Abstract
Acute intravenous infusion of ranolazine (Ran), an anti-ischemic/antiangina drug, was previously shown to improve left ventricular (LV) ejection fraction (EF) without a concomitant increase in myocardial oxygen consumption in dogs with chronic heart failure (HF). This study examined the effects of treatment with Ran alone and in combination with metoprolol (Met) or enalapril (Ena) on LV function and remodeling in dogs with HF. Dogs (n = 28) with microembolization-induced HF were randomized to 3 mo oral treatment with Ran alone [375 mg twice daily (bid); n = 7], Ran (375 mg bid) in combination with Met tartrate (25 mg bid; n = 7), Ran (375 mg bid) in combination with Ena (10 mg bid; n = 7), or placebo (PL; Ran vehicle bid; n = 7). Ventriculographic measurements of LV end-diastolic volume (EDV) and end-systolic volume (ESV) and LV EF were obtained before treatment and after 3 mo of treatment. In PL-treated dogs, EDV and ESV increased significantly. Ran alone prevented the increase in EDV and ESV seen in the PL group and significantly increased EF, albeit modestly, from 35 ± 1% to 37 ± 2%. When combined with either Ena or Met, Ran prevented the increase in EDV, significantly decreased ESV, and markedly increased EF compared with those of PL. EF increased from 35 ± 1% to 40 ± 1% with Ran + Ena and from 34 ± 1% to 41 ± 1% with Ran + Met. Ran alone or in combination with Ena or Met was also associated with beneficial effects at the cellular level on histomorphometric parameters such as hypertrophy, fibrosis, and capillary density as well as the expression for pathological hypertrophy and Ca2+ cycling genes. In conclusion, Ran prevented progressive LV dysfunction and global and cellular myocardial remodeling, and Ran in combination with Ena or Met improved LV function beyond that observed with Ran alone.
Keywords: ventricular remodeling, sodium current, transcription factors, left ventricle
even though combination therapy with β-blockers and angiotensin-converting enzyme (ACE) inhibitors is effective in the treatment of chronic heart failure (HF), many patients experience side effects that include hypotension and bradycardia (18), conditions that often limit optimal dosing of these drugs. Therefore, agents capable of improving left ventricular (LV) function and remodeling, and are devoid of such hemodynamic effects, would be desirable as adjunctive therapy for the management of HF. One possible agent is the novel anti-ischemic drug ranolazine (Ran) in which therapeutic effects are independent of heart rate and blood pressure. Ran is a cytoprotective drug that inhibits the late Na+ current (INa) and consequently reduces intracellular Na+ concentration ([Na+]i)-dependent Ca2+ overload mediated by ischemia-reperfusion (37, 45, 48). Results from randomized placebo-controlled clinical trials such as the Monotherapy Assessment of Ranolazine in Stable Angina (MARISA), the Combination Assessment of Ranolazine in Stable Angina (CARISA), and the Evaluation of Ranolazine in Chronic Angina (ERICA) showed that Ran alone or in combination with atenolol, diltiazem, or amlodipine had clinically significant improvement in exercise duration time to 1-mm ST-depression and reduced the incidence of angina attacks (5, 6, 39).
To date, only a few experimental (7, 22, 23) and observational (41) studies have reported on the effects of Ran in HF. Acute intravenous infusion of Ran was shown to improve LV ejection fraction (EF) in dogs with chronic HF without a concomitant increase in myocardial oxygen consumption and a significant improvement in LV mechanical efficiency (7, 23). Ran has no effect on cardiac function in normal dogs, suggesting that the drug improves contractile function in HF by partially correcting the underlying pathophysiology. Cardiomyocytes isolated from LV of HF dogs and from explanted failed human hearts showed enhanced late INa and poor relaxation. Both abnormalities were reversed by Ran (15, 45, 46). Thus Ran, by reducing the pathological enhanced late INa in cardiomyocytes, can potentially improve intracellular Na+ and Ca2+ homeostasis and thereby prevent deterioration of LV function and remodeling of failing hearts. In the present study, we investigated the effects of long-term treatment with Ran alone, and of enalapril or metoprolol tartrate on top of Ran, on LV function and remodeling in HF. Studies were performed in dogs with HF produced by multiple sequential intracoronary microembolizations (35).
METHODS
Experimental model.
The canine model of chronic HF used in this study was previously described in detail (35). In this preparation, LV dysfunction is produced by intracoronary microembolizations that result in loss of viable myocardium. The model has been fully characterized (35) and is well suited to determine the efficacy of experimental HF treatments (17, 33, 40, 42). HF in this model is produced by multiple sequential intracoronary microembolizations that lead to loss of viable myocardium. The model manifests many of the sequelae of HF in humans including profound systolic and diastolic LV dysfunction, LV dilation and compensatory hypertrophy, increased LV filling pressures, increased systemic vascular resistance, and decreased cardiac output (14, 35). LV dysfunction in this model is accompanied by neurohumoral activation including sustained elevation of plasma norepinephrine, angiotensin II, atrial natriuretic peptide, and endothelin-1 levels. As in patients, the model manifests the downregulation of cardiac β-adrenergic receptors (9), development of mild to moderate functional mitral regurgitation (MR) (27), LV shape changes (remodeling; increased chamber sphericity) (27, 28), development of chronic ventricular arrhythmias (24), third heart sound (13), exercise intolerance (25) ultrastructural abnormalities of residual viable cardiomyocytes (30), and accumulation of collagen in the cardiac interstitium. Of particular importance to the present study is the demonstration of spontaneous and progressive deterioration of LV function long after complete cessation of coronary microembolizations (25, 35). We have also shown that the acute hemodynamic response in dogs with HF to intravenous infusion of prototypical drugs, such as dobutamine, nitroprusside, enalaprilat, and digoxin, is similar to responses observed in patients (29, 33).
In the present study, 28 healthy mongrel dogs, weighing between 19 and 25 kg, underwent serial coronary microembolizations to produce HF. Embolizations were performed 1 to 3 wk apart and were discontinued when LV EF, determined angiographically, was between 30% and 40%. All procedures were performed during cardiac catheterization under general anesthesia and sterile conditions. Animals were sedated with intravenous oxymorphone hydrochloride (0.22 mg/kg) and diazepam (0.17 mg/kg), and a plane of anesthesia was maintained with 1% to 2% isoflurane.
Study protocol and primary end points.
A randomized, blinded, placebo-controlled study design was used. Dogs underwent multiple sequential intracoronary microembolizations as previously described (35) to produce chronic HF. Two weeks after the last embolization, dogs were randomized to 3 mo oral treatment with Ran alone [375 mg twice daily (bid); n = 7], Ran (375 mg bid) in combination with metoprolol (25 mg bid; n = 7), Ran (375 mg bid) in combination with enalapril (10 mg bid; n = 7), or to placebo (Ran vehicle bid; n = 7). Hemodynamic, angiographic, echocardiographic, Doppler, and neurohumoral measurements were made before randomization (pretreatment) and after completion of 3 mo of treatment (posttreatment). After the final hemodynamic assessment, and while dogs were under anesthesia, a left thoracotomy was performed and the heart rapidly removed and tissue prepared for histological and biochemical evaluation. The primary study end points were changes in LV EF determined angiographically and changes in global LV remodeling based on changes in LV end-systolic volume (ESV) and end-diastolic volume (EDV), also determined angiographically. The study was approved by Henry Ford Hospital Institutional Animal Care and Use Committee and adheres to American Physiological Society “Guiding Principles in the Care and Use of Animals.”
Hemodynamic, ventriculographic, echocardiographic, and Doppler flow measurements.
Hemodynamic and ventriculographic measurements were made at baseline, before any microembolization, at the time of randomization, before the initiation of treatment (pretreatment), and at the end of 3 mo of therapy (posttreatment). Aortic and LV pressures were measured with catheter-tip micromanometers (Millar Instruments, Houston, TX). Left ventriculograms were obtained with the dog placed on its right side and recorded on 35-mm cinefilm at 30 frames/s during the injection of 20 ml of contrast material (RENO-M-60; Squibb, Princeton, NJ). Extrasystolic and postextrasystolic beats were excluded from any analysis. LV ESV and EDV volumes, LV EF, cardiac index (CI), and stroke volume (SV) were calculated as previously described (33). Echocardiographic and Doppler studies were performed using a 77030A ultrasound system (Hewlett-Packard) with a 3.5-MHz transducer. All echocardiographic measurements were made with the dog placed in the right lateral decubitus position and recorded on a Panasonic 6300 VHS recorder for subsequent offline analysis. LV end-diastolic circumferential wall stress (EDWS) was calculated as previously described (26). Transmitral inflow velocity was measured using pulsed-wave Doppler echocardiography. The velocity waveforms were used to calculate the ratio between peak mitral flow velocity in early diastole (PE) and peak mitral inflow velocity during left atrial contraction (PA), early mitral inflow deceleration time (DT), and the presence and severity of functional MR as previously described (26).
Histomorphometric measurements.
From each heart, including hearts from seven normal dogs, three transverse slices (∼3-mm thick), one each from basal, middle, and apical thirds of the LV, were obtained. From each slice, transmural tissue blocks were obtained and embedded in paraffin blocks. Transmural tissue blocks were also obtained from the free wall segment of the slice, mounted on cork using Tissue-Tek embedding medium, and rapidly frozen in isopentane precooled in liquid nitrogen and stored at −70°C until used. The volume fraction of replacement fibrosis (VFRF), volume fraction of interstitial fibrosis (VFIF), myocyte cross-sectional area (MCSA), a measure of cardiomyocyte hypertrophy, capillary density (CD), and oxygen diffusion distance (ODD) were measured as previously described (34).
Blood neurohumoral and electrolyte measurements.
All plasma samples were submitted for analysis without treatment regimen identifiers. Evaluation of plasma concentrations of several neurohormones were made at each of the study times. Venous blood samples were obtained in duplicate from conscious dogs before cardiac catheterizations for measurement of plasma norepinephrine, plasma renin activity, and plasma atrial natriuretic factor using radioimmunoassay. Blood samples were also obtained for determination of serum electrolytes, namely Na+, K+, creatinine, and blood urea nitrogen.
Protein expression.
All tissue samples were submitted for analysis without treatment regimen identifiers. Protein expression of β-actin, regulator of pathological cardiac hypertrophy early growth response-1 transcription factor (Egr1) and its repressor NGF1A-binding protein-1 (Nab1), Na+/Ca2+ exchanger (NCX) and its modulator GATA-4, and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a was measured in LV tissue from all dogs.
Protein levels of β-actin, Egr1, Nab1, NCX, GATA-4, and SERCA2a were measured in LV homogenate by Western blots using specific primary antibodies as described previously (20, 21). Briefly, LV homogenate was prepared from ∼100 mg LV powder. Samples were thawed in 1 ml of homogenization buffer that consisted of 50 mM Tris·HCl (pH 7.4), 0.5 mM Na+ EDTA (pH 7.0), 0.3 M, sucrose, and protease inhibitors (0.8 mM benzamidine, 0.8 mg/l each aprotinin and leupeptin, and 0.4 μg/l antipain). Thawed tissue was homogenized by a polytron mixer. SDS extract was prepared from the resulting homogenate, and protein assay was determined by Lowry's method. Protein (∼20–100 μg) of each dog sample was separated on 4–20% SDS-polyacrylamide gel (Bio-Rad), and the separated proteins were electrophoretically transferred to a nitrocellulose membrane. The accuracy of the electrotransfer was confirmed by staining the membrane with 0.1% amido black. For identification of the desired protein, the nitrocellulose blot was incubated with the appropriately diluted primary antibody specific to each protein, based on the supplier's instructions. Antibody binding protein(s) was visualized by autoradiography after treating the blot with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence color, developing reagents according to the supplier. Band intensity was quantified using a Bio-Rad GS-670 imaging densitometer and expressed as densitometric units. In all circumstances, we made sure the antibody was present in excess over the antigen and the density of each protein band was in the linear scale.
Statistical analysis.
All angiographic and histomorphometric analyses were performed in a blinded manner. Within-group comparisons were made between measurements obtained at pretreatment and measurements made at posttreatment using the Student's paired t-test with significance set at P < 0.05. To ensure that all study measures were similar at baseline, before any microembolizations, and at the time of randomization (pretreatment), intergroup comparisons were made using one-way ANOVA with α set at 0.05. To assess treatment effect, the change (Δ) in each measure from pretreatment to posttreatment was calculated for each of the four study groups. Comparisons of the changes between the control group and each of the three treatment groups were also made using ANOVA with α set at 0.05. Histomorphometric and protein expression results obtained in all four treatment arms and in a group of normal dogs were examined using ANOVA with α set at 0.05. In all instances, if significance was attained by overall ANOVA, pairwise comparisons were performed using the Student-Newman-Keuls test, with P < 0.05 considered significant. All data are reported as means ± SE.
RESULTS
All 28 study animals survived the entire study. Baseline cardiac function and blood chemistry were within the normal range and similar among study groups. Similarly, there were no differences among groups for all measures made at pretreatment.
Effects of placebo on the progression of LV dysfunction.
Results are shown in Table 1. There were no differences in heart rate, mean aortic pressure, and LV end-diastolic pressure (EDP) between pre- and posttreatment. At posttreatment, peak LV increase and decrease in pressure over time (+dP/dt and −dP/dt, respectively) decreased significantly, LV EDV and ESV increased significantly, and LV EF and SV decreased significantly compared with those at pretreatment. The ratio of PE to PA and DT all decreased significantly, whereas MR severity and LV EDWS increased. There were no significant changes in plasma neurohormones and electrolytes.
Table 1.
Hemodynamic, angiographic, echocardiographic, Doppler, neurohumoral, and electrolyte measures at pretreatment and posttreatment
|
Placebo |
Ran Alone
|
Ran + Ena
|
Ran + Met
|
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| HR | 79±5 | 82±5 | 77±2 | 80±4 | 82±4 | 84±4 | 84±2 | 80±3 |
| mAoP | 77±4 | 72±3 | 72±3 | 81±7 | 74±4 | 76±5 | 73±3 | 72±5 |
| LVEDP | 14±1 | 15±1 | 14±1 | 10±1* | 13±1 | 9±1* | 14±1 | 7±1* |
| +dP/dt | 1,287±74 | 1,080±203* | 1,253±119 | 1,416±124 | 1,079±77 | 1,349±141 | 1,151±104 | 1,273±103 |
| −dP/dt | 1,458±110 | 1,188±68* | 1,254±74 | 1,460±134 | 1,161±82 | 1,514±205 | 1,416±69 | 1,710±152 |
| EDV | 60±2 | 69±2* | 63±3 | 65±2 | 59±3 | 59±3 | 61±1 | 59±1* |
| ESV | 38±2 | 50±2* | 41±2 | 41±2 | 39±2 | 35±2* | 40±1 | 35±1* |
| EF | 36±1 | 28±1* | 35±1 | 37±2* | 35±1 | 40±1* | 34±1 | 41±1* |
| SV | 22±1 | 19±1* | 22±1 | 24±1* | 21±1 | 24±1* | 20±1 | 24±1* |
| CI | 2.2±0.2 | 1.9±0.1 | 2.0±0.2 | 2.3±0.2* | 2.2±0.1 | 2.6±0.2* | 2.1±0.1 | 2.4±0.1* |
| PE-to-PA | 2.8±0.2 | 2.2±0.2* | 2.7±0.3 | 2.8±0.3 | 2.4±0.3 | 3.1±0.4 | 2.4±0.2 | 3.1±0.3* |
| MR | 11.6±2.3 | 14.1±2.0* | 11.3±1.9 | 10.1±1.3 | 11.9±2.9 | 6.7±1.9* | 10.6±1.8 | 6.0±1.3* |
| DT | 81±3 | 67±3* | 88±3 | 87±4 | 78±4 | 90±5* | 78±6 | 95±5* |
| EDWS | 61±4 | 67±4 | 56±5 | 42±4* | 51±3 | 38±3* | 53±4 | 33±4* |
| Na+ | 148±0.4 | 148±0.7 | 147±0.5 | 147±0.8 | 148±1.0 | 147±0.7 | 148±0.9 | 147±0.8 |
| K+ | 4.6±0.3 | 4.5±0.1 | 4.5±0.1 | 4.7±0.1 | 4.5±0.1 | 4.7±0.1 | 4.6±0.2 | 4.6±0.1 |
| Creat | 0.9±0.1 | 0.9±0.0 | 0.9±0.0 | 1.0±0.1 | 0.9±0.0 | 0.9±0.0 | 0.9±0.0 | 0.9±0.0 |
| BUN | 16±2 | 13±1 | 18±2 | 15±2 | 17±1 | 16±2 | 18±3 | 14±1 |
| PNE | 137±22 | 200±43 | 217±25 | 199±43 | 213±40 | 212±45 | 190±40 | 159±31 |
| PRA | 2.02±0.7 | 2.04±0.1 | 1.69±0.7 | 1.57±0.5 | 1.47±0.3 | 3.49±1.0 | 1.85±0.4 | 1.82±0.6 |
| ANF | 73±8 | 78±3 | 87±15 | 67±11 | 86±16 | 78±3 | 99±13 | 84±12 |
Values are means ± SE. Ran, ranolazine; Ena, enalapril; Met, metoprolol; Pre, pretreatment; Post, postconditioning; HR, heart rate (in beats/min); mAoP, mean aortic pressure (in mmHg); LVEDP, left ventricular (LV) end-diastolic pressure (in mmHg); +dP/dt, peak LV increase in pressure over time (in mmHg/s); −dP/dt, peak LV decrease in pressure over time (in mmHg/s); EDV, LV end-diastolic volume (in ml); ESV, LV end-systolic volume (in ml); EF, LV ejection fraction (in %); SV, stroke volume (in ml); CI, cardiac index (l·min−1·m−2); PE-to-PA, the ratio between peak mitral flow velocity in early diastole and peak mitral inflow velocity during left atrial contraction; MR, severity of mitral regurgitation (in %); DT, deceleration time (in ms); EDWS, LV end-diastolic circumferential wall stress (in gm/cm2); Creat, serum creatinine (in mg/dL); BUN, blood urea nitrogen (in mg/dL); PNE, plasma norepinephrine (in pg/ml); PRA, plasma renin activity (in ng·ml−1·h−1); ANF, atrial natriuretic factor (in pg/ml).
P < 0.05 vs. Pre.
Effects of monotherapy with Ran.
Results are shown in Table 1. In this group, heart rate, mean aortic pressure, LV peak +dP/dt and peak −dP/dt were not significantly changed between pre- and posttreatment, but LV EDP decreased significantly. LV EDV and ESV did not change significantly, whereas EF, SV, and CI increased significantly. The ratio of PE to PA, DT, and MR severity were unchanged, whereas EDWS decreased significantly. There were no significant changes in plasma neurohormones and electrolytes.
Effects of combination therapy with Ran and enalapril.
Results are shown in Table 1. In this group, heart rate, mean aortic pressure, and LV peak +dP/dt and peak −dP/dt were not significantly changed between pre- and posttreatment, but LV EDP decreased significantly. LV EDV remained unchanged and ESV decreased significantly, whereas EF, SV, and CI increased significantly. The PE-to-PA ratio tended to increase, but the increase did not reach statistical difference. The severity of functional MR and LV EDWS decreased significantly. There were no significant differences in plasma neurohormones and electrolytes.
Effects of combination therapy with Ran and metoprolol.
Results are shown in Table 1. In this group, heart rate, mean aortic pressure, and LV peak +dP/dt and peak −dP/dt were not significantly changed between pre- and posttreatment, but LV EDP decreased significantly. LV EDV and ESV decreased significantly, whereas EF, SV, and CI increased significantly. There were significant changes in the PE-to-PA ratio and DT. The severity of MR and EDWS decreased significantly. There were no significant differences in plasma neurohormones and electrolytes.
Comparisons of treatment effect.
The change (Δ) between pre- and posttreatment for all four groups is shown in Table 2. Treatment effect analysis showed no differences among the groups with respect to heart rate and mean aortic pressure. When compared with those of placebo, LV EDP, peak LV +dP/dt, and peak −dP/dt increased significantly in dogs treated with Ran alone and in dogs treated with Ran in combination with either enalapril of metoprolol. LV EDV, ESV, EF, SV, and CI all improved significantly in all three active study groups compared with placebo. The reduction in EDV and ESV and the increase in EF were significantly greater in dogs randomized to combination therapy compared with dogs randomized to Ran alone. When compared with placebo, Ran alone significantly reduced EDWS, whereas combination therapies significantly improved PE-to-PA, severity of MR, DT, and EDWS. There were no significant differences among the four study groups with respect to plasma neurohormones and electrolytes.
Table 2.
Comparison of the change from pretreatment to posttreatment in hemodynamic, angiographic, echocardiographic, Doppler, neurohumoral, and electrolyte measurements between the 4 study groups (treatment effect)
| Placebo | Ran Alone | Ran + Ena | Ran + Met | |
|---|---|---|---|---|
| ΔHR | 3.1±8.3 | 3.3±4.9 | 2.0±4.5 | −2.9±3.1 |
| ΔmAoP | −5.6±3.2 | 9.1±6.9 | 1.7±7.7 | −1.6±6.6 |
| ΔEF | −9.0±−1.0 | 2.0±1.0* | 5.0±1.0* | 8.0±1.0* |
| ΔESV | 12.0±1.0 | 0.0±1.0* | −3.0±1.0* | −5.0±1.0* |
| ΔEDV | 9.0±1.0 | 2.0±1.0* | −1.0±1.0* | −2.0±1.0* |
| ΔLVEDP | 1.0±1.0 | −4.4±1.1* | −4.3±0.6* | −6.9±1.1* |
| Δ+dP/dt | −207±61 | 163±81* | 270±121* | 121±66* |
| Δ−dP/dt | −270±102 | 206±109* | 353±167* | 294 ± 135* |
| ΔSV | −3±1 | 2±1* | 3±1* | 4±1* |
| ΔCI | −0.3±0.2 | 0.3±0.1* | 0.4±0.2* | 0.4±0.1* |
| ΔPE-to-PA | −0.6±0.2 | 0.0±0.4 | 0.7±0.3* | 0.7±0.2* |
| ΔMR | 2.5±0.9 | −1.2±1.1 | −5.2±2.2* | −4.62±1.4* |
| ΔDT | −14±4 | −1±4 | 12±6* | 17±7* |
| ΔEDWS | 6.7±4 | −14.5±5.2* | −13.3±3.3* | −20.4±3.5* |
| ΔNa+ | 1.1±0.9 | −0.3±1.0 | −1.0±1.2 | −0.5±1.2 |
| ΔK+ | −0.1±0.2 | 0.1±0.1 | 0.2±0.1 | 0.0±0.2 |
| ΔCreat | 0.0±0.0 | 0.0±0.1 | 0.0±0.1 | 0.0±0.0 |
| ΔBUN | −2.9±1.3 | −2.1±1.4 | −1.8±2.2 | −4.2±1.9 |
| ΔPNE | 52±33 | −19±42 | −15±61 | −31±35 |
| ΔPRA | 0.03±0.64 | 0.13±0.92 | 2.02±1.04 | −0.45±0.30 |
| ΔANF | 7±8 | 20±14 | −7±19 | −15±17 |
Values are means ± SE.
P < 0.05 vs. placebo.
Histomorphometric findings.
Histomorphometric results are shown in Table 3. When compared with normal dogs, dogs treated with placebo showed a significant increase in MCSA, VFRF, VFIF, and ODD along with a significant decrease in CD. Treatment with Ran alone as well as treatment with combination therapy significantly improved all of the above histomorphometric measures compared with those of placebo. The extent of improvement was significantly greater in dogs treated with combination therapy compared with Ran alone.
Table 3.
Histomorphometric measurements
| Normal | Placebo | Ran Alone | Ran + Ena | Ran + Met | |
|---|---|---|---|---|---|
| MCSA, μm2 | 409±10 | 772±21* | 685±23*† | 558±11*†‡ | 571±14*†‡ |
| VFRF, % | 0±0 | 14.6±1.2* | 10.5±2.1*† | 9.4±0.9*† | 9.3±1.1*†‡ |
| VFIF, % | 3.7±0.1 | 13.0±0.7* | 11.0±0.4*† | 8.8±0.6*†‡ | 7.8±0.7*†‡ |
| CD, capillaries/mm2 | 2,607±80 | 1,706±28* | 1,832±43*† | 2,059±82*† | 2,049±80*† |
| ODD, μm | 8.9±0.2 | 11.5±0.3* | 10.9±0.2* | 10.4±0.4* | 10.6±0.5* |
Values are means ± SE. MCSA, myocyte cross-sectional area; VFRF, volume fraction of replacement fibrosis; VFIF, volume fraction of interstitial fibrosis; CD, capillary density; ODD, oxygen diffusion distance.
P < 0.05 vs. normal;
P < 0.05 vs. placebo;
P < 0.05 vs. Ran alone.
Protein expression.
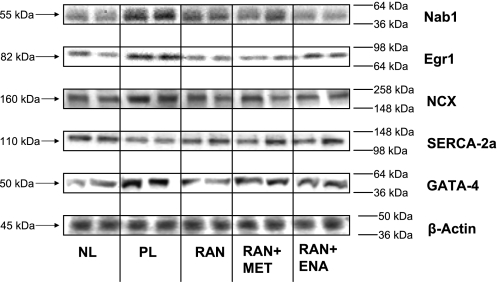
Protein expression of β-actin was not significantly different among study groups and normal dogs. Protein expression of both Nab1 and Egr1 increased significantly in placebo compared with normal dogs, and Ran alone or in combination with enalapril or metoprolol prevented the increase (Fig. 1 and Table 4). Protein expression of SERCA2a was significantly decreased and that of NCX significantly increased in placebo compared with normal dogs. These maladaptations were completely prevented by Ran either alone or in combination with enalapril or metoprolol (Fig. 1 and Table 4). Protein expression of the transcription factor GATA-4, crucial for NCX expression, increased in placebo compared with normal dogs. This increase was attenuated in dogs treated with Ran alone and Ran in combination with enalapril or metoprolol (Fig. 1 and Table 4).
Fig. 1.
Representative Western blot bands showing protein expression of NGF1A-binding protein-1 (Nab1), early growth response-1 transcription factor (Egr1), Na+/Ca2+ exchanger (NCX), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, GATA-4, and β-actin in left ventricular myocardium of 2 normal dogs (NL), 2 placebo dogs (PL), 2 dogs after 3 mo of therapy with ranolazine (Ran), and 2 dogs treated with Ran in combination with enalapril (Ena; Ran + Ena) or Ran in combination with metoprolol (Met; Ran + Met).
Table 4.
Protein expression in densitometric units
| Normal | Placebo | Ran Alone | Ran + Ena | Ran + Met | |
|---|---|---|---|---|---|
| Nab1 | 369±16 | 683±43* | 382±32† | 376±27† | 266±24† |
| Egr1 | 252±23 | 505±18* | 276±30† | 206±8† | 269±29† |
| NCX | 78±2 | 107±4* | 87±4† | 75±4† | 78±4† |
| SERCA2a | 64±1 | 49±2* | 59±2† | 62±4† | 57±2† |
| GATA-4 | 56±7 | 98±2* | 77±4† | 81±3† | 76±6† |
| β-actin | 34±1 | 35±1 | 33±2 | 31±1 | 32±2 |
Values are means ± SE. Nab1, NGF1A-binding protein-1; Egr1, early growth response-1 transcription factor; NCX, Na+/Ca2+ exchanger; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a.
P < 0.05 vs. normal;
P < 0.05 vs. placebo.
DISCUSSION
The present study demonstrates that treatment with Ran prevents progressive LV dysfunction and attenuates LV remodeling in dogs with moderate HF. This conclusion is supported by the finding that 3 mo of treatment with Ran increased LV EF relative to placebo and prevented progressive LV enlargement. Furthermore, the beneficial effects of Ran on LV function and myocardial remodeling were associated, at the cellular level, with a lower VFRF and VFIF, increased CD, improved ODD, and reduced MCSA, a measure of cardiomyocyte hypertrophy. In addition, Ran alone or in combination with either enalapril or metoprolol tended to normalize the protein expression of Nab1, Egr1, SERCA2a, NCX, and GATA-4 in LV tissue. Treatment with Ran in combination with either enalapril or metoprolol was more efficacious than Ran alone. Overall, these findings provide supporting evidence that Ran alone or in combination with commonly used HF drugs prevents and/or retards the progressive worsening of the HF state.
The present study extends previous observations from our laboratory in the same HF model in which acute treatment with Ran improved LV function and mechanical efficiency (7, 23). Three months of oral treatment with Ran prevented LV remodeling and deterioration of LV function. To date, the effects of Ran on LV function or exercise performance have not been investigated in a prospective trial of patients with HF. There is clearly a need for novel drugs for the treatment of HF that act outside the classic neurohormonal axis and do not negatively affect blood pressure or heart rate (10), and the present findings provide impetus for clinical evaluation of Ran in HF patients.
Late INa-induced impairment of Na+ and Ca2+ homeostasis and the mechanism of action of Ran.
Ran does not directly affect heart rate, blood pressure, intrinsic cardiac contractility, or myocardial blood flow (1, 22). The exact mechanisms responsible for the beneficial actions of Ran in HF, shown in the present study, remain to be established. Nonetheless, there are sufficient studies to warrant discussion of potential mechanisms of action. Early during the development of this compound, Ran was thought to act, in part, by eliciting partial fatty acid oxidation inhibition (19). Results of new studies, however, have suggested that Ran may act primarily as a modulator of intracellular Ca2+ homeostasis by inhibiting the pathologically enhanced late INa (2, 37, 45).
HF is associated with imbalances of Na+ and Ca2+ homeostasis (3, 19). In HF, the cardiomyocyte Na+ channel is pathologically altered, leading to increased Na+ influx into the cell (15, 45). The Na+ channel in cardiomyocytes from dogs and humans with HF has a markedly increased late component (late INa) that persists throughout the duration of the action potential plateau (45–47). This results in an increase of [Na+]i, which leads to an increased exchange of intracellular Na+ for extracellular Ca2+ through the NCX, and consequently Ca2+ overload. The latter can lead to both electrical (e.g., afterdepolarizations, increased dispersion of repolarization, and ectopic activity) and mechanical (e.g., delayed and incomplete diastolic relaxation) dysfunction (3). Elevations of cellular Na+ and Ca2+ concentrations are also linked to the regulation of gene expression that contributes to pathophysiological hypertrophy and remodeling (19) of the heart. Ran has been shown to reduce late INa in numerous experimental models, including cardiomyocytes from failing dog hearts, and to reverse the Na+ and Ca2+ overload phenotype caused by an augmentation of late INa (2, 8, 38, 45). Therefore, it is not surprising that in the setting of chronic HF, as in this study, treatment with Ran was associated with improvement of LV function along with the normalization of expression of proteins that involved in the regulation of hypertrophy and intracellular Ca2+ homeostasis.
Effects of Ran on myocardial fibrosis and cardiomyocyte hypertrophy.
HF is associated with excessive accumulation of collagen in the cardiac interstitium and with cardiomyocyte hypertrophy, structural maladaptations that can lead to LV diastolic dysfunction and ultimately systolic dysfunction. In the present study, the VFIF, VFRF, and MCSA, the latter a measure of cardiac hypertrophy, were significantly reduced and CD and ODD significantly increased in dogs treated with Ran compared with placebo. Reactive interstitial fibrosis in the setting of HF is frequently accompanied by a decrease in CD and an increase in ODD (32), a condition that promotes hypoxia of collagen-encircled cardiomyocytes (32). A decrease in interstitial fibrosis along with increased CD is expected to improve oxygen delivery to cardiomyocytes. The combination of enalapril or metoprolol with Ran resulted in a normalization of histomorphometric parameters that were more pronounced than with Ran alone. This finding could be explained by the effects of both enalapril and metoprolol to independently modulate interstitial fibrosis and cardiomyocyte hypertrophy (17, 34).
Other study observations included a decrease in LV EDP and a decrease in LV EDWS in Ran dogs, both desired end points in the treatment of HF. Ran also improved LV fractional area of shortening, PE-to-PA ratio, severity of MR, and DT, but the improvement was more pronounced when Ran was combined with either enalapril or metoprolol. Depending on its severity, functional MR can reduce the effective stroke output of the failing LV, leading to the activation of vasoconstrictor systems to maintain homeostasis (36). From this perspective, functional MR can be viewed as an important component of the progressive deterioration of ventricular pump function that characterizes the HF state. Accordingly, any therapeutic interventions that can ameliorate or prevent functional MR can be considered beneficial in the treatment of HF.
In addition to improving LV function and attenuating progressive global LV remodeling, dogs treated with Ran alone or in combination with either enalapril or metoprolol showed a normalization in protein expression of regulators of pathological cardiac hypertrophy including Egr1 and its repressor Nab1 and of the Ca2+-handling proteins NCX and its modulator GATA-4 and the sarcoplasmic reticulum pump SERCA2a. The Nab1-Egr1 axis is a major regulator of pathological cardiac hypertrophy (4). Nab1 is upregulated in HF in mammals and blocks cardiomyocyte hypertrophy by repressing transcription factor Egr1, a key regulator of cardiac hypertrophy (4). It has been reported that cardiac overexpression of Nab1 in transgenic mice potently inhibits pathological cardiac hypertrophy by repression of Egr1, whereas physiological growth was not affected (4). It has been reported that expression of NCX is increased in HF, whereas SERCA2a is decreased (11). These are key myocardial Ca2+-handling proteins. It is suggested that a robust Ca2+ removal by NCX partially compensates for the impaired removal of diastolic Ca2+ (11, 43, 44). In the present study, it is reasonable to assume that the observed decrease in pathological cardiac hypertrophy in dogs treated with Ran alone or in combination with enalapril or metoprolol may partly be due to the modulation of the Nab1-Egr1 axis and a downregulation in the expression of NCX and GATA-4 and/or upregulation in SERCA2a.
Study limitations.
Treatment with Ran in combination with either enalapril or metoprolol elicited additional improvements in cardiac function and morphology compared with those of Ran alone. A limitation of this study, however, is the lack of an enalapril or metoprolol monotherapy arms. Nevertheless, previous published data from our laboratory showed that monotherapy of dogs with HF with enalapril or metoprolol prevented progressive LV remodeling and dysfunction; in the present study, Ran in combination with enalapril or metoprolol improved LV function beyond that observed with enalapril or metoprolol alone in previous studies from our laboratory (17, 33, 34). It would also have been informative to have demonstrated that Ran, with or without enalapril or metoprolol, decreases intracellular Na+ and Ca2+ overload in failing cardiomyocytes. Lastly, the measurement of ventricular function volume was made under general anesthesia, as in previous studies from our laboratory (12, 16, 31, 49), which may have affected contractile function compared with conscious conditions. However, measurements in all treatment groups were made under the same conditions; thus it is unlikely that our conclusions regarding the effects of Ran are dependent upon isoflurane anesthesia.
Conclusions
The results of this study indicate that monotherapy with Ran prevents the progression of HF, as evidenced by the preservation of LV function and attenuation of LV remodeling. Furthermore, treatment with an ACE inhibitor or a β-blocker, in combination with Ran, resulted in improvement in LV systolic and diastolic function and reversal of global and cellular LV remodeling that was greater than with Ran alone. The results in the present study support an evaluation of Ran as adjunctive therapy in patients with HF.
GRANTS
This work was supported, in part, by research grants from CV Therapeutics and by National Heart, Lung, and Blood Institute Grants RO1-HL-49090 and PO1-HL-074237.
DISCLOSURES
Drs. H. N. Sabbah and W. C. Stanley had research grant support from CV Therapeutics, and Drs. L. Belardinelli and B. Blackburn are full-time employees of CV Therapeutics.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bagger JP, Botker HE, Thomassen A, Nielsen TT. Effects of ranolazine on ischemic threshold, coronary sinus blood flow, and myocardial metabolism in coronary artery disease. Cardiovasc Drugs Ther 11: 479–484, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart 92, Suppl 4: iv6–iv14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic, 2001.
- 4.Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, Ivashchenko Y, Lohse MJ, Engelhardt S. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nat Med 11: 837–844, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 291: 309–316, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, Pepine CJ, Wang W, Nelson JJ, Hebert DA, Wolff AA. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 43: 1375–1382, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, Wolff A, Sabbah HN. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res 91: 278–280, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol 41: 1031–1038, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gengo PJ, Sabbah HN, Steffen RP, Sharpe JK, Kono T, Stein PD, Goldstein S. Myocardial beta adrenoceptor and voltage sensitive calcium channel changes in a canine model of chronic heart failure. J Mol Cell Cardiol 24: 1361–1369, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 44: 954–967, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99: 641–648, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Imai M, Rastogi S, Gupta RC, Mishra S, Sharov VG, Stanley WC, Mika Y, Rousso B, Burkhoff D, Ben-Haim S, Sabbah HN. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol 49: 2120–2128, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Kono T, Rosman H, Alam M, Stein PD, Sabbah HN. Hemodynamic correlates of the third heart sound during the evolution of chronic heart failure. J Am Coll Cardiol 21: 419–423, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Kono T, Sabbah HN, Rosman H, Alam M, Stein PD, Goldstein S. Left atrial contribution to ventricular filling during the course of evolving heart failure. Circulation 86: 1317–1322, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9: 219–227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita H, Khanal S, Rastogi S, Suzuki G, Imai M, Todor A, Sharov VG, Goldstein S, O′Neill TP, Sabbah HN. Selective matrix metalloproteinase inhibition attenuates progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Am J Physiol Heart Circ Physiol 290: H2522–H2527, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Morita H, Suzuki G, Mishima T, Chaudhry PA, Anagnostopoulos PV, Tanhehco EJ, Sharov VG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with metoprolol CR/XL on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Cardiovasc Drugs Ther 16: 443–449, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Pham DQ, Mehta M. Ranolazine: a novel agent that improves dysfunctional sodium channels. Int J Clin Pract 61: 864–872, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res 57: 874–886, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi S, Mishra S, Zaca V, Alesh I, Gupta RC, Goldstein S, Sabbah HN. Effect of long-term monotherapy with the aldosterone receptor blocker eplerenone on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiovasc Drugs Ther 21: 415–422, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi S, Mishra S, Zaca V, Mika Y, Rousso B, Sabbah HN. Effects of chronic therapy with cardiac contractility modulation electrical signals on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiology 110: 230–237, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 95: 311–316, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, Biesiadecki BJ, Blackburn B, Wolff A, Stanley WC. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves left ventricular function in dogs with chronic heart failure. J Card Fail 8: 416–422, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah HN, Goldberg AD, Schoels W, Kono T, Webb C, Brachmann J, Goldstein S. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: relation to haemodynamics and sympathoadrenergic activation. Eur Heart J 13: 1562–1572, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Sabbah HN, Hansen-Smith F, Sharov VG, Kono T, Lesch M, Gengo PJ, Steffen RP, Levine TB, Goldstein S. Decreased proportion of type I myofibers in skeletal muscle of dogs with chronic heart failure. Circulation 87: 1729–1737, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M. Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. Am J Cardiol 99: 41A–46A, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J 123: 961–966, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Sabbah HN, Kono T, Stein PD, Mancini GB, Goldstein S. Left ventricular shape changes during the course of evolving heart failure. Am J Physiol Heart Circ Physiol 263: H266–H270, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Sabbah HN, Levine TB, Gheorghiade M, Kono T, Goldstein S. Hemodynamic response of a canine model of chronic heart failure to intravenous dobutamine, nitroprusside, enalaprilat, and digoxin. Cardiovasc Drugs Ther 7: 349–356, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 24: 1333–1347, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Sabbah HN, Sharov VG, Gupta RC, Mishra S, Rastogi S, Undrovinas AI, Chaudhry PA, Todor A, Mishima T, Tanhehco EJ, Suzuki G. Reversal of chronic molecular and cellular abnormalities due to heart failure by passive mechanical ventricular containment. Circ Res 93: 1095–1101, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 147: 29–34, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 89: 2852–2859, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation 102: 1990–1995, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Shimoyama H, Sabbah HN, Rosman H, Kono T, Alam M, Goldstein S. Effects of long-term therapy with enalapril on severity of functional mitral regurgitation in dogs with moderate heart failure. J Am Coll Cardiol 25: 768–772, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–222, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol 44: 192–199, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 48: 566–575, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki G, Morita H, Mishima T, Sharov VG, Todor A, Tanhehco EJ, Rudolph AE, McMahon EG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation 106: 2967–2972, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Taegtmeyer H Cardiac metabolism as a target for the treatment of heart failure. Circulation 110: 894–896, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tanimura M, Sharov VG, Shimoyama H, Mishima T, Levine TB, Goldstein S, Sabbah HN. Effects of AT1-receptor blockade on progression of left ventricular dysfunction in dogs with heart failure. Am J Physiol Heart Circ Physiol 276: H1385–H1392, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Terracciano CM, Philipson KD, MacLeod KT. Overexpression of the Na+/Ca2+ exchanger and inhibition of the sarcoplasmic reticulum Ca2+-ATPase in ventricular myocytes from transgenic mice. Cardiovasc Res 49: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Terracciano CM, Souza AI, Philipson KD, MacLeod KT. Na+-Ca2+ exchange and sarcoplasmic reticular Ca2+ regulation in ventricular myocytes from transgenic mice overexpressing the Na+-Ca2+ exchanger. J Physiol 512: 651–667, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 17, Suppl 1: S169–S177, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci 55: 494–505, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38: 475–483, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther 321: 213–220, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Zaca V, Rastogi S, Imai M, Wang M, Sharov VG, Jiang A, Goldstein S, Sabbah HN. Chronic monotherapy with rosuvastatin prevents progressive left ventricular dysfunction and remodeling in dogs with heart failure. J Am Coll Cardiol 50: 551–557, 2007. [DOI] [PubMed] [Google Scholar]