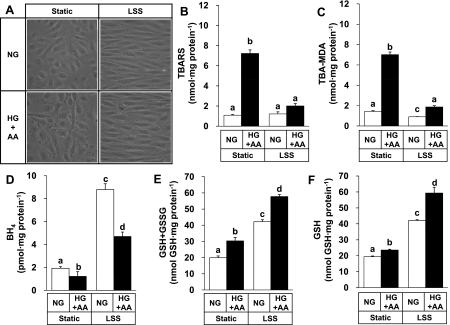
The next experiment tested if LSS could provide an antioxidant effect in this model system. HUVECs were exposed to an arterial level of LSS (12 dyn/cm2) in an NG medium or HG + AA medium. LSS treatment for 24 h resulted in an alignment of the cells along the direction of fluid movement in both cases (Fig. 3A). LSS also reduced the cytotoxic effect of HG + AA observed at static conditions, in agreement with the anti-apoptotic effect of LSS against oxidative stress (7). Both TBARS and TBA-MDA were increased by HG + AA treatments, and these changes were suppressed markedly by LSS (Fig. 3, B and C), demonstrating a unique effect of LSS attenuating lipid peroxidation under oxidative stress.
Fig. 3.
Laminar shear stress (LSS) prevents the lipid peroxidation induced by HG + AA and increases the cellular content of BH4 and GSH. HUVECs were exposed to LSS at 12 dyn/cm2 for 24 h in an NG (5.0 mM glucose) or HG + AA medium (30 mM glucose + 40 μM AA). Cell images were captured under a microscope (original magnification, ×400; A). Lipid peroxidation was measured by a spectrophotometric TBARS assay (B). TBA-MDA adducts (C), BH4 content (D), and GSH content (F) were determined by an HPLC method, and total GSH content (GSH + GSSG) was determined by an enzymatic cycling assay method (E). Data represent means ± SE (n = 3∼5). Bars not sharing the same letter are significantly different from each other (P < 0.05).
Because BH4 and GSH prevented the lipid peroxidation (Fig. 1, E and F), the effects of LSS on the cellular contents of these molecules were then examined. LSS significantly increased BH4 content by three- to approximately fourfold (Fig. 3D) and GSH + GSSG content by approximately twofold (Fig. 3E) in both NG and HG + AA media. GSH contents determined by an HPLC method (Fig. 3F) were very close to GSH + GSSG contents determined by an enzymatic cycling method in most cases, indicating that GSH is the predominant form. The only exception was static cells under HG + AA whose GSH content was significantly smaller than GSH + GSSG content, probably because of oxidation to GSSG.
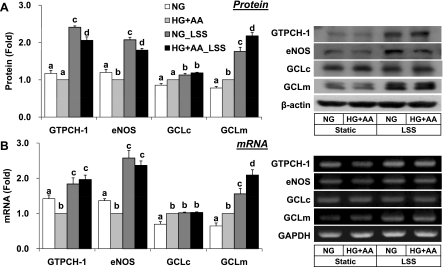
De novo synthesis of BH4 and GSH requires GTPCH-1 and GCL, respectively, as the rate-limiting enzymes. Consistently with the changes of BH4 and GSH + GSSG content, LSS increased GTPCH-1 and GCLm at the protein and mRNA levels, whereas β-actin or GAPDH expression was not altered (Fig. 4). The expression level of eNOS was upregulated by LSS as expected, but GCLc level appeared to be less sensitive to LSS, implying that GCLm rather than GCLc may play a regulatory role in the biosynthesis of GSH in response to LSS.
Fig. 4.
Effect of LSS on the expression of GTP cyclohydrolase-1 (GTPCH-1), endothelial nitric oxide synthase (eNOS), GCL catalytic subunit (GCLc), and GCL modulatory subunit (GCLm) in HUVECs exposed HG + AA. Cells were treated as in Fig. 3. Expression of GTPCH-1, eNOS, GCLc, GCLm, β-actin, and/or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at the protein and mRNA levels was monitored by Western blotting (A) and RT-PCR (B), respectively. Data are presented as fold change compared with HG + AA (means ± SE, n = 3∼5). Bars not sharing the same letter are significantly different from each other (P < 0.05).
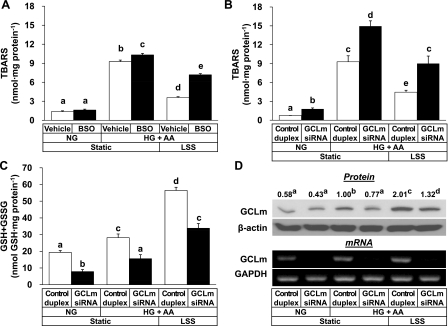
The study further examined if the LSS effect preventing lipid peroxidation requires GSH synthesis. Treatment with BSO, a specific inhibitor of GCL enzyme, resulted in a significant reduction of the antioxidant effect of LSS (Fig. 5A). BSO effect depleting GSH was verified (data not shown).
Fig. 5.
Inhibition of GCL activity by BSO and small-interfering RNA (siRNA)-mediated knockdown of GCLm reduces the antioxidant effect of LSS. In A, HUVECs were pretreated with vehicle or 100 μM DL-buthionine-(S,R)-sulfoximine (BSO) in an NG medium (5.0 mM glucose) for 1 h and then exposed to LSS at 12 dyn/cm2 for 24 h in an HG + AA medium (30 mM glucose + 40 μM AA). In B–D, cells were transfected with 25 nM GCLm siRNA or control duplex oligoribonucleotide for 5 h and then cultured for 36 h before exposure to LSS at 12 dyn/cm2 for 24 h in an HG + AA medium. Expression of GCLm, β-actin, and/or GAPDH at the protein and mRNA levels was monitored by Western blotting and RT-PCR, respectively. Blot and gel images are representative of 3 experiments. The numbers on GCLm Western blot denotes relative expression levels of GCLm protein (means, n = 3). Bars represent means ± SE (n = 3). Data not sharing the same letter are significantly different from each other (P < 0.05).
To further address the essential role of GSH synthesis for the antioxidant effect of LSS, GCLm expression was knocked down with siRNA targeted to the human GCLm gene. LSS at 12 dyn/cm2 prevented the lipid peroxidation induced by HG + AA significantly in control duplex-treated cells, but the effect was much weaker in GCLm siRNA-treated cells (Fig. 5B). GCLm knockdown lowered GSH + GSSG content significantly (Fig. 5C). Specific knockdown of GCLm gene expression was confirmed at the protein and mRNA levels (Fig. 5D).
DISCUSSION
The mechanisms in which HG and AA induce oxidative stress in endothelial cells are of importance because these risk factors in combination are closely associated with the development of diabetic complications (11). Although free fatty acids are generally kept at low micromolar concentrations in plasma (20), their concentration increases up to millimolar range in diabetic patients (29). Excessive influx of acetyl-CoA, both derived from glycolysis of glucose and β-oxidation of free fatty acids, in the tricarboxylic acid cycle can generate an accumulation of mitochondrial NADH in excess of electron transport capacity (3, 21). This condition may lead to an overproduction of ROS at the mitochondrial level, which in turn stimulates NAD(P)H oxidase and other enzymes associated with amplification of oxidative stress and inflammation (15, 26). Indeed, metabolic overload-induced oxidative stress appears to be an important common mechanism of diabetes and cardiovascular disease (3).
The data of the present study suggest that uncoupled eNOS might contribute to the lipid peroxidation induced by HG plus AA (Fig. 1E) and that the lipid proxidation could be mitigated by exogenous antioxidants (Fig. 1F). The present study further demonstrated the novel effect of LSS inhibiting the lipid peroxidation induced by HG plus AA (Fig. 3).
It is of interest to see that NAC was more effective than GSH for the inhibition of lipid peroxidation (Fig. 1F). Because NAC is assumed to be the biosynthetic precursor of GSH, one would have expected comparable antioxidant effects of these two compounds. It is known that NAC is readily taken up by cells and deacetylated, providing l-cysteine, a limiting amino acid for the synthesis of GSH (9). In contrast, extracellular GSH cannot enter cells directly but should be broken down into its constituting amino acids (9). Therefore, external addition of GSH may not lead to rapid increase of intracellular GSH in certain cellular contexts. In this sense, different antioxidant activity of NAC and GSH may be attributed to their different efficiency in increasing intracellular GSH.
The LSS effect inhibiting the lipid peroxidation induced by HG plus AA was partly attributable to the increased biosynthesis of BH4. As an essential cofactor, BH4 may be in greater demand under HG + AA to maintain eNOS activity. The lowed content of BH4 under HG + AA (Fig. 3D) and the inhibitory effect of externally added BH4 on the lipid peroxidation (Fig. 1E) conforms to this notion. A recent study reported that LSS increased BH4 content by activating GTPCH-1 through posttranslational modifications (34). In the current study, LSS was observed to increase the expression GTPCH-1 at the protein and mRNA levels as well, suggesting LSS regulates BH4 biosynthesis at multiple stages.
Although LSS induced GTPCH-1 expression to a similar extent in both NG and HG + AA media (Fig. 4A), BH4 contents were very different from each other (Fig. 3D). This discrepancy, however, could be attributed to the oxidative loss of BH4 under HG + AA conditions. Indeed, BH4 is very prone to oxidative degradation (19), and similar discrepancy between BH4 content and GTPCH-1 expression has been observed in the cells treated with hydrogen peroxide (17).
The current study provided multiple lines of evidence that GSH biosynthesis underlies the LSS effect preventing the oxidative stress induced by metabolic disturbance. First, LSS increased GSH + GSSG contents by approximately twofold in both the NG and HG + AA media (Fig. 3). GSH + GSSG contents varied from ∼20 nmol/mg protein in the static cells in an NG medium to ∼60 nmol/mg protein in the LSS-exposed cells under HG + AA (Fig. 3). The overall variation of GSH + GSSG contents might seem to be very big considering a product inhibition mechanism of GSH synthesis, but similar fold changes have been previously observed in endothelial cells treated with an NO donor (24).
The present study also demonstrated an LSS effect increasing GCLm expression at both the protein and mRNA levels (Fig. 4). Because GCLm increases catalytic activity of GCLc by lowering the Michaelis constant for the substrate l-glutamyl cysteine and decreasing the inhibition by GSH, its expression level could have an influence on GSH biosynthesis. In agreement with this notion, the changes of GCLm expression level due to LSS and HG + AA (Fig. 4) were very comparable to those of intracellular GSH (+GSSG) content (Fig. 3).
LSS has been shown to stimulate the antioxidant response element (ARE), a cis-acting regulatory element that regulates genes encoding enzymes involved in phase II metabolism of xenobiotics and antioxidant defense, in a different manner to oscillatory shear stress (4, 14). GCL is among the enzymes whose expression is controlled by ARE, and previous studies have shown that LSS increased GCLm, but not GCLc, expression at the mRNA level (4, 33). Our data shown in Fig. 4 are in agreement with those studies.
The association of GSH synthesis with the antioxidant effect of LSS was further demonstrated by intervention of GCLm activity and expression (Fig. 5). The antioxidant effect of LSS was significantly reduced by pharmacological inhibition of GCL activity with BSO and siRNA-mediated knockdown of GCLm (Fig. 5).
Of interest, the siRNA-dependent knockdown of GCLm itself increased lipid peroxidation and enhanced the lipid peroxidation by HG + AA (Fig. 5B). BSO treatment also resulted in an increase of lipid peroxidation under HG + AA condition although its effect under the NG condition was insignificant (Fig. 5A). The data suggest that GSH depletion and HG + AA can cause oxidative stress additively or synergistically. Minor difference between BSO and GCLm siRNA effects may be attributed to a different degree and/or duration of GSH depletion.
GCLm siRNA effectively depleted GCLm mRNA and significantly inhibited the LSS-dependent increase of GCLm protein under HG + AA conditions (Fig. 5D). A relatively smaller change of the GCLm protein level than its mRNA level may be an indication of a slow turnover rate of GCLm protein. Nonetheless, the inhibition of GCLm expression by an siRNA approach resulted in a significant decline of intracellular GSH content (Fig. 5C).
The LSS effect inhibiting lipid peroxidation due to HG + AA was significantly attenuated but not completely abolished by BSO or GCLm siRNA treatments. This may be due to incomplete depletion of intracellular GSH or the presence of other defense mechanisms such as superoxide dismutase and peroxiredoxins (16, 25). Although our data indicated that GSH plays a critical role in antioxidant defense by LSS, association of other antioxidants such as l-ascorbic acid and α-tocopherol cannot be excluded. Further studies are needed to examine if the antioxidant effect of LSS involves these antioxidants.
Even though data presented in this study were obtained by using HUVECs that were chosen because such cells are readily available, many of them could be reproduced in other endothelial cells. For example, HG and AA synergistically decreased cell viability and increased lipid peroxidation in bovine aortic endothelial cells (data not shown). However, considering heterogeneity in the vascular endothelium, further studies using different cell types are warranted to generalize the findings of the present study as a common physiology of endothelial cells.
This study suggested that certain strategies to enhance LSS may be potentially useful for the prevention of oxidative stress in endothelial cells. In this regard, frequent exercise would be one of the best choices because it can provide repeated episodes of elevated shear stress. A recent study demonstrated a correlation between brachial artery shear stress and intensity of walking exercise (27). Additionally, pharmacological approaches that can mimic or enhance the LSS effect would be helpful to reduce oxidative stress.
In conclusion, the present study demonstrated a beneficial effect of LSS attenuating oxidative stress due to HG and AA, the important cardiovascular risk factors. To the best of our knowledge, this is the first direct demonstration of the antioxidant effect of LSS inhibiting lipid peroxidation. The antioxidant effect of LSS could be attributed to increased biosynthesis of BH4 and GSH. BH4 could prevent eNOS from the production of ROS, which triggers lipid peroxidation, and GSH could be used for the removal of lipid peroxide by GSH peroxidases. The unique effect of sustained LSS enhancing antioxidant capacity would help understand the key roles of LSS in maintaining endothelial function and vascular health, especially under pathological conditions.
GRANTS
This work was supported by a Korea Science and Engineering Foundation grant funded by the Korea government (R01-2007-000-10580-0).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson ME Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113: 548–555, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388–3396, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A Effects of macronutrient excess and composition on oxidative stress: relevance to diabetes and cardiovascular disease. Curr Atheroscler Rep 8: 472–476, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem 278: 703–711, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res 89: 1073–1080, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89: 271–277, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res 83: 334–341, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576: 557–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith OW Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27: 922–935, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab 27: 436–447, 2001. [PubMed] [Google Scholar]
- 11.Hjelte LE, Nilsson A. Arachidonic acid and ischemic heart disease. J Nutr 135: 2271–2273, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hojo Y, Saito Y, Tanimoto T, Hoefen RJ, Baines CP, Yamamoto K, Haendeler J, Asmis R, Berk BC. Fluid shear stress attenuates hydrogen peroxide-induced c-Jun NH2-terminal kinase activation via a glutathione reductase-mediated mechanism. Circ Res 91: 712–718, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Hong YL, Yeh SL, Chang CY, Hu ML. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clin Biochem 33: 619–625, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280: 27244–27250, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939–1945, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Inoue N, Ramasamy S, Fukai T, Nerem RM, Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Kalivendi S, Hatakeyama K, Whitsett J, Konorev E, Kalyanaraman B, Vasquez-Vivar J. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide–A role for GFRP? Free Rad Biol Med 38: 481–491, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Lee SI, Lee DH, Smith D, Jo H, Schellhorn HE, Boo YC. Ascorbic acid synthesis due to l-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem Biophys Res Commun 345: 1657–1662, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Leaf A Plasma nonesterified fatty acid concentration as a risk factor for sudden cardiac death: the Paris Prospective Study. Circulation 104: 744–745, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Leverve X Hyperglycemia and oxidative stress: complex relationships with attractive prospects. Intensive Care Med 29: 511–514, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Li YSJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271–278, 1978. [DOI] [PubMed] [Google Scholar]
- 24.Moellering D, Mc AJ, Patel RP, Forman HJ, Mulcahy RT, Jo H, Darley-Usmar VM. The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: a role for gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase. FEBS Lett 448: 292–296, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem 283: 1622–1627, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Padilla J, Harris RA, Rink LD, Wallace JP. Characterization of the brachial artery shear stress following walking exercise. Vasc Med 13: 105–111, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Rieder MJ, Carmona R, Krieger JE, Pritchard KA Jr, Greene AS. Suppression of angiotensin-converting enzyme expression and activity by shear stress. Circ Res 80: 312–319, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97: 2859–2865, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 47: 1727–1734, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Szabados E, Fischer GM, Gallyas F Jr, Kispal G, Sumegi B. Enhanced ADP-ribosylation and its diminution by lipoamide after ischemia-reperfusion in perfused rat heart. Free Radic Biol Med 27: 1103–1113, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Vovk T, Bogataj M, Roskar R, Kmetec V, Mrhar A. Determination of main low molecular weight antioxidants in urinary bladder wall using HPLC with electrochemical detector. Int J Pharm 291: 161–169, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med 42: 260–269, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Widder JD, Chen W, Li L, Dikalov S, Thony B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res 101: 830–838, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Wyne KL Free fatty acids and type 2 diabetes mellitus. Am J Med 115, Suppl 8A: 29S–36S, 2003. [DOI] [PubMed]